Abstract
Background
Evidence shows that wait times before bladder cancer surgery have been increasing, and wait time can negatively affect survival. We aimed to determine if a long delay caused by an indirect referral before a first urologist visit affects the survival of patients undergoing radical cystectomy for bladder cancer.
Methods
We analyzed data from 1271 patients who underwent surgery for bladder cancer during the decade 2000–2009. The cohort was obtained by linking two administrative databases in the province of Quebec. Patients were considered to have been directly referred to a urologist if they had 5 or fewer visits with a general practitioner before their first urologist visit; otherwise, they were considered to have been indirectly referred. The effect on survival after surgery of a longer delay before a first urologist visit was assessed using Cox regression models.
Results
Median referral delay for the study population was 30 days (56 days for women, 23 days for men; p < 0.0001). Indirect referral was observed for 49% of women and 33% of men. Compared with patients who were directly referred, those who were indirectly referred after first symptoms of bladder cancer experienced poorer survival (hazard ratio: 1.29; 95% confidence interval: 1.10 to 1.52). Women who were indirectly referred had a significant 47% greater risk of death after radical cystectomy. Men who were indirectly referred also experienced decreased survival (adjusted hazard ratio: 1.25; 95% confidence interval: 1.03 to 1.51).
Conclusions
Patients indirectly referred to a urologist had an increased risk of mortality after surgery. Compared with men, women had longer wait times and poorer survival.
Keywords: Urologist referral delay, bladder cancer, survival, radical cystectomy, cohort study
1. INTRODUCTION
Each year, approximately 390,000 people worldwide are diagnosed with bladder cancer1. The disease is the 2nd most common urologic cancer and the 5th most frequently diagnosed malignancy in Canada2. Unlike superficial bladder cancer, muscle-invasive disease is often treated with surgical removal of the bladder (radical cystectomy)3. Even though it is well accepted that treatment should be instituted once a diagnosis of bladder cancer has been made, there is growing evidence that preoperative wait times for cancer surgery have been increasing4,5. In addition, it has been suggested that delaying radical cystectomy for bladder cancer is associated with poorer survival6,7.
Limited studies on the effect of sex in the continuum of health care for cancer suggest that urinary tract malignancies might be diagnosed less promptly in women than in men8. Although bladder cancer occurs with a higher incidence in men than in women, women tend to present with more advanced disease9. Moreover, women with bladder cancer are more likely than men to have pre-referral consultations with a general practitioner (gp) or a gynecologist and to experience a longer wait time between first symptoms and urologist referral10. Those sex differences indicate that gps might interpret the clinical importance of first symptoms of bladder cancer differently in women, potentially attributing them to other causes, such as urinary tract infections. Previous work suggests worse survival outcomes for women with bladder cancer11. However, it is not clear whether the detrimental effect on survival is caused by a wait time between onset of symptoms and referral to a urologist that is longer for women than for men. We therefore conceived this study, which set out to determine whether a long wait time between the first gp visit and the first urologist visit (“referral delay”) has a negative effect on survival after radical cystectomy, and to explore sex-related differences in survival. Long referral delay can be caused by patients having additional gp or non-urologic specialist consultations before a first urologist visit.
2. METHODS
2.1. Study Design
This retrospective cohort study used data from 2778 patients who underwent radical cystectomy for bladder cancer in Quebec between January 1, 2000, and September 30, 2009.
2.2. Data Source
The cohort was built by linking two administrative databases: the medical billing records database of the Régie de l’assurance maladie du Québec (ramq), and the Registre des événements démographiques of the Institut de la statistique du Québec.
The ramq is the government body that administers provision of health care in the province. All health care services are recorded in ramq administrative databases and claims files. The ramq medical claims database provides information about medical services dispensed to all Quebec residents. It includes patient diagnoses (coded according to the International Classification of Diseases, 9th revision), relevant therapeutic procedures and their calendar dates (visits to gps, urologists, and other specialists; radical cystectomy; etc.), patient characteristics, health care providers, hospital facilities, and the costs involved. The ramq does not collect information on disease stage or grade and on the patient’s functional status.
