Abstract
Background
Characterization of myocardial structural changes in heart failure (HF) with preserved ejection fraction (HFpEF) has been hindered by limited availability of human cardiac tissue. Cardiac hypertrophy, coronary artery disease (CAD), coronary microvascular rarefaction and myocardial fibrosis may contribute to HFpEF pathophysiology.
Methods and Results
We identified HFpEF patients (n=124) and age-appropriate control patients (non-cardiac death, no HF diagnosis; n=104) who underwent autopsy. Heart weight and CAD severity were obtained from the autopsy reports. Using whole field digital microscopy and automated analysis algorithms in full thickness left ventricular (LV) sections, microvascular density (MVD), myocardial fibrosis and their relationship were quantified. Subjects with HFpEF had heavier hearts (median 538 g; 169% of age/sex/body size expected heart weight vs. 335 g; 112% in controls), more severe CAD (65% with ≥ one vessel with >50% diameter stenosis in HFpEF vs 13% in controls), more LV fibrosis (median % area fibrosis, 9.6 vs. 7.1) and lower MVD (median 961 vs. 1316 vessels per mm2) than control (p <0.0001 for all). Myocardial fibrosis increased with decreasing MVD in controls (r = − 0.28, p=0.004) and HFpEF (r = − 0.26, p=0.004). Adjusting for MVD attenuated the group differences in fibrosis. Heart weight, fibrosis and MVD were similar in HFpEF patients with vs without CAD.
Conclusions
In this study, patients with HFpEF had more cardiac hypertrophy, epicardial CAD, coronary microvascular rarefaction and myocardial fibrosis than controls. Each of these findings may contribute to the LV diastolic dysfunction and cardiac reserve function impairment characteristic of HFpEF.
Keywords: Autopsy, coronary microvessel, endothelium, diastolic heart failure, fibrosis, pathology
BACKGROUND
Heart failure (HF) with preserved ejection fraction (HFpEF) is common and increasing in prevalence.1 HFpEF occurs in association with advanced age and cardiovascular, metabolic and pro-inflammatory comorbidities.2, 3
At the integrative level, patients with HFpEF display impaired left ventricular (LV) relaxation and increased diastolic LV stiffness.4, 5 While arterial and LV systolic elastance (stiffness) are increased in HFpEF, resting contractile function is subtly impaired, as is the ability to enhance arterial, chronotropic and LV systolic and diastolic performance with exercise (impaired reserve function).5–8 Chronic elevation of LV filling pressures leads to left atrial remodeling and dysfunction, mixed pulmonary hypertension and ultimately, right ventricular (RV) remodeling and dysfunction.5, 9
Increased LV stiffness suggests passive myocardial stiffening due to fibrosis and/or altered cardiomyocyte function.6, 10 However, the underlying myocardial alterations in HFpEF are incompletely defined as endomyocardial biopsy and surgical specimens commonly available in HF with reduced EF (HFrEF), are rarely available in HFpEF. A small number of studies obtained endomyocardial biopsies in highly selected, younger HFpEF patients and reported myocyte hypertrophy, interstitial fibrosis, incomplete myocardial relaxation and increased cardiomyocyte stiffness, as well as evidence of systemic and myocardial inflammation and oxidative stress.11–17 Based on these elegant studies, a new paradigm for the pathophysiology of HFpEF has been proposed wherein comorbidities lead to a systemic pro-inflammatory state and coronary microvascular endothelial inflammation, impairment in endothelial-cardiomyocyte nitric oxide signaling, inflammatory cell infiltration and production of pro-fibrotic cytokines resulting in diastolic dysfunction due to altered cardiomyocyte function and extracellular matrix.3 Microvascular endothelial inflammation is also associated with endothelial dysfunction and microvascular rarefaction.18 The resultant reduction in coronary microvascular density (MVD) may impair oxygen delivery with stress, limiting LV systolic and diastolic reserve function.19, 20 However, studies in human HFpEF myocardium are limited and MVD in particular has not been assessed in HFpEF.
We hypothesized that cardiac hypertrophy, microvascular rarefaction and myocardial fibrosis are common and related in patients with HFpEF. To test this hypothesis, we obtained transmural LV specimens from patients who had undergone postmortem examination with an ante mortem diagnosis of HFpEF and age-appropriate control patients. Whole field digital microscopy and automated digital histopathologic analyses were used to quantify fibrosis and MVD while hypertrophy was assessed by age-, sex-, and body size-adjusted cardiac weight and histological characterization (by cardiovascular pathologists). Severity of epicardial coronary artery disease (CAD) was assessed by serial coronary artery sectioning and gross and histologic evaluation was performed by a pathologist.
METHODS
This study was approved by the Mayo Clinic institutional review board and the Mayo Clinic biospecimens subcommittee.
Study subjects
Consecutive adult subjects with a prior HF hospitalization (primary dismissal diagnosis of HF (ICD-9-CM code 428.xx and the diagnosis related-group (DRG) code 127) between 1986 and 2010 (except the timeframe between January 1, 2002 to September, 2003, when no data was included) or an outpatient diagnosis of HF (ICD-9-CM code 428) between 1980 and 2009 with an LVEF≥40% within a median of 1 day of the HF event were identified. Subjects with more than mild aortic or mitral stenosis, infiltrative or hypertrophic cardiomyopathy, complex congenital heart disease or heart transplant recipients were excluded from the study. This list was cross-referenced to the Mayo Clinic Tissue Registry archives and those HFpEF patients who underwent autopsy constituted the HFpEF autopsy cohort.
From the Mayo Clinic Tissue Registry archives (1977 to 2010), we identified autopsies performed on subjects who died of non-cardiovascular causes (approximately 10 men and 10 women per decade of life from the 5th to the 10th decades), constituting the control cohort. Charts were reviewed to confirm the absence of an ante mortem diagnosis of HF.
To assess the relative severity of microvascular rarefaction and fibrosis in HFpEF as compared to HFrEF using the technologies employed in this study, we also identified a subgroup of HFrEF patients (EF < 40% at HF diagnosis, n=27) who underwent autopsy using similar methods as above.
Data abstraction
Clinical and pathology characteristics were manually abstracted from the medical records and autopsy reports. Comorbidities were defined as described previously,2 and as outlined in Supplemental methods.
Autopsy and tissue processing
Autopsies were conducted according to the Mayo institutional guidelines and standardized protocols as described previously.21 Semi-quantitative assessment of gross remodeling (ventricular and atrial sizes, presence of hypertrophy, fibrosis or infarction) was defined by the performing pathologist. Absolute heart weight was reported, as well as the percentage of expected heart weight, derived from established age nomograms based on sex, body weight and body height.21
The epicardial coronary arteries were sectioned serially and atherosclerosis was graded as 0–4 with grade 0 indicating no stenosis and grade 4 indicating ≥ 75% luminal area stenosis (analogous to ≥ 50% angiographic diameter stenosis).22
After gross examination, hearts were serially sectioned along the short axis at apical and mid-ventricular levels. From the mid-ventricle, representative transmural sections of the left ventricular wall and septum were procured, fixed in 10% neutral buffered formalin and embedded in paraffin for histologic analysis. For standardization, the inferior wall was the region analyzed, unless a gross infarct was seen. Alternately, an adjacent non-infarcted portion of the wall was chosen in the following order of availability: inferolateral, lateral, anterolateral, anterior, anteroseptal or inferoseptal.
