Abstract
Chronic restraint stress alters hippocampal-dependent spatial learning and memory in a sex-dependent manner, impairing spatial performance in male rats and leaving intact or facilitating performance in female rats. Moreover, these stress-induced spatial memory deficits improve following post-stress recovery in males. The current study examined whether restraint administered in an unpredictable manner would eliminate these sex differences and impact a post-stress period on spatial ability and limbic glutamic acid decarboxylase (GAD65) expression. Male (n=30) and female (n=30) adult Sprague-Dawley rats were assigned to non-stressed control (Con), chronic stress (Str-Imm), or chronic stress given a post-stress recovery period (Str-Rec). Stressed rats were unpredictably restrained for 21 days using daily non-repeated combinations of physical context, duration, and time of day. Then, all rats were tested on the radial arm water maze (RAWM) for two days and given one retention trial on the third day, with brains removed 30 minutes later to assess GAD65 mRNA. In Str-Imm males, deficits occurred on day 1 of RAWM acquisition, an impairment that was not evident in the Str-Rec group. In contrast, females did not show significant outcomes following chronic stress or post-stress recovery. In males, amygdalar GAD65 expression negatively correlated with RAWM performance on day 1. In females, hippocampal CA1 GAD65 positively correlated with RAWM performance on day 1. These results demonstrate that GABAergic function may contribute to the sex differences observed following chronic stress. Furthermore, unpredictable restraint and a recovery period failed to eliminate the sex differences on spatial learning and memory.
Keywords: Chronic stress, spatial learning, GABA, hippocampus, amygdala, sex differences, recovery
1. Introduction
It is increasingly apparent that chronic stress impairs hippocampal-dependent spatial learning and memory in a sex-dependent manner. In males, chronic stress impairs performance on the radial arm maze (Luine et al., 1994; Sweis et al., 2013), Y-Maze (McLaughlin et al., 2007), Morris water maze (Sousa et al., 2000; Kitraki et al., 2004a,b; Radecki et al., 2005; Song et al., 2006; McFadden et al., 2011; Green and McCormick, 2013), and the radial arm water maze task (RAWM, Hoffman et al., 2011; Hutchinson et al., 2012; For review see: Conrad 2010; Popoli et al., 2012; McEwen et al., 2012). Conversely, several studies report that chronic stress enhances spatial memory ability in females on the radial arm maze (Bowman et al., 2001), Y-maze (McLaughlin et al., 2007), object placement task (Beck and Luine, 2002), and Morris water maze (Kitraki et al., 2004a,b; McFadden et al., 2011; reviewed in Luine et al., 2007; Simpson & Kelly, 2012). These studies suggest that the sex of an individual influences the underlying neural mediators of spatial ability.
A putative target for chronic stress actions is the hippocampus, as it is essential for spatial memory (O’Keefe and Dostrovsky, 1971; Moser et al., 1993; Eichenbaum 1999; Conrad 2010) and contains an abundance of receptors for glucocorticoid stress hormones (McEwen et al., 1968). In male rats, chronic stress leads to a simplification of apical dendritic arbors within the CA3 region of the hippocampus (Watanabe et al., 1992a, 1992b; Galea et al., 1997; Conrad, 2006) and reduced glucocorticoid receptor number (Sapolsky et al., 1984a, 1984b), both of which correspond to deficits in spatial tasks (Hoffman et al., 2011; Wright et al., 2006). When chronically stressed males are given time to recover following the end of chronic stress, spatial ability improves in parallel with the restoration of the CA3 dendritic architecture (Hoffman et al., 2011; Ortiz et al., 2014). In contrast, chronically stressed, gonadally-intact female rats show mild or negligible basal dendritic retraction in the CA3 region (Galea et al., 1997; McLaughlin et al., 2010), which does not necessarily correspond with spatial memory (McLaughlin et al., 2005). Mitigating variables that might contribute to chronic stress effects on the hippocampus and spatial memory in females include the type of stressor (Park et al., 2008; McCormick et al., 2008), task (Wood & Shors, 1998; McLaughlin et al., 2008; McCormick et al., 2010), estrogen status (Shansky et al., 2006; Conrad et al., 2012; Ortiz et al., 2013) and even experimenter handling of the rats (Dobrakovová et al., 1993; Bohacek and Daniel, 2007; Hoffman et al., 2010). Whether females would return to their previous status in spatial ability following a post-stress recovery period has not been investigated. One goal of the present study is to investigate the potential sex differences arising from the effects of chronic stress and a post-stress recovery period on spatial learning and memory. Successful spatial memory recovery from chronic stress has been documented in male rats using the radial arm water maze (Hoffman et al., 2011; Ortiz et al., 2014) and so a similar task will be used in the current study.
An important variable that could impact sex differences in spatial memory performance is the type of stressor used. Restraint stress is commonly used in rodents due to its relative ease of use, however chronic restraint elicits detriments in spatial learning and memory in male (Sousa et al., 2000; Hoffman et al., 2011; Gomez et al., 2012; Ortiz et al., 2014), but not female rats (Kitraki et al., 2004a; McLaughlin et al., 2010). Stressors can be categorized as being physical, psychological, or both and chronic restraint is thought to include components of both physical and psychological stressors (Buynitsky and Mostofsky, 2009). In humans, psychological stressors produce a stress response when the stressor consists of any one of three characteristics: loss of control, novelty, and unpredictability (Mason, 1968; Cohen, 1980; Miller, 1981). In rodents, administration of chronic restraint stress might consist of a loss of a sense of control, but lacks components in the other categories (i.e. novelty and unpredictability). Furthermore, repeatedly administering restraint leads to predictability, as male rats demonstrate habituation of the stress hormone response (Galea et al., 1997). Therefore, one purpose of the present study was to determine whether enhancing the unpredictable nature of chronic restraint would lead to impaired spatial ability in both male and female rats.