The Institut de la statistique du Québec administers the Registre des événements démographiques, which provides demographic data on all births and deaths in Quebec.
The linkage between ramq and Institut data is made possible by matching records based on an anonymous patient identifier (generated from the individual’s health insurance number, a unique identifier for all legal residents of Quebec).
The use of the data was approved by Quebec’s Commission d’accès à l’information, the provincial agency that grants authorization for the use of linked administrative databases. The study protocol was approved by the Research Ethics Board of the McGill University Health Centre.
2.3. Study Population
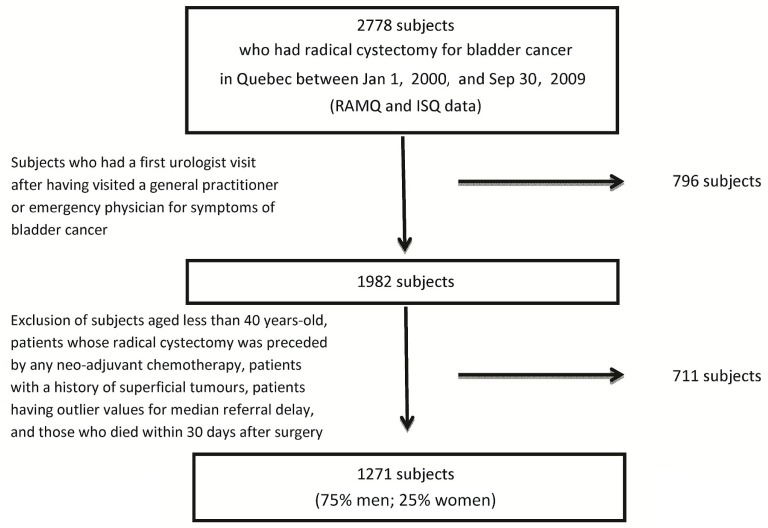
The analysis considered data from a sub-cohort of the initially identified 2778 patients. Patients in the sub-cohort had attended their first urologist visit after having visited a gp or emergency physician for bladder cancer symptoms (Figure 1). The analysis excluded patients less than 40 years of age (the cut-off for a microhematuria work-up in Canada), patients whose radical cystectomy was preceded by any neoadjuvant chemotherapy, patients with a history of superficial tumours, patients having outlier values for median referral delay, and patients who died within the 30 days after their surgery. “Outlier values” were defined as wait times longer than the upper quartile of the delay distribution plus 3 times the interquartile range5. Given that information on tumour stage was lacking, a “history of superficial tumours” was presumed for patients who underwent more than 1 transurethral resection of bladder tumour more than 4 months apart. The index date for a given patient was the date on which that individual entered the cohort (calendar date of the radical cystectomy).
FIGURE 1.
Selection of the study population. ramq = Régie de l’assurance maladie du Québec; isq = Institut de la statistique du Québec.
2.4. Comparison Groups
We analyzed survival after radical cystectomy, dividing the cohort into patients who were directly or indirectly referred to a urologist after consultation with a gp, emergency physician, or other specialist during which a diagnostic code associated with a bladder tumour was recorded. International Classification of Diseases codes were used to identify symptoms associated with bladder cancer—for example, cystitis, dysuria, and hematuria. Patients were considered “directly referred” if they visited a gp 5 or fewer times before making their first urologist visit. Those patients were not seen by another specialist before their first urologist visit. Patients were considered “indirectly referred” if they made more than 5 visits to a gp, emergency physician, or other specialist before making their first urologist visit8. To investigate sex-related differences in survival that might be associated with referral patterns, we performed independent analyses stratified by patient sex.
2.5. Statistical Analysis
Descriptive statistics summarize the characteristics of the study population. Univariate and multivariate Cox proportional hazards regression models were used to assess the effect of referral patterns on long-term survival. Effects were quantified as hazard ratios (hrs) with 95% confidence intervals (95% cis). Analyses were adjusted for four age categories (40–59 years, 60–64 years, 65–79 years, and −80 years). We assessed the assumption of proportional hazards by examining graphs of scaled Schoenfeld residuals. All analyses were two-sided, and p ≤ 0.05 was considered significant. The SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.) was used to perform the calculations.