Histochemistry for fibrosis detection
Sulfated Alcian blue (SAB) with van Gieson’s counterstain was used to differentially stain amyloid (green) and collagen (red). Four µm-thick paraffin embedded LV sections were deparaffinized in xylene and rehydrated with alcohol. Sections were immersed in acetic acid, stained with SAB, hematoxylin then picric acid and finally counterstained with van Gieson’s. Histologic slides were reviewed by a cardiovascular pathologist (WDE or JJM) who was blinded to the group; those with amyloid deposition were excluded from the study.
Immunohistochemistry for coronary microvascular detection
Epitopes were retrieved from deparaffinized, rehydrated parallel tissue sections and non-specific binding was blocked. Sections were then incubated with a monoclonal mouse anti-human platelet endothelial cell adhesion molecule–1 (PECAM-1)/cluster of differentiation 31(CD31) antibody (Dako, Carpinteria, Calif) in a 1:200 dilution. The bond was detected by a horseradish peroxidase linked secondary antibody conjugate kit (Leica Biosystems, Newcastle Upon Tyne, UK), and visualized using 3,3’diaminobenzidine tetrahydrochloric acid chromogen. Tissue sections were counterstained with hematoxylin for visualization of the nuclei.
Digital image acquisition and processing
Digital images were captured at 20× magnification with a resolution of 1.3 megapixels and scanned into Baccus/NDPI format using NanoZoomer Digital Pathology whole field microscopy (Hamamatsu photonics K.K; Hamamatsu city, Japan).
Quantitative fibrosis image analysis
Images were processed using an automated custom designed quantitative analysis software (Definiens Developer XD ® object-oriented image analysis software; Definiens®, München, Germany). The process consisted of 3 main steps: (1) automated separation of tissue from glass/background, (2) detection of whitespace within tissue, (3) detection of SAB stain (Supplemental Figure 1).
Images were loaded into the software in Baccus native file format, down-sampled to 2.5% and a Gaussian blur with a kernel size of 7×7 was applied. Tissue was segmented from the glass slide using an automated and adaptive threshold. The automatic threshold algorithm utilized a combination of intensity histogram-based methods and a homogeneity measurement to calculate a threshold that divides the selected set of pixels into two subsets to maximize heterogeneity. The generated classification mask of the tissue region was then overlaid onto a 5× copy of the image.
The auto-adaptive threshold was then applied in a 2-stage approach. First, a threshold was calculated for the set of pixels within the tissue region only. The resulting segmentation separated the high intensity, homogeneous whitespace regions from the tissue body. A second threshold was then calculated using the remaining set of pixels in the tissue, segmenting the SAB stain. Fibrosis was quantified as a percent of the total tissue area.
Quantitative coronary MVD image analysis
An automated quantitative analysis bright-field vessel detection and classification algorithm (Definiens Tissue Studio 3.5; Definiens®, München, Germany) was applied to PECAM-1 stained slides. The process consisted of 3 main steps: (1) manual region of interest selection (2) vessel detection (PECAM-1; CD31 stain) and (3) vessel classification according to vessel size (Supplemental Figure 2). Images were loaded into the software in Hamamatsu NDPI/Baccus file format. The region of interest was defined manually by dividing the myocardium area into 4 regions: (1) sub-epicardium, (2) mid-wall, (3) sub-endocardium and (4) papillary muscle (Supplemental Figure 3).
A modifiable threshold algorithm was used for vessel detection. To maximize discrimination between brown stain and background stain, the stain values of the brown chromogen were based on conversion of the Red, Blue, and Green (RBG) space to the Hue-Saturation-Density (HSD) model which has been shown to be superior.23 To exclude non-specific binding, stain fragments and artifacts, a stain intensity threshold of 0.225 and a minimum stain area of 10 µm2 were set. This minimum stain area was selected based on pilot measurements of the area of the smallest vessels. Sides were analyzed in tiles at 10× magnification. Sensitivity analysis was performed quantifying total vessel density with and without a minimum stain area restriction (Supplemental methods and results).
Microvessels were defined as the combination of capillaries (endothelial monolayer and area between 10 µm2 and 78.5 µm2; average luminal diameter ≤ 10 µm)24, 25 and small pre-capillary arterioles (area 78.5–314 µm2; average luminal diameter 10 to 20 µm).26
After vessel detection and classification, the program defined microvessel count, tissue area and microvascular density (vessel to tissue ratio; per mm2).27
Variability in cardiomyocyte fiber and microvessel orientation may potentially impact the accuracy of MVD analysis in full thickness LV sections. To address this: (1) microvessel size discrimination assumed a circular shape excluding longitudinally cut microvessels that exceeded the maximum area cut off and (2) MVD was analyzed by region (sub-epicardium, mid-mural, and sub-endocardium) with the sub-epicardium and sub-endocardium providing two thirds of the myocardial area with parallel fiber and microvessel alignment allowing short axis cuts of both (Supplemental Figure 4).
Echocardiograms
Transthoracic echocardiograms closest to the date of HF diagnosis were obtained. LV EF was available on all HF subjects, however availability of other echocardiographic parameters was inconsistent. An LVEF≥40% was used as a cut off for definition of HFpEF. Sensitivity analyses excluding HFpEF patients with EF 40–49% at diagnoses were performed. For assessment of correlation of echocardiographic parameters with histology, the last echocardiographic variable obtained closest to death was used.
Electrocardiograms (ECGs)
ECGs closest to death were interpreted and voltages were measured manually blinded to the group (n= 224). LVH was determined by voltage criteria (Cornell and Sokolow).
Statistical Analysis
Variables are summarized as median (25th–75th percentiles) or % frequency. To test for differences in characteristics between HFpEF and control, we used Wilcoxon rank sum test for continuous variables and Chi square test of independence or Fischer exact test for categorical variables as appropriate. We used least squares linear regressions to compare fibrosis and MVD between groups (HFpEF and control) adjusting for pertinent covariates. Variables which were not normally distributed were log transformed for statistical analysis. All analyses were 2 tailed and a p value <0.05 indicated statistically significant differences.
RESULTS
Clinical characteristics
The study included 228 subjects (124 HFpEF and 104 controls) who underwent autopsy between 1977 and 2010 (median 2001; 25th–75th percentiles: 1995 – 2006). There were no significant differences in age or sex distribution between the groups (Table 1). HFpEF subjects had more cardiovascular comorbidities (hypertension, diabetes mellitus (and CAD), conduction system disease and ECG evidence of LV hypertrophy (Table 1).
Table 1.