Another purpose of this study was to examine the role of inhibitory tone as it pertains to chronic stress-induced changes in spatial learning and memory. Glutamate and γ-aminobutyric acid (GABA) neurotransmitters are primarily responsible for regulating inhibitory tone. In male rats, chronic stress leads to significant increases in hippocampal extracellular glutamate (Joëls et al., 2004) and decreases in hippocampal GABA (Grønli et al., 2007; O’Mahony et al., 2011). Hippocampal GABA levels in males might be lowered following chronic stress, in part, due to decreases in glutamic acid decarboxylase (GAD) levels, the synthesizing enzyme for GABA (El-faramawy et al., 2009). Furthermore, other limbic areas may display a dysregulated inhibitory tone following chronic stress, which may also impact spatial learning and memory. MeA input into the HPA axis is critical for HPA axis activation following restraint stress (Dayas et al., 1999). Cells in the MeA exhibit the greatest level of c-fos activation following a psychological stressor compared to other amygdala nuclei (e.g. central amygdala). These findings suggest that the MeA is implicated in the response to stressors. Taken together, these studies provide support for the notion that the spatial memory impairments induced by chronic stress are mediated by changes in inhibitory tone the limbic system. Whether these changes contribute to the sex differences observed in spatial learning and memory tasks following chronic stress are unknown and will be investigated.
The current study tested the hypothesis that the unpredictability of a chronic stress paradigm impacts the sensitivity of females to the immediate and long-term (i.e., recovery from) effects of chronic stress. The behavioral endpoint of spatial learning and memory was chosen due to the well-established immediate and long-term effects of chronic restraint on male rats. In addition, this study explored the effect of chronic unpredictable restraint stress on GAD expression in limbic regions, as a likely mediator of chronic stress effects and a potential mediator of sex differences.
2. Material and methods
2.1. Animals
Sixty young adult Sprague-Dawley male and female rats (males=30, females=30) approximately 2 months of age were purchased from Charles River Laboratories (Wilmington, MA) and were pair housed with a same-sex cage mate at Arizona State University housing facilities. Male and female rats were housed in separate rooms and control and experimental groups were additionally separated into different chambers on a reverse light cycle (12:12; lights off at 06:00am). Rats were given one week to acclimate before any behavioral procedures were performed. Food and water were available ad libitum except during the restraint procedure when both control and stressed animals did not have access. Rats were weighed once a week throughout the experiment. Behavioral testing was conducted during the dark phase of the light cycle. All procedures were conducted according to federal guidelines outlined in the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996) and institutional guidelines set forth by the Institutional Animal Care and Use Committee at Arizona State University.
2.2. Group Assignments
Rats were randomly divided into six groups (n=10/group): male and female non-stressed control (Con), male and female stressed rats that were tested immediately (Str-Imm), and male and female stressed rats that were given a 21 day post-stress recovery period (Str-Rec). The experimental groups and timeline are depicted in (Figure 1A).
Figure 1. Experimental Timeline and Physiological Data.
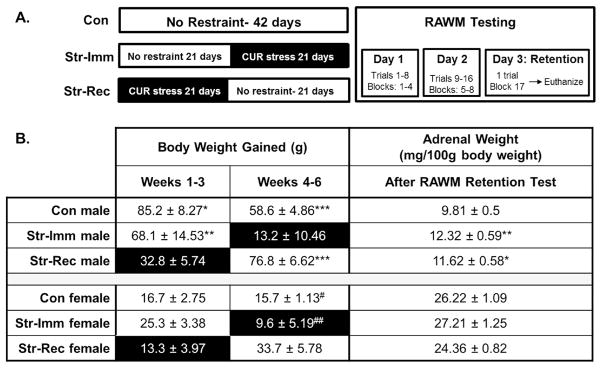
A. Experimental Timeline. Male and female rats were assigned to a chronic unpredictable restraint (CUR) stress for 21 days and then given a 21 day post-stress recovery period (Str-Rec) before radial arm water maze (RAWM) testing, or tested the day after restraint ended (Str-Imm), or left undisturbed (Con). All rats underwent 3 days of training and testing on the RAWM and were then sacrificed 30 minutes after the last testing session. Note that darkened blocks are shown to indicate when CUR stress occurred. B. Stressor Effectiveness. In males, CUR significantly attenuated body weight gain. Unstressed males gained significantly more weight than stressed males during CUR administration. During weeks 1–3, Str-Rec rats gained significantly less weight than both Con (* p < 0.05) and Str-Imm (** p < 0.01) males. During weeks 4–6, Str-Imm males gained significantly less weight than Con (*** p < 0.001) and Str-Rec (* p < 0.001) males. In females, CUR did not significantly attenuate body weight gain. However, during recovery (weeks 4–6), Str-Rec females exhibited significantly increased body weight gain compared to both Con (# p < 0.05) and Str-Imm (## p < 0.01) females. In males, CUR significantly increased relative adrenal weight in both Str-Imm (** p < 0.01) and Str-Rec * p <0.05) groups compared to Con males. In females, CUR had no effect on adrenal weight. Note that darkened blocks are shown to indicate when CUR stress occurred.
2.3. Chronic Unpredictable Restraint Stress Procedure
Rats were transported to different rooms each day and were restrained using restrainers made from wire mesh (purchased from Flynn and Enslow, San Francisco, CA, USA) with the ends sealed with grip guard sealer (ACE Hardware). The ends were secured with black binder clips. The chronic unpredictable restraint (CUR) schedule was comprised of different start time periods (ranges: 05:00–07:00, 08:00–10:00, 11:00–13:00, 14:00–16:00, 17:00–19:00), physical contexts (four different rooms), extract odors (almond, banana, orange), and restraint durations (no restraint, 30 minutes, 60 minutes, 90 minutes, and 120 minutes). After acclimation, the Str-Rec group was unpredictably restrained for 21 days then given a 21 day recovery period from restraint before behavioral testing. Str-Imm, however, were left undisturbed during the first 21 days, then unpredictably restrained during the subsequent 21 days and behaviorally tested the day after restraint ended. Con rats were left undisturbed until behavioral testing. Since the past literature indicates that even the subtle manipulation of handling to determine estrous status can impact female performance (Dobrakovová et al., 1993; Bohacek and Daniel, 2007; Hoffman et al., 2010), the gonadally-intact male and female rats were not manipulated to determine female estrous stage status.
2.4. Radial Arm Water Maze (RAWM)
2.4.1 Apparatus
The RAWM was constructed of black polypropylene with eight symmetrical arms (27.9 cm long by 12.7 cm wide) protruding from a circular center (48 cm diameter). The water (20°C–22°C) was made opaque with non-toxic powdered black tempera paint in order to obscure the platform. Males and females were tested in separate rooms. Each room contained salient cues along the walls of the room. Groups were counterbalanced across experimenters.