3. RESULTS
3.1. Characteristics of the Study Population
Table i summarizes the characteristics of the study population. Of the 1271 patients who underwent radical cystectomy for bladder cancer and who met the eligibility criteria, 28% were women—a proportion similar to that seen in Canadian and international statistics. Among the eligible patients, 58% were more than 65 years of age, and 64% lived near a metropolitan area. Median overall referral delay was 30 days (with a mean of 5.6 pre-referral visits); among women, the median referral delay was 56 days. The latter delay was more than double the delay experienced by men (23 days, p < 0.0001). Indirect referral was observed in 37% of the study cohort and was more prevalent for women than for men (49% vs. 33%). Compared with indirectly referred men, indirectly referred women experienced a significantly longer median wait time (p < 0.0001). Although the difference in the mean number of pre-referral visits was numerically small (6.7 for women vs. 5 for men), the tails of the distributions varied more widely.
TABLE I.
Characteristics of the study population
| Characteristic | Referral type | Overall | p Valuea | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Women | Men | |||||||
|
|
|
|||||||
| Direct | Indirect | Overall | Direct | Indirect | Overall | |||
| Population [n (%)] | 177 (51) | 170 (49) | 347 (27.3) | 619 (67) | 305 (33) | 924 (72.7) | 1271 (100) | |
| Age [n (%)] | ||||||||
| <65 Years | 71 (40.1) | 69 (40.6) | 140 (40.3) | 282 (45.5) | 109 (35.7) | 391 (42.3) | 531 (41.8) | 0.52 |
| >65 Years | 106 (59.9) | 101 (59.4) | 207 (59.7) | 337 (54.5) | 196 (64.3) | 533 (57.7) | 740 (58.2) | |
| Residency [n (%)] | ||||||||
| Montreal and region | 90 (50.8) | 92 (54.1) | 182 (52.4) | 269 (43.7) | 150 (49.2) | 419 (45.5) | 601 (47.4) | 0.07 |
| Quebec City | 19 (10.7) | 31 (18.2) | 50 (14.4) | 100 (16.2) | 62 (20.3) | 165 (17.6) | 215 (16.7) | |
| Other | 68 (38.4) | 47 (27.6) | 115 (33.1) | 247 (40.1) | 93 (30.5) | 340 (37) | 455 (35.8) | |
| Median (sd) delay (days)b | 40 (103) | 75 (119) | 56 (113) | 21 (81) | 30 (107) | 23 (93) | 30 (99) | |
| Mean (sd) visits (n)c | 2.4 (1.1) | 11.2 (19) | 6.7 (14) | 2.2 (1) | 11 (10) | 5 (7.5) | 5.6 (9.8) | 0.01 |
For comparisons between men and women.
Between first general practitioner visit and first urologist visit.
Before first urologist visit.
sd = standard deviation.
3.2. Effect of Referral Patterns on Overall Survival
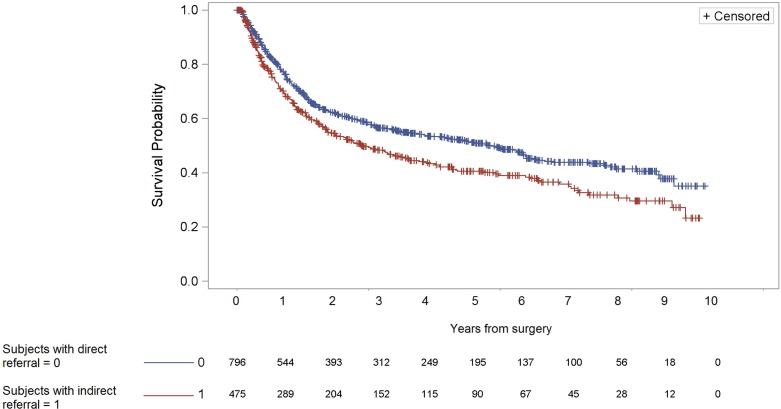
Median follow-up in the cohort was 4.9 years, and the overall 5-year survival rate was 46% (Table ii). Controlling for age, patients who were indirectly referred after first symptoms of bladder cancer had a risk of long-term mortality after radical cystectomy that was 1.29 times (95% ci: 1.10 to 1.52) the risk experienced by patients who were directly referred to a urologist (Figure 2). Analyses stratified by sex showed that, compared with their directly referred counterparts, women who were indirectly referred had a 47% greater risk of death after radical cystectomy (95% ci: 1.08 to 1.99). Men who were indirectly referred also had poorer survival (adjusted hr: 1.25; 95% ci: 1.03 to 1.51).