Clinical characteristics.
| Control | HFpEF | p value | |
|---|---|---|---|
| N | 104 | 124 | |
| Sex, men | 34% | 44% | 0.10 |
| Age at heart failure event, years | NA | 75 (66–83) | |
| Age at death, years | 74 (61–85) | 78 (68–85) | 0.13 |
| Hypertension | 31% | 79% | <0.0001 |
| Diabetes mellitus | 11% | 42% | <0.0001 |
| Clinical diagnosis of coronary artery disease | 0% | 65% | <0.0001 |
| Permanent pacemaker | 0% | 23% | <0.0001 |
| Creatinine, mg/dl | 1.2 (0.8–1.7) | 1.6 (1.2–2.4) | <0.0001 |
| GFR, ml/min/1.73m2 | 54 (35–84) | 38 (24–51) | <0.0001 |
| Ejection fraction at heart failure event, % | NA | 56 (50–62) | |
| LVH (Cornell criteria) | 4% | 15% | 0.01 |
| LVH (Sokolow criteria) | 0% | 5% | 0.03 |
| QRS duration, msec | 84 (76–92) | 108 (92–150) | <0.0001 |
| QTc interval, msec | 265 (223–315) | 337 (280–398) | <0.0001 |
ECG data were available on 224 subjects
Clinical characteristics of HFpEF subjects in this series were generally comparable to observational studies (Supplemental Table 1). The immediate cause of death as defined by the autopsy report differed by group with more HFpEF patients dying of HF and other cardiovascular causes (Supplemental Table 2).
Autopsy Findings
As compared to controls, HFpEF patients had a higher body mass index (BMI) and body surface area (BSA) at the time of autopsy (Table 2).
Table 2.
Autopsy characteristics.
| Control | HFpEF | p value | |
|---|---|---|---|
| N | 104 | 124 | |
| Body mass index, kg/m2 | 25.6 (21.2–30.4) | 28.0 (23.7–34.5) | 0.006 |
| Body surface area, m2 | 1.8 (1.6–2.0) | 1.9 (1.7–2.1) | 0.003 |
| Heart weight at autopsy, gram | 335 (280–380) | 538 (440–659) | <0.0001 |
| Heart weight at autopsy/BSA, gram/ m2 | 190 (171–207) | 275 (233–344) | <0.0001 |
| Heart weight at autopsy/Ht, gram/ m | 203 (173–233) | 323 (266–389) | <0.0001 |
| Percent expected heart weight, % | 112 (101–132) | 169 (144–202) | <0.0001 |
| Gross pathology | |||
| Left ventricular hypertrophy | 15% | 74% | <0.0001 |
| Right ventricular hypertrophy | 9% | 50% | <0.0001 |
| Left ventricular dilation | 2% | 37% | <0.0001 |
| Right ventricular dilation | 13% | 48% | <0.0001 |
| Atrial dilation | 7% | 52% | <0.0001 |
| Infarct (old) | 2% | 42% | <0.0001 |
| infarct (acute) | 1% | 11% | 0.002 |
| Fibrosis | 1% | 25% | <0.0001 |
| Microscopic pathology | |||
| Hypertrophy | 15% | 31% | 0.007 |
| Infarct | 0% | 20% | <0.0001 |
| Fibrosis | 43% | 58% | 0.04 |
| Coronary artery stenosis total score | 6 (4–8) | 12 (8–14) | <0.0001 |
| Quantitative histology | |||
| MVD, microvessels/mm2 | 1316 (1148–1467) | 967 (800–1370) | <0.0001 |
| % Area fibrosis | 7.1 (5.1–9.0) | 9.6 (6.8–13.5) | <0.0001 |
Heart Weights
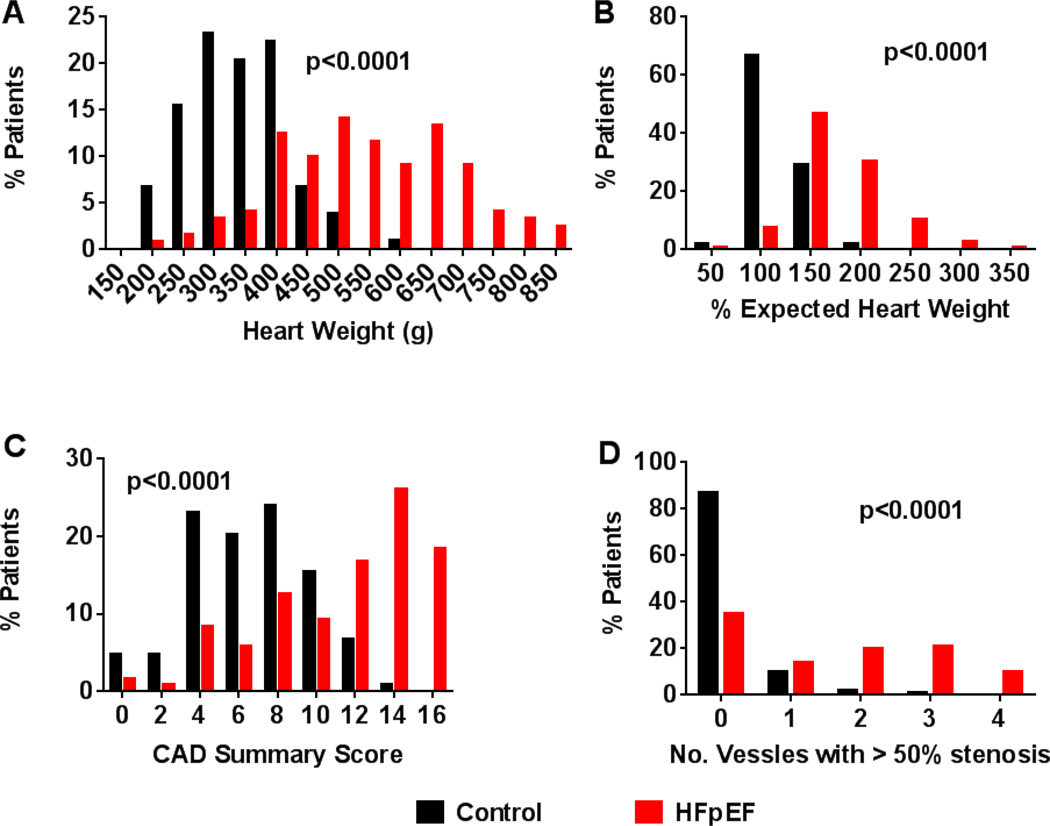
The absolute heart weight (HW) and age, sex and body size adjusted heart weight (percentage of expected HW) were higher in HFpEF than controls (Table 2 and Figure 1). The distribution of HW and % expected HW were both skewed but log transformed % expected HW was normally distributed. Group explained 40% of the variability in log % expected HW. Adjusting for group, neither history of diabetes mellitus nor hypertension nor the severity of CAD (CAD sum score) were associated with HW (p>0.05 for both) (Table 3) and percentage of expected HW was similar in HFpEF patients with (168 (144–206) %) or without (168 (144–195) %; p=0.75) one or more epicardial vessels with >50% diameter stenosis.
Figure 1.
Cardiac hypertrophy and CAD in HFpEF and control. Frequency distributions indicate higher absolute heart weight (A) and percent expected (for age, sex and body size) heart weight (B), higher coronary artery disease (CAD) score (C) and more frequent multi-vessel coronary disease (D) in HFpEF than control.