2.4.2. Procedure
A testing platform was placed at the end of one arm, 2.5 cm below the surface of the opaque water. The platform location was kept constant throughout the trials for each rat, but was counterbalanced between rats. Testing occurred over three consecutive days between 9:00am and 3:00pm. The first two training days consisted of eight trials, with one retention trial given on the third day. A trial began as soon as the rat was released into a non-platformed arm (start arm), which varied across trials so that a rat did not start in the same start arm on consecutive trials. Additionally, a trial was never initiated in an arm directly across from the platformed arm. Rats were given a maximum of three minutes to find the hidden platform. If a rat failed to find the hidden platform, the experimenter guided the rat to it. Rats remained on the platform for 15 seconds before they were removed and returned to the testing cage underneath heat lamps. After each trial, the maze was cleared of any debris, and the water was stirred to prevent subsequent rats from using odor cues. To avoid exhaustion, rats of different groups were tested in cohorts of six to eight at a time. After a trial was completed, rats were placed back into the testing cage to rest while the other rats in the cohort were tested. Time between each trial for an individual rat ranged from 5 minutes to 20 minutes. An entry into an arm was scored when the tip of the rat’s nose passed 11 cm into an arm.
2.5. Brain Collection and Tissue Processing
Thirty minutes after the start time of the retention trial on the final day of testing, rats were transported to a necropsy room and deeply anesthetized with isoflurane and rapidly decapitated. Adrenal glands were dissected and weighed. Brains were removed, blocked for hippocampus and amygdala, flash frozen in 2-methylbutane chilled with dry ice, and stored in a −80°C freezer until shipped frozen to Dr. Lucas for in situ hybridization processing.
2.6. In Situ Hybridization
In situ hybridization was utilized to quantify GAD65 mRNA expression in the cornu ammonis 1 (CA1) and dentate gyrus (DG) regions of the hippocampus, as well as the ventromedial hypothalamus (VMH) and medial amygdala (MeA). Brains were maintained at −70°C until transfer to cryostat for sectioning (at −20°C) and subsequently kept at −20°C until used in the in situ protocol. Twenty micrometer coronal brain sections were thaw-mounted on to glass microscope slides (Superfrost Plus, VWR, West Chester PA) on a cryostat (Leica).
Antisense mRNA was generated from a cDNA plasmid (generously provided by Dr A.J. Tobin, UCLA) linearized with HdIII and transcribed with RNA Polymerase T3 (Promega) with [35-S]-UTP (Perkin-Elmer) to a specific activity of 4.5 × 108 in a 550 nucleotide mRNA fragment. Unincorporated nucleotides were removed by Rneasy® MinElute™ Cleanup Kit (Qiagen). Prehybridization of tissue sections included a brief drying at room temperature after removal from −20°C storage, postfixation in 4% formaldehyde/PBS, rinsing in PBS, and acetylation in 0.25% acetic anhydride/0.1 M triethanolamine. Slides were passed through a dehydrating ethanol gradient and incubated with probe in hybridization solution at a saturated concentration (~ 28 Kcpm/μL) under glass coverslips at 45 °C for 18 h. Coverslips were removed in 4× standard sodium citrate and non-specifically bound probe was removed by treatment with RNase (Sigma-Alrich) for 30 min. Sections were run through stringency washes of 1× SSC and 0.5× SSC at 37 °C, and 0.1× SSC at 45 °C. Sections were then dehydrated, air-dried and exposed to Kodak BioMax X-ray film for 3 days along with microscale 14C standards (Amersham Biosciences). Autoradiographic images were transferred to a desktop illuminator (Kaiser Fototechnik, Buchen, Germany) with a CCD video camera (Hamamatsu C8484) with a MacroNikkor lens (Nikon) attached. X-ray film brain sections were digitized using computer assisted densitometry (Compix Imaging Systems, Sewickly PA). Background illumination was recorded (and digitally subtracted from subsequent images) and optical density was plotted as a function of microscale calibration values. Regions of interest in the CA1, DG, VMH, and MeA were selected among 4 to 6 coronal sections bilaterally, digitized and a calibrated mean optical density value was obtained for each location resulting in one averaged value of each region per animal.
2.7. Statistical Analyses
Statistical analyses were performed using SPSS (version 22). Physiological and behavioral data were analyzed with multifactorial or repeated measures analyses of variance (ANOVAs). Main effects and interactions were followed by Fisher’s LSD post hoc tests when p < 0.05. Pearson’s correlations were used to help determine the relationship between GAD65 expression and RAWM performance. Data are represented as means ± S.E.M. Results were considered statistically significant if p < 0.05.
3. Results
3.1 Physiological Measures
3.1.1 Body Weight
Attenuation of body weight gain throughout the study confirmed the effectiveness of CUR. Before experimental manipulation, the weight of male rats ranged from 282 grams to 332 grams, with an average weight of 317.5 grams. Female rats weight ranged from 194 grams to 230 grams, with an average weight of 210.3 grams. A multifactorial repeated measures ANOVA for stress history and sex over the two treatment periods (weeks 1–3: restraint on Str-Rec; weeks 4–6: restraint on Str-Imm) revealed significant main effects of stress (F(2,54) = 11.26, p < 0.001) and sex (F(1, 54) = 194.69, p < 0.001) and an interaction between treatment period and stress (F(2, 54) = 15.48, p < 0.001). Unstressed males gained significantly more weight than did stressed males during CUR administration (weeks 1–3: Con > Str-Rec, p < 0.01, Str-Imm > Str-Rec, p < 0.05; weeks 4–6: Con/Str-Rec > Str-Imm, p < 0.001; Figure 1B). In addition, during the recovery period the Str-Rec males exhibited weight gain similar to Con males (Figure 1B). Interestingly, the same pattern was not evident in females. Unstressed females did not gain significantly more weight than stressed females during the restraint periods (Figure 1B). However, during the recovery period (weeks 4–6), the Str-Rec females exhibited a significant increase in weight gain compared to both Con (p < 0.05) and Str-Imm (p < 0.01) females.