TABLE II.
Effect of indirect referral on survival
| Patient group | Comparator | Patients [n (%)] | Analysis type | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Crude | Adjusted for age | |||||
|
|
|
|||||
| hr | 95% ci | hr | 95% ci | |||
| Overall | 1271 (100) | |||||
| Directly referred | 796 (62.6) | Reference | Reference | |||
| Indirectly referred | 475 (37.4) | 1.32 | 1.12 to 1.54 | 1.29 | 1.10 to 1.52 | |
| Women | 347 (27.3) | |||||
| Directly referred | 177 (51) | Reference | Reference | |||
| Indirectly referred | 170 (49) | 1.46 | 1.1 to 1.99 | 1.47 | 1.08 to 1.99 | |
| Men | 924 (72.7) | |||||
| Directly referred | 619 (67) | Reference | Reference | |||
| Indirectly referred | 305 (33) | 1.28 | 1.05 to 1.55 | 1.25 | 1.03 to 1.51 | |
hr = hazard ratio; ci = confidence interval.
FIGURE 2.
Survival curves for patients who were indirectly or directly referred to a urologist after a first general practitioner visit.
4. DISCUSSION
Bladder cancer is a disease characterized by sex-dependent variations in its epidemiologic profile. Its higher incidence in men has been linked to several factors, including increased exposure to carcinogens, differences in pelvic anatomy and vascularity, and the involvement of androgens in bladder carcinogenesis12. Recent evidence showed that women present with disease of more advanced stage at the time of diagnosis, which can negatively affect survival6,13. The effect of tumour stage on survival has been challenged in some studies that observed an increased risk of mortality among women even after adjustment for tumour stage6,10, and most of the available evidence indicates that more advanced stage at the time of diagnosis might be the result of a long diagnosis delay. Diagnosis delay can be partly explained by the incorrect attribution of early cancer symptoms to more common conditions10.
In our study population, patients who were indirectly referred to a urologist for symptoms of bladder cancer (and who therefore experienced a greater median referral delay) had an increased risk of mortality after radical cystectomy. That detrimental effect on survival was more evident among women. Our results corroborate earlier studies on sex disparities in the delivery of care for bladder cancer8–10,13,14. In this instance, disparities in referral delay were associated with adverse survival outcomes. In addition, our data add to the evidence suggesting that a negative impact on survival caused by a long referral delay can be correlated with women having a cancer of more advanced stage at the time of diagnosis. To the best of our knowledge, this population-based study is the first to address sex-related differences in survival by referral pattern of patients with bladder cancer.
The median wait time before a first urologist visit has increased from 20 days in the 1990s5 to 30 days in our study population. The fact that women waited more than twice as long as men is an indication that gps might fail to appreciate the importance of first symptoms of bladder cancer in women. However, the question of whether women have less explicit symptoms or whether they fail to disclose their symptoms appropriately has to be clarified10.
This concerning referral delay might in part be attributable to difficulty in obtaining a timely urologist appointment, which could be a marker of limited access to care or poorer quality of care in urology. Indeed, overall time in the continuum of care for urology patients was shown to be beyond the duration considered acceptable by the Canadian Society of Surgical Oncology15. Patients with less access to care might receive less medical follow-up after initial presentation with symptoms. Poorer quality of care might also translate into less prompt diagnosis, less accurate grading and staging, and potentially into suboptimal care.