Table 3.
Factors associated with heart weight, CAD sum score, MVD or Fibrosis.
| Model R2 |
Regression coefficient (SE) |
Variable p | |
|---|---|---|---|
| Percent expected heart weight | |||
| Group (HFpEF) | 0.40 | 30.47 (2.52) | <0.0001 |
| + Diabetes history | 0.99 | ||
| + Hypertension history | 0.30 | ||
| + CAD score | 0.79 | ||
| CAD score sum | |||
| Group (HFpEF) | 0.30 | −2.30 (0.02) | <0.0001 |
| + Age | 0.33 | 0.06 (0.02) | 0.002 |
| + Sex (women) | 0.35 | −0.69 (0.24) | 0.004 |
| + Diabetes history | 0.38 | 0.91 (0.27) | 0.0009 |
| + Hypertension history | 0.58 | ||
| Microvascular Density (vessels/mm2) | |||
| Group (HFpEF) | 0.23 | −155.3 (18.9) | <0.0001 |
| + Age (year) | 0.25 | 3.3 (1.4) | 0.02 |
| + Sex (women) | 0.43 | ||
| + Diabetes history | 0.27 | ||
| + Hypertension history | 0.45 | ||
| + CAD score | 0.43 | ||
| +Log % Expected heart weight | 0.31 | −658 (183) | 0.0004 |
| Log % Area Fibrosis | |||
| Group (HFpEF) | 0.090 | 0.072 (0.016) | <0.0001 |
| + Age | 0.28 | ||
| + Sex (women) | 0.12 | ||
| + Diabetes history | 0.19 | ||
| + Hypertension history | 0.68 | ||
| + CAD score | 0.96 | ||
| + Log % Expected heart weight | 0.63 |
Findings were similar when HFpEF patients with an EF 40–49 % at diagnosis (n=27) were excluded (% expected HW in controls 112 (101–132) % vs HFpEF 170 (143–205) %; p<0.0001) and percentage of expected HW was similar in HFpEF patients with EF ≥50% (170 (143–205) %) or EF 40–49% (166 (148–197) %; p=0.88) at HF diagnosis.
Gross Pathology
Biventricular hypertrophy, biventricular and atrial dilatation, old and new infarction and macroscopic evidence of fibrosis were all more common in HFpEF patients (Table 2). Of HFpEF patients, 28 (23%) had no gross hypertrophy of either chamber, 35 (28%) had only LVH, 4 (3%) had only RVH, while 57 (46%) had both.
Microscopic pathology
Histologic LV cardiomyocyte hypertrophy, infarction and fibrosis were all more common in HFpEF patients (Table 2).
Coronary artery disease in control and HFpEF
Serial coronary artery sections showed more extensive CAD in HFpEF patients with a higher CAD total score and a greater frequency of vessels with ≥50% luminal diameter stenosis (Figure 1). Of the 119 HFpEF patients with serial coronary sections, 77 (65%) had at least one vessel with ≥50% stenosis. Of patients without a clinical diagnosis of coronary disease (n=41), 13 (32%) had at least one vessel with >50% stenosis while 64 (82%) of the 78 patients with a clinical diagnosis had at least one stenosis ≥50%. HFpEF patients with EF≥ 50% at diagnosis tended to have less severe CAD at autopsy (CAD total score 12 (7–14)) than HFpEF patients with EF 40–49% at HF diagnosis (12.5 (11–15), p=0.06). The percent of patients with at least one vessel with >50% stenosis was lower in HFpEF patients with EF≥50% (61%) than in those with EF 40–49% (81%, p=0.04). CAD sum score was significantly associated with age at death, sex (higher in men) and diabetes mellitus. Adjusting for these variables, CAD was more extensive in HFpEF than control (Table 3).
The coronary microvasculature in control and HFpEF
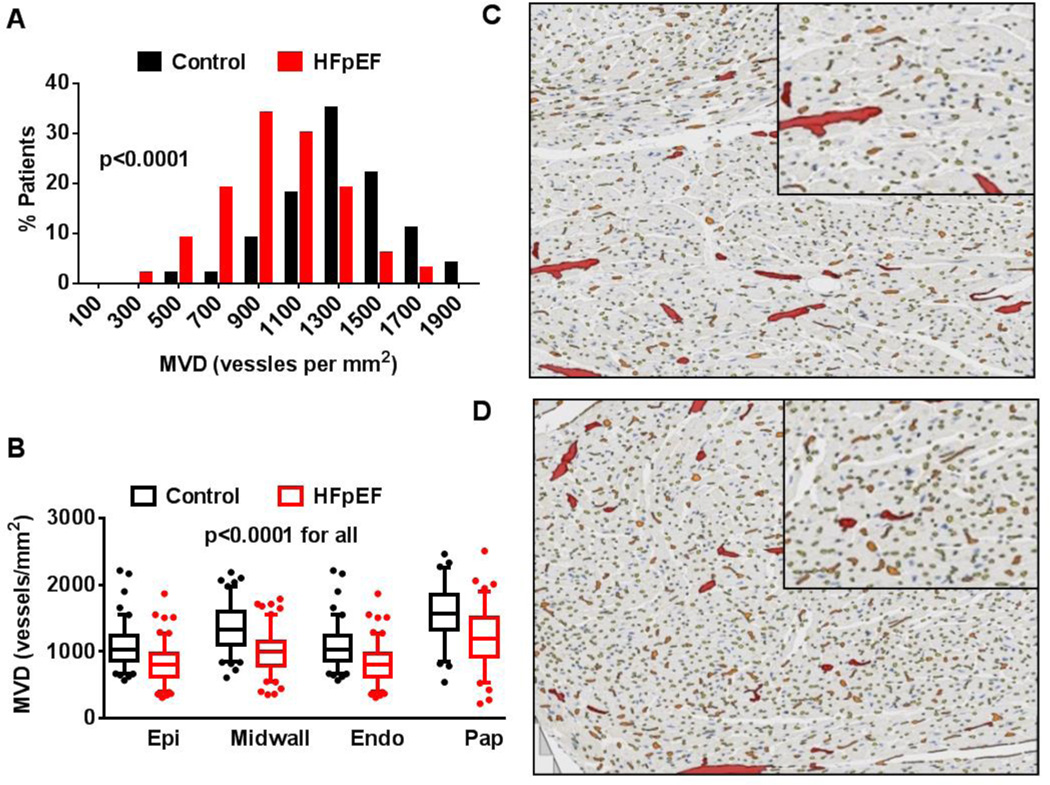
In the study population as a whole, the median tissue area analyzed was 2.29 cm2. MVD was normally distributed. MVD was diminished in HFpEF compared with controls, both overall (27% reduction in median MVD) and within each myocardial region (Table 2 and Figure 2). HFpEF patients had lower MVD than controls when HFpEF patients with an EF of 40–49% at diagnosis (n=27) were excluded (control 1316 (1148–1467) vessels/mm2 vs HFpEF 1032 (802–1243) vessels/mm2; p<0.0001),
Figure 2.