3.1.2 Adrenal weight
Restraint stress produced a significant increase in adrenal weights relative to body weight in males, but not in females (Figure 1B). Adrenal weights (mg) were analyzed per 100 g body weight. A 2 × 3 ANOVA for sex and stress revealed a significant main effect of sex (F(1, 50) = 401.134, p < 0.001) and a tendency for an effect of stress history (F(2,50) = 2.53, p = 0.09) on adrenal weights, with no significant interaction of sex by stress (F(2,50) = 2.17, p = 0.125). This analysis was followed up with separate one-way ANOVAs in males and females to examine stress history. In males, a significant effect of stress was found (F(2, 26) = 4.872, p < 0.05; Figure 1B), while the females failed to show an effect of stress on adrenal weight (Figure 1B). Post hoc analyses in male rats revealed a significant increase in relative adrenal weight in both Str-Rec (p < 0.05) and Str-Imm (p < 0.01) groups compared to Con males (Figure 1B).
3.2 Radial Arm Water Maze
3.2.1 Acquisition
Significant acquisition effects of CUR were observed across training and this interacted with stress history. A repeated-measures ANOVA for stress history and sex across training blocks (two trials each, blocks 1–8) revealed a significant effect of block (F(7,364) = 32.41, p < 0.001) on entry errors. Errors decreased as trials progressed, indicating that all rats learned the task (Figure 2). In addition, an interaction between stress history and block was detected (F(14,364) = 1.84, p < 0.05), indicating that not all groups performed similarly across time and this was probed further by day, as described next.
Figure 2. Radial Arm Water Maze.
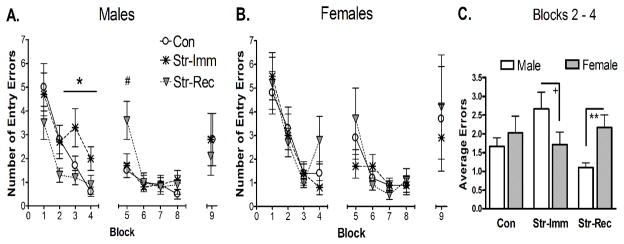
A and B. All groups demonstrated improved performance during acquisition (Day 1: blocks 1–4, Day 2: blocks 5–8). Data points represent mean entry errors ± SEM for each block. A. In males, significant acquisition effects were found on day 1. After the first block of information gathering (block 1), Str-Imm males made significantly more errors compared to both CON and Str-Rec males (* p < 0.05, blocks 2–4). Str-Rec and Con males showed statistically similar number of errors on day 1. However, at the beginning of the second day of training, Str-Rec rats made more errors than both Con and Str-Imm males (# p < 0.05, block 5), possibly indicating greater overnight forgetting. No other effects of stress history were found in males on day 2 of training (blocks 5–8) or on the retention trial (block 9). B. In females, no main effect of history was detected on any day or block. C. Sex differences were evident on day 1, blocks 2–4, where males demonstrated significant effects of stress history. Str-Rec females made significantly more errors than Str-Rec males (** p < 0.01) while Str-Imm females demonstrated a pattern of making fewer errors than Str-Imm males (+ p = 0.1). Con males and females did not differ. Bars represent mean ± SEM entry errors on blocks 2–4.
Training Day One
To reduce the impact of variability during the information gathering trials, the first block of day 1 was parsed out and analyzed separately. A two-way ANOVA for stress history and sex on the first block (2 trials) of training revealed no main effects of treatment or sex on entry errors (Figure 2A and 2B), confirming that all groups made a similar number of entry errors at the beginning of training. The subsequent blocks on day 1 (blocks 2–4) reflect behavior impacted by information gathered in the first and subsequent blocks. A two-way repeated measures ANOVA across blocks 2–4 on entry errors revealed a significant interaction between stress history and sex (F(2,52) = 4.72, p < 0.05) with no significant main effects of stress or sex. To further probe the sex-specific effects of stress history on acquisition, subsequent analyses assessed day 1 performance in males and females separately. In males, a repeated measures ANOVA on blocks 2–4 revealed a significant main effect of stress history (F(2,26) = 7.58, p < 0.01). Post hoc analyses revealed that Str-Imm males made more errors than did Con (p < 0.05; Figure 2A) and Str-Rec males (p < 0.001; Figure 2A). Con and Str-Rec males performed similarly on blocks 2–4. In females, no main effect of stress history was detected on blocks 2–4 (Figure 2B).
Sex Differences on Day One
Additional ANOVAs were used to probe the stress by sex interaction on blocks 2–4. An effect of sex was probed for each stress cohort separately. A significant effect of sex was found between Str-Rec males and females on blocks 2–4 (F(1,18) = 8.57, p < 0.01), such that Str-Rec females made more errors than did Str-Rec males (2.17 ± 0.34 vs. 1.1 ± 0.12; Figure 2C). For the Str-Imm comparison, a tendency for an effect of sex was also identified (F(1,17) = 3.00, p = 0.1), such that Str-Imm females made fewer errors than did Str-Imm males (1.72 ± 0.33 vs. 2.67 ± 0.45; Figure 2C). No difference in performance between Con females and males was evident (2.09 ± 0.46 vs. 1.67 ± 0.22; Figure 2C).
Training Day Two
On the second day of training (8 trials; blocks 5–8), no significant main effects of stress or sex were identified with a repeated measures ANOVA on entry errors (Figure 3). However, an interaction between block and stress was observed (F(6,156) = 3.08, p < 0.01). To further probe performance on day two, repeated measures ANOVAs were run separately for males and females. This analysis indicated that the stress effect was carried by the males (block by stress interaction; F(6,78) = 3.89, p < 0.01) but not the females. Block by block analysis of male performance revealed a significant effect of stress on block 5 only. Post hoc analyses indicated that this effect was caused by the Str-Rec males, such that Str-Rec males made more errors on block 5 than both Con and Str-Imm males (p < 0.05; Figure 2A). No effect of stress history on day two was identified in females.
Figure 3. Correlation between GAD65 expression and Acquisition on the RAWM.