We chose indirect referral as a surrogate for the delay elapsed between first cancer symptoms and urologist referral because of its logical appeal and clinical intuitiveness. The idea that early diagnosis and treatment should lead to improved survival has strong face validity. However, the use of observational data makes it difficult to define the length of time from symptoms to diagnosis that is sufficiently short to lower the risk to patients. Given that randomized clinical trials designed to determine optimal delay are impractical and unethical, the question remains largely controversial16. Indeed, several earlier studies that investigated the association between delay (as a direct measure of time) and survival had methodology flaws such as selection bias and lead time bias6. Such threats to internal validity can undermine the useful interpretation and clinical use of some of the studies.
Our findings of an increased risk of mortality among patients (especially women) experiencing long wait times raise, for patients and gps alike, the importance of awareness of first bladder cancer symptoms and prompt management. Earlier work has already shown a detrimental effect on long-term survival for patients with longer delays between transurethral resection of bladder tumour and radical cystectomy5,7. Taken together, such longer delays can result in a higher frequency of disease progression and poorer long-term survival16.
Available guidelines recommend that all patients with bladder cancer symptoms (particularly those with hematuria and without evidence of infections or other factors) undergo immediate cystoscopy and urinary tract imaging17,18. Nevertheless, research indicates that gps often do not adhere to guidelines for prompt investigation of patients presenting with hematuria19,20. A proposed alternative to increased detection of patients at risk would be the implementation of screening for hematuria at gp offices. In fact, a large study that used hematuria urine test strips to detect incident bladder tumours reinforced the perception of early detection by providing evidence that dipstick-detected tumours are identified at a lower stage and are less likely to be lethal21. However, the positive predictive value of hematuria for bladder cancer is very low. Furthermore, many patients with bladder cancer present without hematuria. Large-scale implementation of screening protocols would therefore require that health economic analyses explore its cost-effectiveness8.
Our study had some limitations inherent to the use of administrative databases. We were unable to measure some confounding factors—such as grade, stage, and severity of the tumour—that can play an important role in long-term overall survival. Data on patient comorbidities and functional state are also lacking. Residual confounding might therefore be present. On the other hand, the universal health care coverage provided by the ramq allows for collection of prospective information on a large sample and increases the external validity of the findings. Data about mortality are valid, and the database linkage used a unique patient identifier, which allows for very reliable correspondence between medical services data and vital status.
5. CONCLUSIONS
The median wait time from first gp visit to first urologist visit for patients with bladder cancer has been increasing since the early 1990s. Compared with men, women tend to experience more than double the referral wait time, which has a negative effect on survival after surgery. Patients indirectly referred to a urologist after a first gp visit also experienced an increased risk of mortality. Stakeholders in the field must focus their efforts to determine why these sex-related variations in referral occur and to propose solutions that result in more effective approaches. Our findings reinforce the importance of highlighting for gps the symptoms related to bladder cancer and the need for prompt urology referral.
6. ACKNOWLEDGMENTS
This study was supported by the Canadian Institutes of Health Research (funding reference number 127596), in partnership with Bladder Cancer Canada and the McGill Integrated Cancer Research Training Program.
7. CONFLICT OF INTEREST DISCLOSURES
Each author participated actively and sufficiently in this study. FS conducted the study, performed the analyses, and led the writing of the manuscript. AD and WK helped to interpret the results and revised the manuscript for important intellectual content. EF helped with the statistical methods and interpretation of results. AA conceived and supervised the study. All authors read and approved the final version of the manuscript and its submission.
We have read and understood Current Oncology’s policy on disclosing conflicts of interest and declare that we have none.
8. REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: globocan 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. [Google Scholar]
- 3.Yafi FA, Aprikian AG, Fradet Y, et al. Surveillance guidelines based on recurrence patterns after radical cystectomy for bladder cancer: the Canadian Bladder Cancer Network experience. BJU Int. 2012;110:1317–23. doi: 10.1111/j.1464-410X.2012.11133.x. [DOI] [PubMed] [Google Scholar]
- 4.May M, Nitzke T, Helke C, Vogler H, Hoschke B. Significance of the time period between diagnosis of muscle invasion and radical cystectomy with regard to the prognosis of transitional cell carcinoma of the urothelium in the bladder. Scand J Urol Nephrol. 2004;38:231–5. doi: 10.1080/00365590410029141. [DOI] [PubMed] [Google Scholar]
- 5.Fahmy N, Kassouf W, Jeyaganth S, et al. An analysis of preoperative delays prior to radical cystectomy for bladder cancer in Quebec. Can Urol Assoc J. 2008;2:102–8. doi: 10.5489/cuaj.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenbeck BK, Dunn RL, Ye Z, et al. Delays in diagnosis and bladder cancer mortality. Cancer. 2010;116:5235–42. doi: 10.1002/cncr.25310. [DOI] [PubMed] [Google Scholar]
- 7.Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol. 2006;175:78–83. doi: 10.1016/S0022-5347(05)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Lyratzopoulos G, Abel GA, McPhail S, Neal RD, Rubin GP. Gender inequalities in the promptness of diagnosis of bladder and renal cancer after symptomatic presentation: evidence from secondary analysis of an English primary care audit survey. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002861. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shariat SF, Sfakianos JP, Droller MJ, Karakiewicz PI, Meryn S, Bochner BH. The effect of age and gender on bladder cancer: a critical review of the literature. BJU Int. 2010;105:300–8. doi: 10.1111/j.1464-410X.2009.09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henning A, Wehrberger M, Madersbacher S, et al. Do differences in clinical symptoms and referral patterns contribute to the gender gap in bladder cancer? BJU Int. 2013;112:68–73. doi: 10.1111/j.1464-410X.2012.11661.x. [DOI] [PubMed] [Google Scholar]
- 11.Rachet B, Maringe C, Nur U, et al. Population-based cancer survival trends in England and Wales up to 2007: an assessment of the nhs cancer plan for England. Lancet Oncol. 2009;10:351–69. doi: 10.1016/S1470-2045(09)70028-2. [DOI] [PubMed] [Google Scholar]
- 12.Scosyrev E, Trivedi D, Messing E. Female bladder cancer: incidence, treatment, and outcome. Curr Opin Urol. 2010;20:404–8. doi: 10.1097/MOU.0b013e32833c7a9b. [DOI] [PubMed] [Google Scholar]
- 13.Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–71. doi: 10.1016/S0090-4295(99)00481-1. [DOI] [PubMed] [Google Scholar]
- 14.Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology. 2008;72:498–502. doi: 10.1016/j.urology.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 15.Fleshner N, Dranitsaris G, Finelli A, Tsihlias J, Bell D, Gleave M, on behalf of the Canadian Surgical Wait Times initiative Surgical wait times for patients with urological cancers: a survey of Canadian surgeons. Can J Urol. 2006;13(suppl 3):3–13. [PubMed] [Google Scholar]
- 16.Porter MP. Examining the association between delay in diagnosis and decreased survival in bladder cancer. Cancer. 2010;116:5122–5. doi: 10.1002/cncr.25290. [DOI] [PubMed] [Google Scholar]
- 17.Smith JA, Jr, Labasky RF, Cockett AT, Fracchia JA, Montie JE, Rowland RG. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162:1697–701. doi: 10.1016/S0022-5347(05)68208-0. [DOI] [PubMed] [Google Scholar]
- 18.U.K. National Institute for Health and Care Excellence (nice) Referral Guidelines for Suspected Cancer. London, U.K.: NICE; 2005. nice clinical guideline 27. [Google Scholar]
- 19.Nieder AM, Lotan Y, Nuss GR, et al. Are patients with hematuria appropriately referred to Urology? A multi-institutional questionnaire based survey. Urol Oncol. 2010;28:500–3. doi: 10.1016/j.urolonc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Yafi FA, Aprikian AG, Tanguay S, Kassouf W. Patients with microscopic and gross hematuria: practice and referral patterns among primary care physicians in a universal health care system. Can Urol Assoc J. 2011;5:97–101. doi: 10.5489/cuaj.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messing EM, Madeb R, Young T, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006;107:2173–9. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]