Coronary microvascular density (MVD) in HFpEF and control. The frequency distribution for total MVD (A) is shifted downward in HFpEF. Tukey box plots (box: median, 75th, and 25th percentiles; whiskers: highest value within 75th percentile plus 1.5*IQR and lowest value within the 25th percentile minus 1.5*IQR, symbols show outliers if present) of regional MVD (B) demonstrate similar reduction in MVD across the sub-epicardial (Epi), mid-myocardial (Midwall), sub-endocardial (Endo) and papillary muscle (Pap) in HFpEF. Representative examples of anti-CD 31 stained left ventricular sections with algorithm-defined capillaries (yellow), pre-capillary arterioles (orange) and larger intra-myocardial arteries (red) illustrate the lower MVD in HFpEF (C) as compared to control (D) subjects.
Findings were also similar when only density of capillaries was assessed (control 1044 (911–1188) vessels/mm2 vs HFpEF 788 (616–941) vessels/mm2; p<0.0001).
Group explained 23% of the variability in MVD (Table 3). Adjusting for cohort group, MVD increased slightly with increasing age at death but sex was not significantly associated with MVD. Adjusting for group and age at death, neither history of diabetes nor systemic hypertension nor severity of coronary atherosclerosis at death (as assessed by the CAD total score) were associated with MVD (Table 3). Further, in both HFpEF patients and controls, those without or with a history of hypertension had similar MVD (Supplemental Figure 5A).
When analysis was restricted to patients with no epicardial coronary artery stenosis >50% (91 controls and 40 HFpEF), MVD was lower in HFpEF (1042 (823–1220) vessels/mm2) than controls (1300 (1141–1462) vessels/mm2; p < 0.0001) and MVD was similar in HFpEF patients with (940 (794–1180) vessels/mm2) or without (1042 (823–1220) vessels/mm2; p=0.30) any epicardial coronary artery stenosis >50%.
Adjusting for group and age at death, the severity of hypertrophy as assessed by Log % expected heart weight) was inversely associated with MVD (Table 3).
Group differences in MVD persisted when no minimal stain area was used for analysis (Supplemental results).
LV fibrosis in control and HFpEF
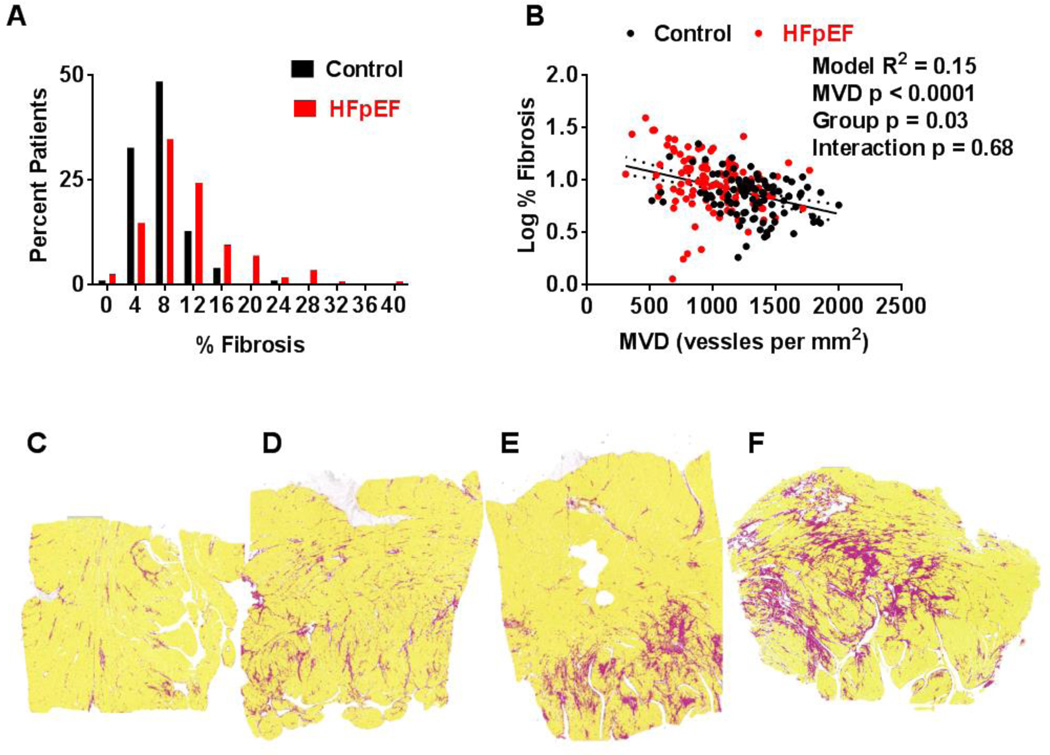
In the study population as a whole, % area fibrosis was skewed but log % area fibrosis was normally distributed and used for statistical comparisons. HFpEF patients had greater % area fibrosis than control (Table 2 and Figure 3). Findings were similar when HFpEF patients with an EF 40–49% at diagnosis were excluded (7.1 (5.1–9.0)% control vs 9.6 (6.8–13.5)% HFpEF; p<0.0001,
Figure 3.
Cardiac fibrosis in HFpEF and control. The frequency distribution for percent area fibrosis (A) is shifted upward in HFpEF. In B, log-transformed percent area fibrosis increases similarly with decreasing MVD in HFpEF and controls but remains higher in HFpEF than control at any level of MVD. In C–F, representative examples of SAB stained left ventricular sections with algorithm-defined fibrosis (red), myocardium (yellow) and space (white) from HFpEF patients with 3% (C), 7% (D), 10% (E) and 21 % (F) fibrosis.
Cohort group explained 9 % of the variability in log % area fibrosis (Table 3). Adjusting for cohort group, there was no significant association between age at death, sex, history of diabetes or systemic hypertension or severity of hypertrophy (as assessed by log % expected heart weight) and log % area fibrosis (Table 3). Further, in both HFpEF patients and controls, those without or with a history of hypertension had similar percent fibrosis (Supplemental Figure 5B).
Adjusting for group, severity of coronary atherosclerosis at death (as assessed by the CAD total score) was not significantly associated with log % area fibrosis (Table 3). When analysis was restricted to patients with no epicardial coronary artery stenosis > 50% (88 control and 38 HFpEF), % area fibrosis was higher in HFpEF (9.4 (6.9–12.8)%) than control (7.4 (5.6–9.0)%; p = 0.002) patients and % area fibrosis was similar in HFpEF patients with (9.5 (6.8–14.6) %) or without (9.4 (6.9–12.8) %; p=0.70) any epicardial coronary artery stenosis > 50%.
Association between fibrosis and MVD
Log % fibrosis increased with decreasing MVD in both control (r=−0.28, p=0.004) and HFpEF (r=−0.26, p=0.004). Adjusting for MVD attenuated but did not eliminate group differences in Log % fibrosis (Figure 3B).
Echocardiographic characteristics, MVD and myocardial fibrosis
LV mass (r=−0.29, p=0.01, n=78) and E/e’ ratio (r=−0.42, p= 0.02, n=30) were each negatively associated with MVD in HFpEF (Supplemental Figure 6). While the extent of fibrosis tended to correlate with E/e’ (r=0.35, p=0.06), there was no significant association between fibrosis and LV mass (Supplemental Table 3).