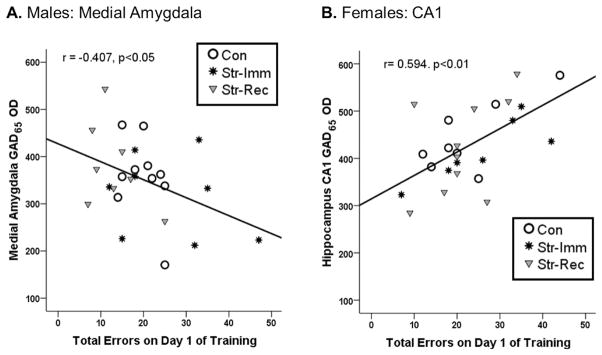
A. In males, GAD65 expression in the medial amygdala was negatively correlated with total errors on day 1 of training (r = −0.407, p < 0.05), such that increased errors were associated with decreased GAD65 expression. B. In females, GAD65 expression in the CA1 was positively correlated with total errors on day 1 of training (r = 0.594, p < 0.01), such that increased errors were associated with increased GAD65 expression.
3.2.2 Retention
Twenty-four hours after the final day of training, all groups were given a single retention trial to investigate long-term memory. A two-way ANOVA for stress history and sex did not reveal a significant main effect of either stress history or sex on either memory domain (Figure 2). Further probing of the retention trial with separate ANOVAs for males and females did not reveal any sex-specific effects of stress history.
Sample Size
One female Con rat was a statistical outlier and excluded, and one male Str-Imm rat was excluded for health issues. The final sample sizes were as follows: Male Con: n=10, male Str-Rec: n=10, male Str-Imm: n=9, female Con: n=9, female Str-Rec: n=10, female Str-Imm: n=10.
3.3 GAD65 expression
In a first pass analysis, an omnibus 2 × 3 ANOVA for the variables of sex and stress history on GAD65 expression was performed on each brain region analyzed. There were no significant main effects for sex, stress history, or interaction in any of the four areas examined (Table 1). Since the variable of stress was expected to alter GAD65 expression in limbic structures (El-faramawy et al., 2009), planned comparisons were performed.. However, no significant effects of stress history on GAD65 expression were revealed in any of the four brain regions analyzed in either males or females (Table 1).
Table 1. Mean GAD65 expression.
Calibrated optical densities from in situ hybridization results of GAD65 mRNA (nCi/g) expression from all brain regions analyzed. No differences between sex or stress conditions were found to be significant in any of the brain regions analyzed. Data are represented as mean ± SEM.
| Mean GAD65 expression (nCi/g) | ||||
|---|---|---|---|---|
| CA1 | MeA | DG | VMH | |
| Con male | 437.7 ± 33.07 | 358.0 ± 26.16 | 533.6 ± 27.01 | 355.8 ± 44.34 |
| Str-Imm male | 459.5 ± 20.86 | 317.1 ± 31.00 | 538.4 ± 15.25 | 356.9 ± 57.69 |
| Str-Rec male | 438.7 ± 19.63 | 378.8 ± 31.78 | 553.0 ± 26.08 | 335.1 ± 37.90 |
| Con female | 444.1 ± 26.00 | 334.6 ± 31.45 | 542.1 ± 9.66 | 345.3 ± 56.82 |
| Str-Imm female | 423.8 ± 32.28 | 333.0 ± 24.28 | 543.1 ± 21.28 | 241.6 ± 38.16 |
| Str-Rec female | 415.8 ± 24.24 | 335.1 ± 28.12 | 555.6 ± 22.89 | 258.2 ± 30.39 |
We found a wide range of variability in GAD65 expression across animals. We took advantage of this variability and conducted correlation analyses to determine whether individual differences in GAD65 expression were associated with RAWM performance. Pearson’s correlations were conducted to determine the extent to which GAD65 expression in the CA1, dentate gyrus (DG), ventromedial hypothalamus (VMH) and medial amygdala (MeA) correlated with performance on the RAWM (total errors on each day of training). GAD65 expression significantly correlated with total errors on Day 1 of training in a sex and region-specific manner. In males, a significant negative correlation was identified between GAD65 expression in the MeA and total errors on day 1 of training (r = −0.41, p < 0.05; Figure 3), such that increased errors were associated with decreased GAD65 expression. In females, a significant positive correlation was identified between GAD65 expression in the CA1 and total errors on day 1 of training (r = 0.59, p < 0.01; Figure 3), such that increased errors were associated with increased GAD65 expression. In both males and females, no significant correlations were found between GAD65 expression and day 2 of training in any of the brain regions analyzed.
4. Discussion
4.1 Summary of Findings
The present study administered chronic unpredictable restraint (CUR) stress to investigate whether CUR would impact spatial learning and memory in male and female rats similarly with regard to the immediate and long-term effects of chronic stress. The effectiveness of CUR in male rats was confirmed by attenuated body weight gain and adrenal hypertrophy. In male rats that received CUR followed by a recovery period (Str-Rec), body weight gain returned to normal, while adrenal weights did not. Str-Imm female rats were resistant to the measured physiological effects of CUR administration, though Str-Rec females gained significantly more weight than did Con and Str-Imm females during the recovery period. Male rats that were tested on the RAWM immediately after CUR administration (Str-Imm) exhibited significant impairments during the acquisition phase of the task, as demonstrated by increased total errors on day one of testing. However, male rats that were given a 21-day recovery period (Str-Rec) performed better than Str-Imm males and similarly to non-stressed control males. CUR administration did not affect acquisition performance in females tested immediately (Str-Imm) or after a recovery period (Str-Rec). For the retention trial phase of the RAWM (day 3), the effects of CUR did not affect performance in either males or females. Interestingly, RAWM acquisition correlated with GAD65 mRNA expression in a sex- and region-specific manner. In male rats, GAD65 expression in the MeA was negatively correlated with total errors on day 1 of RAWM training. Conversely, in female rats GAD65 expression in the hippocampal CA1 region was positively correlated with total errors on day 1 of RAWM training.