Electrocardiographic characteristics, MVD and myocardial fibrosis
QRS duration and QTc interval were each negatively associated with MVD and positively associated with fibrosis severity (in HFpEF and control combined) (Supplemental Figure 7).
Microvascular density and fibrosis in HFpEF versus HFrEF
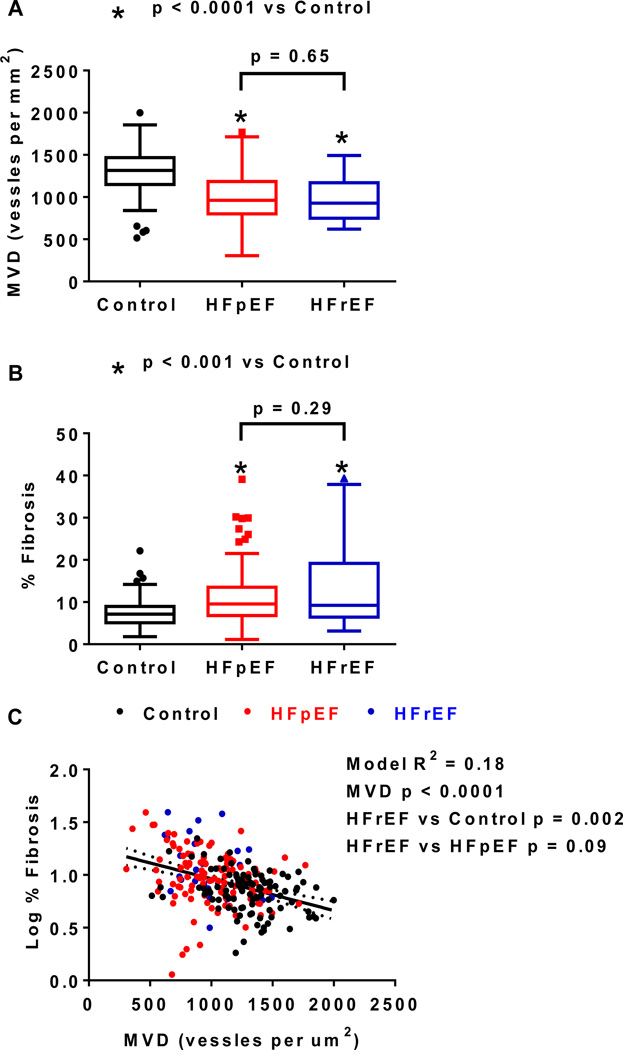
The clinical and autopsy characteristics of HFpEF and HFrEF patients were similar except for more left ventricular dilatation in HFrEF, more gross but not microscopic fibrosis, and more microscopic but not gross evidence of infarction in HFrEF (Supplemental Table 4). On quantitative analysis, neither MVD nor fibrosis differed between HFpEF and HFrEF (Figure 4 A and B). In all patients, adjusting for group (dummy variables), log % fibrosis remained inversely related to MVD (Figure 4 C). Adjusting for MVD, % fibrosis was higher in HFrEF as compared to control subjects but similar in HFpEF and HFrEF.
Figure 4.
Coronary microvascular density (MVD) and fibrosis in HFpEF and HFrEF. Microvascular Density (MVD, A) and percent fibrosis (B) are compared among controls (n=104), HFpEF (n=124) and HFrEF (n=27) patients. Data are displayed as Tukey box plots. In C, log-transformed % fibrosis increases with decreasing MVD but remains higher in HFrEF than control. In HF patients, adjusting for MVD, fibrosis is similar in HFpEF and HFpEF.
DISCUSSION
In this autopsy study, patients with an ante mortem diagnosis of HFpEF had multiple comorbidities at diagnosis. Autopsy reports indicated higher prevalence of gross and microscopic hypertrophy and fibrosis in HFpEF than control patients. Total cardiac weight was increased in HFpEF and diffuse coronary disease was common at autopsy irrespective of clinical diagnosis of coronary disease. HFpEF patients had lower coronary MVD and more severe fibrosis than control patients regardless of the severity of epicardial coronary disease. In both control and HFpEF patients, the severity of myocardial fibrosis was inversely associated with MVD. Group differences in LV fibrosis were attenuated after adjustment for MVD suggesting that reduced MVD or processes leading to coronary microvascular rarefaction contribute to myocardial fibrosis. The severity of microvascular rarefaction and fibrosis were similar in HFpEF and HFrEF. These findings provide several potential mechanisms for the LV systolic and diastolic dysfunction and reserve impairment in HFpEF. Further, the association of microvascular rarefaction and fibrosis lends support to a role for coronary microvascular endothelial inflammation in HFpEF pathophysiology by contributing to microvascular rarefaction, myocardial fibrosis and other myocardial perturbations leading to HFpEF.3
Previous studies analyzing LV tissue specimens in HFpEF
To our knowledge, this is the first autopsy study of HFpEF and differs substantially from the few previous smaller studies which have elucidated myocardial structure and function in endomyocardial biopsy specimens from carefully phenotyped patients with HFpEF and variable comparator groups. The current study included all patients with HFpEF and autopsy specimens and excluded only those patients with infiltrative or hypertrophic cardiomyopathies or severe valve disease irrespective of clinically suspected or autopsy defined coronary disease.
Previous biopsy studies excluded patients with significant angiographic evidence of coronary disease and enrolled patients who were younger at HF diagnosis (mean age early 50’s −60’s vs 75 years here), used a combination of RV and LV biopsies, had much smaller and exclusively endocardial specimens for review, did not quantify extent of non-critical coronary disease and did not assess MVD or its association with myocardial fibrosis. Thus, the current study expands upon previous studies and provides information regarding LV structure in elderly HFpEF patients more typical of the HF epidemic.13, 14, 28
Hypertrophy in HFpEF
Hypertrophy was present in HFpEF as cardiac weight was higher in HFpEF patients than controls and there was gross and microscopic LV myocardial hypertrophy as well as RV hypertrophy and atrial enlargement in HFpEF. The extent to which the increase in cardiac weight was due to LV versus other chamber hypertrophy or epicardial adiposity cannot be ascertained in our study as neither chamber specific weights nor dissection of epicardial fat was performed. Notably, the mean cardiac weight in HFpEF here (544 g) is lower than previously reported in autopsy studies of adults with idiopathic dilated cardiomyopathy (605 g),29 aortic stenosis (780 g)30 or hypertrophic cardiomyopathy (600–719 g)31 but the lack of age, sex and body size adjusted values in previous studies hinder direct comparisons and heart weight was similar in HFrEF and HFpEF patients in this autopsy series.
Imaging studies indicate that concentric LV remodeling and hypertrophy, while common, are not severe in HFpEF32 and a recent observational study found that height indexed LV mass was increased by 35% in elderly HFpEF versus age/sex matched healthy controls.2 Here the percentage of expected cardiac weight was 50% higher in HFpEF than controls, suggesting multi-chamber remodeling and/or epicardial adiposity contributes to the increases in cardiac weight in HFpEF.