Physiological effects, such as attenuated body weight gain and enlarged adrenal glands, are common metrics used to determine chronic stress effectiveness. In the current study, chronically stressed male rats displayed increased relative adrenal weights compared to non-stressed controls, an effect that persisted even after a post-stress recovery period. In female rats, the adrenal to body weight ratio and body weight gain were unaltered by CUR. (Figure 1B). This contrasts with other studies showing that chronic restraint stress (McLaughlin et al., 2005; Conrad et al., 2012) and chronic unpredictable stress (McFadden et al., 2011) attenuate body weight gain in female rodents. Thus, it appears that females are less sensitive to CUR when compared to other stress paradigms. The predictability of chronic restraint stress administration was decreased in order to increase the effectiveness of the stressor on altering spatial performance, because repeated exposure to the same stressor leads to habituation of the HPA axis (Viau and Sawchenko, 2002). Habituation to a stressor depends on an organism’s ability to recognize the familiarity of stressor and if an animal encounters a novel stressor then the response of the HPA axis can be enhanced (Bhatnagar and Dallman, 1998). Indeed, some studies have shown that the physical context in which a repeated stressor is administered affects subsequent HPA responses to the stressor (Grissom et al., 2009). The present data suggest that manipulating the predictability in which a stressor is delivered, without changing the stressor itself per se, has little impact on altering body weight, adrenal size and spatial ability in female rats, but it does affect male rats in a manner consistent with other chronic stressors.
An extensive literature reveals that the effects of chronic stress on spatial learning and memory are determined by the sex of the animal (for reviews, see Conrad, 2010; Conrad and Bimonte-Nelson, 2010; McLaughlin et al., 2009). In males, some reports show that chronic stress impairs the learning aspect of spatial tasks (Luine et al., 1994; Sousa et al., 2000; Wright and Conrad, 2008), while others show that chronic stress affects only the memory for the task (Hoffman et al., 2011; Ortiz et al., 2014) or both (Rao and Raju, 2000; Radecki et al., 2005). Despite these discrepancies, the data reported here corroborate the established pattern of chronic stress having detrimental effects on learning in males, as administration of CUR to male rats produced deficits during the acquisition of the RAWM. Consistent with the literature, the CUR stressor in this study did not lead to learning and memory deficits in female rats (Kitraki et al., 2004a; McLaughlin et al., 2010). Although no significant effects were found, if any pattern was to be generously discerned, CUR may have even potentiated spatial learning in female rats, as documented in other reports on learning and memory (see Bowman et al., 2001; Kitraki et al., 2004b; Conrad et al., 2003; McLaughlin et al., 2005; Conrad et al., 2012). Thus, it appears that when restraint stress was made to be unpredictable, a non significant effect on spatial learning was observed in female rats. The common theme of these studies is that female rats appear to be resilient to the effects of chronic stress, especially when compared to male rats.
While CUR impaired spatial ability in the males, we found that a post-stress recovery period following chronic stress reversed the spatial learning deficits. The improvement of spatial ability in males following a post-stress recovery period is corroborated by other studies (Luine et al., 1994; Sousa et al., 2000; Hoffman et al., 2011; Bian et al., 2012; Ortiz et al., 2014), with outcomes that correspond to a restoration of hippocampal CA3 apical dendritic complexity following a post-stress recovery period (Conrad et al., 1999; Sousa et al., 2000; Hoffman et al., 2011). Unexpectedly, Str-Rec males showed impaired performance on day 2 (trial 1), implying that they did not retain the previous day’s information, an outcome that contrasts with our previous findings (Hoffman et al., 2011, Ortiz et al., 2014) and others (Sousa et al., 2000). However, the Str-Rec male performance on day 2 is confounded by acquisition differences occurring on day one. Specifically, Con and Str-Imm males made more arm entries during acquisition on day one than did Str-Rec males, which means that all male groups had different arm entry exposures on day 1. Thus, these a priori behavioral differences on day 1 confound any interpretations for performance on day 2. Despite these discrepancies, we add to the growing body of literature to show that allowing a post-stress recovery period following chronic stress allows for the return of spatial learning and memory.
The mechanisms underlying chronic stress-induced spatial learning deficits, and subsequent recovery in males, are unclear, but may involve altered inhibitory tone in limbic brain regions. GABA, the main inhibitory neurotransmitter, works in concert with glutamate to maintain a balanced excitatory/inhibitory tone in many brain circuits (Herman et al., 2004). Disruption of the delicate balance of these tones, as seen following chronic stress, is associated with impaired learning and memory and compromised neuronal integrity (Magariños et al., 1999; Watanabe et al., 1992b; Yoon et al., 2008). In the current study, GAD65 expression was measured in the CA1 subfield of the hippocampus, the medial amygdala, the dentate gyrus, and the ventromedial hypothalamus; brain regions known to display sexual dimorphisms (Matsumoto et al., 1983; Cooke et al., 1999; Madeira et al., 2001; MacLusky et al., 2004), to be influenced by chronic stress (Alfarez et al., 2003; Heine et al., 2004; Jankord and Herman, 2008; Radahmadi et al., 2014) and/or to mediate spatial learning and memory (Moser et al., 1993; Whitlock et al., 2006; Hunsaker et al., 2007; Goodrich-Hunsaker et al., 2008). We predicted that CUR would impact GAD65 expression in a region and sex dependent manner. However, we found no significant main effects of CUR or sex in GAD65 expression in any of the brain regions analyzed. We followed up with exploratory correlational analyses to determine the extent to which individual differences in GAD65 expression contributed to performance on the RAWM. It was observed that GAD65 expression in the medial amygdala (MeA) of males negatively correlated with spatial learning: increased errors were associated with less GAD65 expression in the MeA. This brain region is intriguing because it is responsible for emotional regulation (Phelps and Ledoux, 2005) and is impacted by stress (Prewitt and Herman, 1997; Dayas and Day, 2002; Vyas et al., 2002, 2004; Rosenkranz et al., 2010). It is fascinating that the distribution of the data show the Str-Imm males clustered together and with low GAD65 expression. In contrast, spatial learning in females positively correlated with GAD65 expression in the CA1 region of the hippocampus: increased errors correlated with higher CA1 GAD65 expression and the groups (Con, Str-Imm, Str-Rec) are dispersed throughout the graph. The lack of a correlation between spatial learning and amygdalar GAD65 expression in females is consistent with CUR not impacting spatial ability in females. However, the CA1 region is involved with spatial ability and shows plasticity with spatial learning (Moser et al., 1993; Whitlock et al., 2006). In sum, it appears that GAD65 expression in stress-sensitive limbic regions may be associated with sex differences on spatial ability tasks. A caveat in the current study is that animals underwent three days of RAWM testing, which can increase GAD expression in of itself (Bianchi et al., 2007; de Groote and Linthorst, 2007). Additionally, estradiol can regulate the inhibitory tone in the hippocampus of female rats through GAD65 and GAD67 mRNA expression (McCarthy et al., 1995), without necessarily affecting on GABAA receptor subunit mRNA expression (Weiland and Orchinik, 1995). Thus, inhibitory tone in limbic regions is affected by a number of factors, with chronic stress being one, but with other mediators (e.g. RAWM testing and sex hormones) that were present in the current study also regulating GAD65 expression. Future studies are needed to elucidate the influence that inhibitory tone, chronic stress, and a post-stress recovery period have on spatial learning between the sexes.