Coronary disease in HFpEF
The prevalence of coronary disease in HFpEF is poorly described with older studies using variable ascertainment methods reporting prevalence from 0–67%33 Two recent large observational studies and a recent catheterization laboratory based study report a clinical or angiographic diagnosis of coronary disease in 50% – 68% of HFpEF patients.2, 9, 34
There is a potential for over- or under-diagnosis of coronary disease in elderly HFpEF patients.33 Our findings are consistent with this as significant coronary atherosclerosis existed in subjects without known CAD, while among those with a clinical diagnosis of CAD, significant atherosclerosis was variably present. This may result from a bias against coronary angiography in older HF patients with normal as opposed to reduced EF and the limited sensitivity and specificity of non-invasive CAD detection in HFpEF as recently described.34
Decreased coronary MVD in HFpEF
To our knowledge, this is the first study to examine MVD and its association with LV fibrosis in HFpEF. In the few studies that have assessed MVD in biopsies or autopsy specimens from humans with cardiovascular disease, histological and analytical methodologies and microvasculature definitions vary widely, thereby hampering comparisons of absolute values for MVD observed in HFpEF and controls to that observed in other cardiovascular diseases. However, the overall reduction in MVD in HFpEF (27%) was similar to that observed in HFrEF patients studied here and similar to that observed in other studies in HFrEF patients where a 30%–40% reduction in mean MVD in HFrEF versus controls was observed.35, 36
Advanced age and common HFpEF comorbidities such as obesity, systemic hypertension and diabetes mellitus have been shown to be associated with coronary microvascular dysfunction.37, 38 Microvascular endothelial dysfunction is associated with microvascular rarefaction18 and both are believed to contribute to chronic ischemia in cardiovascular disease20, 35, 39–41 as endothelial dysfunction, decreased MVD and stenotic microvascular remodeling may all limit coronary blood flow during reactive hyperemia.25, 42, 43 MVD was lower in HFpEF than control in subjects with or without a history of hypertension and among HFpEF patients, MVD was similar in those with or without hypertension. These findings suggest that comorbidities other than hypertension may perpetuate microvascular rarefaction.
Microvascular rarefaction signifies imbalance between vessel destruction and regeneration18 and the current study cannot determine whether microvascular destruction or insufficient angiogenesis are responsible for the reduction in MVD observed in HFpEF or establish the physiologic impact of altered cardiac MVD in HFpEF. Of note however, pre-eclampsia occurs in response to tissue (placental) ischemia producing imbalance between circulating pro- and anti-angiogenic factors44 with widespread (including coronary) microvascular rarefaction.45, 46 Individuals with cardiovascular risk factors similar to HFpEF (obesity, diabetes mellitus and hypertension) are more prone to pre-eclampsia. Removal of the ischemic placenta results in regression of most manifestations although residual dysfunction and risk for future cardiovascular events may persist.44 Impairment of cardiac function in pre-eclampsia and overlap between pre-eclampsia and peripartum cardiomyopathy47 lends support to the concept that coronary microvascular rarefaction may contribute to myocardial dysfunction in HFpEF. Correlation of microvascular rarefaction with severity of hypertrophy, diastolic dysfunction at echo and ante-mortem conduction system disease further supports this concept.
While variability in cardiomyocyte fiber and microvessel orientation may affect MVD quantification, this is unlikely to have impacted our results because error created by exclusion of the longitudinal vessels in the mid-wall is systematic and likely equivalent in HFpEF and control subjects. Analysis of MVD per region (sub-epicardium, mid-wall and sub-endocardium) showed consistent and proportionate reduction in HFpEF. Finally, MVD in the three regions were not significantly different, although there was an expected trend towards lower MVD in the mid-wall.
Fibrosis in HFpEF
Endomyocardial biopsy studies in HFpEF and variable comparator groups have demonstrated enhanced fibrosis in HFpEF compared to controls as evidenced by collagen I and III gene expression16 or collagen volume fraction12, 17 and similar degrees of fibrosis in HFpEF and HFrEF, consistent with our findings.13, 14, 28 In previous studies, the values for collagen volume fraction in HFpEF (2–13%) and comparator (2–4%) groups have varied widely, likely owing to variability in tissue procurement, histologic and analytical methods.12–14, 17, 28 We found mean % fibrosis area of 7.5% in control and 11.2% in HFpEF (median 7.1 and 9.6% respectively). The higher fibrosis area in controls may reflect differences in histologic techniques or analytical methods, the differences in tissue procurement (autopsy) or the more advanced age of controls. The correlation between fibrosis as assessed here and ante-mortem conduction system disease provides support for the validity of fibrosis measurement and its physiologic impact in HFpEF.48
The extent of fibrosis was higher in HFpEF than control in subjects with or without a history of hypertension and among HFpEF patients, fibrosis was similar in those with or without hypertension. These findings suggest that comorbidities other than hypertension perpetuate fibrosis. While significant, the difference in fibrosis between HFpEF and controls was modest and the difference in fibrosis between HFpEF and HFrEF patients was not significant suggesting that mechanisms other than fibrosis contribute to diastolic and systolic dysfunction in HF.12, 14
Relationship between microvascular rarefaction and fibrosis in HFpEF
The inverse association of MVD with fibrosis in both control and HF patients suggests that microvascular rarefaction contributes to chronic ischemia and micro-scars20, 49 and/or that a common process (such as microvascular endothelial inflammation) contributes to both microvascular rarefaction and myocardial fibrosis.3, 18
Limitations
The use of autopsy specimens procured over a significant time span precludes detailed phenotypic characterization and cardiac function at the time of death is not known. Nonetheless, autopsy studies have been widely used to define the morphologic features of a variety of cardiovascular diseases. This study was limited to subjects who underwent autopsy, which is a small subset of the HFpEF population. Autopsy tissue banking does not include skeletal muscle precluding assessment of skeletal muscle MVD in our study. The number of HFrEF patients was small but the focus of this study was on HFpEF and the HFrEF group was included to provide an estimate of the relative severity of microvascular rarefaction and fibrosis in HFpEF and HFrEF.
Conclusions
This autopsy study describes the cardiac morphologic features of patients with an ante mortem diagnosis of HFpEF and age-appropriate controls. HFpEF patients had more severe cardiac hypertrophy, diffuse coronary disease, microvascular rarefaction and modesty more myocardial fibrosis than controls. Decreases in MVD were associated with greater fibrosis in both HFpEF and control patients. As recently proposed, microvascular endothelial inflammation3, 16 is a plausible trigger for the microvascular rarefaction and myocardial fibrosis observed here as well as other myocardial perturbations leading to HFpEF.
Supplementary Material
Acknowledgments
The authors are grateful to Janis Donovan for her assistance with research coordination and tissue specimen retrieval.
Funding Sources: This study (HL72435 and UL1 TR000135) and/or the investigators (MMR: U10 HL 110262, PO1HL 76611 and HL105418; SFM: T32-HL07111) were supported by the National Institute of Health and Mayo Clinic. Dr. Mohammed is a heart failure clinical research network clinical research skills development fellow (U10 HL 110262).