5. Conclusions
In this study, administration of CUR induced spatial learning deficits in male rats and this negatively correlated with GAD65 mRNA expression in the MeA an area important for emotional regulation. Conversely, administration of CUR led to no effects on spatial learning in female rats. Consequently, increasing the unpredictability of chronic restraint stress did not impact the resilience of female rats to chronic stress on these outcomes. Nevertheless, female rat performance on RAWM correlated with GAD65 mRNA expression in the CA1 region, an area that is highly plastic and responsive to ovarian hormones (McLaughlin et al., 2010; Conrad et al., 2012). The present findings represent an important first step in identifying the brain regions and neural mechanisms that contribute to sex differences in behavior and plasticity in response to chronic stress. Further investigations are necessary to determine how inhibitory tone in the amygdala and hippocampus contribute to the stress-induced behavioral differences observed between the sexes.
Highlights.
In males, chronic unpredictable restraint impaired spatial learning.
Stress-induced spatial learning deficits in males improved after a recovery period.
Spatial learning negatively correlated with medial amygdala GAD65 in males.
In females, chronic unpredictable restraint had no effect on RAWM performance.
Female RAWM spatial learning positively correlated with hippocampal CA1 GAD65.
Acknowledgments
The authors gratefully acknowledge the contributions of Danya P. Anouti, Amanda Krigbaum, Agnieszka Mika, and Jeffery J. Hanna for their assistance with the study. This work was funded in part by Arizona State University’s College of Liberal Arts and Sciences (Conrad), Loyola University Research Support (Lucas), the National Institutes of Health Initiative to Maximize Student Development program (R25GM099650 to Stuart Newfeld for funding Ortiz), the National Science Foundation Graduate Research Fellowship Program (DGE-1311230, Ortiz).
Abbreviations
- ANOVA
analysis of variance
- CA1
cornu ammonis 1
- CA3
cornu ammonis 3
- Con
control
- CUR
chronic unpredictable restraint
- GABA
γ-aminobutyric acid
- GAD65
glutamic acid decarboxylase
- RAWM
radial arm water maze
- Str-Imm
Stress-Immediate
- Str-Rec
Stress-Recovery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. Bryce Ortiz, Email: j.bryce.ortiz@asu.edu.
Sara B. Taylor, Email: taylor@hendrix.edu.
Ann N. Hoffman, Email: hoffmana7@ucla.edu.
Alyssa N. Campbell, Email: alyssa.campbell3@gmail.com.
Louis R. Lucas, Email: louis.r.lucas@gmail.com.
Cheryl D. Conrad, Email: conradc@asu.edu.
References
- Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. European Journal of Neuroscience. 2003;17(9):1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiology & Behavior. 2002;75(5):661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bian Y, Pan Z, Hou Z, Huang C, Li W, Zhou B. Learning memory and glial cell changes following recovery from chronic unpredictable stress. Brain Research Bulletin. 2012;88:471–476. doi: 10.1016/j.brainresbull.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Ballini C, Colivicchi MA, Della Corte L, Giovannini MG, Pepeu G. Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochemical Research. 2003;28(3–4):565–573. doi: 10.1023/a:1022881625378. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Hormones and Behavior. 2007;52(2):237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bowman R, Zrull M, Luine V. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Research. 2001;904(2):279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neuroscience & Biobehavioral Reviews. 2009;33(7):1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Cohen S. Aftereffects of stress on human performance and social behavior: a review of research and theory. Psychological Bulletin. 1980;88(1):82. [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behavioral & Cognitive Neuroscience Reviews. 2006;5(1):41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2010;34(5):742–55. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Bimonte-Nelson HA. Impact of the Hypothalamic-Pituitary-Adrenal/Gonadal Axes on Trajectory of Age-Related Cognitive Decline. In: Martini L, editor. Progress in Brain Research. Vol. 182. Elsevier; New York, NY: 2010. pp. 31–76. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory. 2003;79:32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113(5):902. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: Relationship with hippocampal CA1 spine density and dendritic complexity. Behavioral Neuroscience. 2012;126(1):142. doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. European Journal of Neuroscience. 1999;11(7):2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Day TA. Opposing roles for medial and central amygdala in the initiation of noradrenergic cell responses to a psychological stressor. European Journal of Neuroscience. 2002;15(10):1712–1718. doi: 10.1046/j.1460-9568.2001.02011.x. [DOI] [PubMed] [Google Scholar]
- Dobrakovová M, Kvetňanský R, Opršalová Z, Jeẑová D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18(3):163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- de Groote L, Linthorst ACE. Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience. 2007;148(3):794–805. doi: 10.1016/j.neuroscience.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103(2):123–33. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- El-Faramawy YA, El-banouby MH, Sergeev P, Mortagy AK, Amer MS, Abdel-tawab AM. Changes in glutamate decarboxylase enzyme activity and tau-protein phosphorylation in the hippocampus of old rats exposed to chronic mild stress: reversal with the neuronal nitric oxide synthase inhibitor 7-nitroindazole. Pharmacology Biochemistry and Behavior. 2009;91(3):339–344. doi: 10.1016/j.pbb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex difference in dendritic atrophy of CA3 pyramidal neuron in response to chronic restraint stress. Neuroscience. 1997;81(3):689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Lewis MJ, Luine VN. The interaction of chronic restraint stress and voluntary alcohol intake: Effects on spatial memory in male rats. Alcohol. 2012;46(5):499–504. doi: 10.1016/j.alcohol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavorial Neuroscience. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Green MR, McCormick CM. Effects of social instability stress in adolescence on long-term, not short-term, spatial memory performance. Behavioural Brain Research. 2013;256:165–171. doi: 10.1016/j.bbr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiology of Learning and Memory. 2009;92(2):215–224. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J, Fiske E, Murison R, Bjorvatn B, Sørensen E, Ursin R, Portas CM. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behavioural Brain Research. 2007;181(1):42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. European Journal of Neuroscience. 2004;19(1):131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and Glutamate Circuitry in Hypothalamo Pituitary Adrenocortical Stress Integration. Annals of the New York Academy of Sciences. 2004;1018(1):35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD. Chronic stress, cyclic 17β-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiology of Learning and Memory. 