Footnotes
Disclosures: None.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: A community based study. Circ Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. Epub 2012 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 4.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 5.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: Implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–409. doi: 10.1016/j.jacc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. doi: 10.1161/CIRCHEARTFAILURE.113.000854. Epub 2013 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 11.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ. Hypophosphorylation of the stiff n2b titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. Epub 2009 Jan 29. [DOI] [PubMed] [Google Scholar]
- 12.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 13.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 14.van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbely A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 15.van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase g activity in heart failure with preserved ejection fraction. Circulation. 2012;126:830–839. doi: 10.1161/CIRCULATIONAHA.111.076075. [DOI] [PubMed] [Google Scholar]
- 16.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 17.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C. Diastolic tissue doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. doi: 10.1016/j.jacc.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Goligorsky MS. Microvascular rarefaction: The decline and fall of blood vessels. Organogenesis. 2010;6:1–10. doi: 10.4161/org.6.1.10427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aroesty JM, McKay RG, Heller GV, Royal HD, Als AV, Grossman W. Simultaneous assessment of left ventricular systolic and diastolic dysfunction during pacing-induced ischemia. Circulation. 1985;71:889–900. doi: 10.1161/01.cir.71.5.889. [DOI] [PubMed] [Google Scholar]
- 20.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. 2008;6:292–300. doi: 10.2174/157016108785909779. [DOI] [PubMed] [Google Scholar]
- 21.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part ii (maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 22.Edwards WE. Pathology of myocardial infarction and reperfusion. In: Gersh BJ, Rahimtoola SH, editors. Acute myocardial infarction. New York: Chapman & Hall; 1997. pp. 16–50. [Google Scholar]
- 23.van Der Laak JA, Pahlplatz MM, Hanselaar AG, de Wilde PC. Hue-saturation-density (hsd) model for stain recognition in digital images from transmitted light microscopy. Cytometry. 2000;39:275–284. [PubMed] [Google Scholar]
- 24.Kaul S, Jayaweera AR. Coronary and myocardial blood volumes: Noninvasive tools to assess the coronary microcirculation? Circulation. 1997;96:719–724. [PubMed] [Google Scholar]
- 25.Kaul S, Jayaweera AR. Myocardial capillaries and coronary flow reserve. J Am Coll Cardiol. 2008;52:1399–1401. doi: 10.1016/j.jacc.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Hiemann NE, Wellnhofer E, Knosalla C, Lehmkuhl HB, Stein J, Hetzer R, Meyer R. Prognostic impact of microvasculopathy on survival after heart transplantation: Evidence from 9713 endomyocardial biopsies. Circulation. 2007;116:1274–1282. doi: 10.1161/CIRCULATIONAHA.106.647149. [DOI] [PubMed] [Google Scholar]
- 27.Rakusan K. Verification of coronary angiogenesis by quantitative morphology. Mol Cell Biochem. 2004;264:45–49. doi: 10.1023/b:mcbi.0000044373.61812.f7. [DOI] [PubMed] [Google Scholar]
- 28.Aoki T, Fukumoto Y, Sugimura K, Oikawa M, Satoh K, Nakano M, Nakayama M, Shimokawa H. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. -comparison between preserved and reduced ejection fraction heart failure. Circ J. 2011;75:2605–2613. doi: 10.1253/circj.cj-11-0568. [DOI] [PubMed] [Google Scholar]
- 29.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: Analysis of 152 necropsy patients. Am J Cardiol. 1987;60:1340–1355. doi: 10.1016/0002-9149(87)90618-7. [DOI] [PubMed] [Google Scholar]
- 30.Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Tavora F, Cresswell N, Li L, Ripple M, Fowler D, Burke A. Morphologic features of exertional versus nonexertional sudden death in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:532–537. doi: 10.1016/j.amjcard.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury L, Gheorghiade M, Bonow RO. Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol. 2002;89:719–722. doi: 10.1016/s0002-9149(01)02345-1. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014 Jul 1;63(25 Pt A):2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Tsagalou EP, Anastasiou-Nana M, Agapitos E, Gika A, Drakos SG, Terrovitis JV, Ntalianis A, Nanas JN. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–1398. doi: 10.1016/j.jacc.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 36.Drakos SG, Kfoury AG, Hammond EH, Reid BB, Revelo MP, Rasmusson BY, Whitehead KJ, Salama ME, Selzman CH, Stehlik J, Clayson SE, Bristow MR, Renlund DG, Li DY. Impact of mechanical unloading on microvasculature and associated central remodeling features of the failing human heart. J Am Coll Cardiol. 2010;56:382–391. doi: 10.1016/j.jacc.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: A disease of the microcirculation? Hypertension. 2006;48:1012–1017. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 38.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemura G, Takatsu Y, Fujiwara H. Luminal narrowing of coronary capillaries in human hypertrophic hearts: An ultrastructural morphometrical study using endomyocardial biopsy specimens. Heart. 1998;79:78–85. doi: 10.1136/hrt.79.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998;97:230–233. doi: 10.1161/01.cir.97.3.230. [DOI] [PubMed] [Google Scholar]
- 41.Cecchi F, Sgalambro A, Baldi M, Sotgia B, Antoniucci D, Camici PG, Sciagra R, Olivotto I. Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2:452–461. doi: 10.1007/s12265-009-9142-5. [DOI] [PubMed] [Google Scholar]
- 42.Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C, Kaul S. Role of capillaries in determining cbf reserve: New insights using myocardial contrast echocardiography. Am J Physiol. 1999;277:H2363–H2372. doi: 10.1152/ajpheart.1999.277.6.H2363. [DOI] [PubMed] [Google Scholar]
- 43.Escaned J, Flores A, Garcia-Pavia P, Segovia J, Jimenez J, Aragoncillo P, Salas C, Alfonso F, Hernandez R, Angiolillo DJ, Jimenez-Quevedo P, Banuelos C, Alonso-Pulpon L, Macaya C. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: Validation with endomyocardial sampling in cardiac allografts. Circulation. 2009;120:1561–1568. doi: 10.1161/CIRCULATIONAHA.108.834739. [DOI] [PubMed] [Google Scholar]
- 44.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nama V, Manyonda IT, Onwude J, Antonios TF. Structural capillary rarefaction and the onset of preeclampsia. Obstet Gynecol. 2012;119:967–974. doi: 10.1097/AOG.0b013e31824ea092. [DOI] [PubMed] [Google Scholar]
- 46.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: Effect of tumor necrosis factor blockade. J Hypertens. 2011;29:1203–1212. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- 47.Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:1715–1723. doi: 10.1016/j.jacc.2013.08.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox JE, Rosenberg J, Vallakati A, Gheorghiade M, Shah SJ. Usefulness of electrocardiographic qt interval to predict left ventricular diastolic dysfunction. Am J Cardiol. 2011;108:1760–1766. doi: 10.1016/j.amjcard.2011.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganz P, Hsue PY. Assessment of structural disease in the coronary microvasculature. Circulation. 2009;120:1555–1557. doi: 10.1161/CIRCULATIONAHA.109.899336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.