2010;94(3):422–433. doi: 10.1016/j.nlm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika a, Hutchinson KM, Bimonte-Nelson Ha, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. European Journal of Neuroscience. 2011;34(6):1023–30. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson KM, McLaughlin KJ, Wright RL, Ortiz JB, Anouti DP, Mika A, Diamond DM, et al. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiology of Learning and Memory. 2012;97(2):250–60. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behavioral Neuroscience. 2007;121(4):742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Annals from the New York Academy of Sciences. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, Van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–231. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience. 2004a;125(1):47–55. doi: 10.1016/j.neuroscience.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Annals of the New York Academy of Sciences. 2004b;1018:323–7. doi: 10.1196/annals.1296.039. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen B. Repeated stress cases reversible impairments of spatial memory performance. Brain Research. 1994;639(1):167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ. Chronic stress and neural function: Accounting for sex and age. Journal of Neuroendocrinology. 2007;19(10):743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145(9):4154–4161. doi: 10.1210/en.2004-0477. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. Journal of Comparative Neurology. 2001;432(3):329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Magariños AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European Journal of Pharmacology. 1999;371(2):113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the sympathetic-adrenal medullary system. Psychosomatic Medicine. 1968;30:631–53. doi: 10.1097/00006842-196809000-00022. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinologia Japonica. 1983;30(3):277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Kaufman LC, Brooks PJ, Pfaff DW, Schwartz Giblin S. Estrogen modulation of mRNA levels for the two forms of glutamic acid decarboxylase (GAD) in female rat brain. Journal of Comparative Neurology. 1995;360(4):685–697. doi: 10.1002/cne.903600412. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J. Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behavioural Brain Research. 2010;208(1):23–29. doi: 10.1016/j.bbr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behavioural Brain Research. 2008;187(2):228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology. 2012;62(1):3–12. doi: 10.1016/j.neuropharm.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L. Sex-dependent effects of chronic unpredictable stress in the water maze. Physiology & Behavior. 2011;102(3–4):266–275. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Molecular Neurobiology. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135(4):1045–1054. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Hormones and Behavior. 2008;54(3):386–395. doi: 10.1016/j.yhbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Research. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. chronic 17b-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized females: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM. Predictability and human stress: Toward a clarification of evidence and theory. Advances in Experimental Social Psychology. 1981;14:203–256. [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. The Journal of Neuroscience. 1993;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Mahony CM, Clarke G, Gibney S, Dinan TG, Cryan JF. Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression. Pharmacology Biochemistry and Behavior. 2011;97(4):690–699. doi: 10.1016/j.pbb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Ortiz JB, Mathewson CM, Hoffman AN, Hanavan PD, Terwilliger EF, Conrad CD. Hippocampal BDNF mediates recovery from chronic stress-induced spatial reference memory deficits. European Journal of Neuroscience. 2014 doi: 10.1111/ejn.12703. [DOI] [PubMed] [Google Scholar]
- Ortiz JB, McLaughlin KJ, Hamilton GF, Baran SE, Campbell AN, Conrad CD. Cholesterol and perhaps estradiol protect against corticosterone-induced hippocampal CA3 dendritic retraction in gonadectomized female and male rats. Neuroscience. 2013;246:409–421. doi: 10.1016/j.neuroscience.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learning & Memory. 2008;15(4):271–280. doi: 10.1101/lm.721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews Neuroscience. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt CM, Herman JP. Anatomical interactions between the central amygdaloid nucleus and the hypothalamic paraventricular nucleus of the rat: a dual tract-tracing analysis. Journal of Chemical Neuroanatomy. 1998;15(3):173–186. doi: 10.1016/s0891-0618(98)00045-3. [DOI] [PubMed] [Google Scholar]
- Radahmadi M, Hosseini N, Nasimi A. Effect of Chronic Stress on Short and Long-Term Plasticity in Dentate Gyrus; Study of Recovery and Adaptation. Neuroscience. 2014;280:121–129. doi: 10.1016/j.neuroscience.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15(2):246–53. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Rao S, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Current Science. 2000;79(11):1581–1584. [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biological Psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proceedings of the National Academy of Sciences. 1984a;81(19):6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress Down-Regulates Corticosterone Receptors in a Site-Specific Manner in the Brain*. Endocrinology. 1984b;114(1):287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behavioral and Brain Functions. 2006;2(8) doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behavioural Brain Research. 2012;229(1):289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacology Bochemistry and Behavior. 2006;83(2):186–93. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov N, Madeira M, Almeida O, Paula-Barbosa M. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;101(2):483. doi: 10.1016/s0306-4522(00)00465-6. [DOI] [PubMed] [Google Scholar]
- Sweis BM, Veverka KK, Dhillon ES, Urban JH, Lucas LR. Individual differences in the effects of chronic stress on memory: Behavioral and neurochemical correlates of resiliency. Neuroscience. 2013;246:142–159. doi: 10.1016/j.neuroscience.2013.04.052. [DOI] [PubMed] [Google Scholar]
- Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress: rapid publication. Journal of Comparative Neurology. 2002;445(4):293–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress Induces Atrophy of Apical Dendrites of Hippocampal Ca3 Pyramidal Neurons. Brain Research. 1992a;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992b;2:431–436. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Molecular Brain Research. 1995;32(2):271–278. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313(5790):1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proceedings of the National Academy of Sciences. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behavioural brain research. 2008;187(1):41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. European Journal of Neuroscience. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Okada J, Jung MW, Kim JJ. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning and Memory. 2008;15(3):97–105. doi: 10.1101/lm.850808. [DOI] [PMC free article] [PubMed] [Google Scholar]


