Abstract
Songbirds learn individually unique songs through vocal imitation and use them in courtship and territorial displays. Previous work has identified a forebrain auditory area, the caudomedial nidopallium (NCM),that appears specialized for discriminating and remembering conspecific vocalizations. In zebra finches, only males produce learned vocalizations, but both sexes process these and other signals. The present study assessed sex differences in auditory processing by recording extracellular multi-unit activity at multiple sites within NCM. Juvenile female zebra finches (n=46) were reared in individual isolation and artificially tutored with song. In adulthood, songs were played back to assess auditory responses, stimulus-specific adaptation, neural bias for conspecific song, and memory for the tutor’s song, as well as recently heard songs. In a subset of females (n=36), estradiol (E2) levels were manipulated to test the contribution of E2, known to be synthesized in the brain, to auditory responses. Untreated females (n= 10) showed significant differences in response magnitude and stimulus-specific adaptation compared to males reared in the same paradigm (n=9). In hormone-manipulated females, E2 augmentation facilitated the memory for recently heard songs in adulthood, but neither E2 augmentation (n=15) nor E2 synthesis blockade (n=9) affected tutor song memory or the neural bias for conspecific song. The results demonstrate subtle sex differences in processing communication signals, and show that E2 levels in female songbirds can affect the memory for songs of potential suitors, thus contributing to the process of mate selection. The results also have potential relevance to clinical interventions that manipulate E2 in human patients.
Keywords: zebra finch, sex differences, learning, memory, estradiol
INTRODUCTION
In many species, individuals communicate with each other through acoustic signals. In order to effectively relay information between conspecifics, these signals must be perceived and discriminated by the listener. In humans, the production and discrimination of communication signals depend on learned processes, shaped by the auditory and social contexts experienced during a sensitive period of development. Songbirds also use a complex system of acoustic signals to communicate, and provide an ideal model in which to study auditory processing because they 1) communicate through vocalizations in a variety of social interactions (Williams, 2004; Zann,1996); 2) learn individually unique vocalizations through a process of imitation that parallels human speech acquisition (Doupe and Kuhl, 1999); and 3) have forebrain auditory areas specialized for processing species-specific sounds (Chew et al., 1995, 1996a and b; Mello et al., 1992). Songbirds also serve as an accessible model to examine how hormonal state affects processing of these signals, because neurons within these regions are both sensitive to and can synthesize hormones from steroid precursors (Saldanha et al., 2011, 2000).
Many songbird species show sexual dimorphism in the production of song, but few direct comparisons have been drawn between the ways that the male and female auditory systems process these communication signals. In the zebra finch, only the male sings (although both sexes produce unlearned calls) and uses his song as a sexual advertisement to attract females, who listen to the songs of individual males and select a mate based on learned preferences (reviewed in Nowicki and Searcy, 2004). The auditory system plays an essential role in shaping these social interactions, since developmental exposure to a singing tutor is required for a male to learn to sing a quality song (tutored song: Tchernikovski, 1999) and for a female to learn to prefer it (Riebel, 2000; Miller, 1979; Clayton, 1988; Lauay et al, 2003; Hernandez et al., 2008; Hauber et al., 2010).
An auditory area in the songbird forebrain, the caudomedial nidopallium (NCM), is specialized for discriminating and remembering the vocalizations of other birds. NCM receives projections from neurons of the avian primary auditory area, the Field L complex (Karten, 1991; Wang et al., 2010) and shares reciprocal connections with another auditory area, the caudomedial mesopallium (CMM). NCM neurons respond more robustly to conspecific than to heterospecific vocalizations or tones (Chew et al., 1996a) and exhibit stimulus-specific adaptation (SSA) to the songs of individual birds (Chew et al., 1995, 1996a and b; Stripling et al., 1997). SSA in NCM is long-lasting and provides an index of a song’s familiarity that can be interpreted as a form of neuronal auditory memory (Chew et al., 1995 and 1996a and b; Phan et al., 2006; Mello et al., 1992). NCM neurons also express estrogen receptors, can synthesize estradiol (E2) from androgen precursors (Saldanha et al., 2011, 2000), and rapidly produce E2 in response to hearing song (Remage-Healey et al., 2012, 2008). Together, these unique characteristics of NCM enable us to investigate the possibility of sex differences in the auditory brain circuits that underlie behavioral responses to conspecific vocalizations and how hormonal state modulates neural responses to communication signals.
In the present study, we use methods previously employed in males in our lab (Phan et al., 2006) to tutor females and test neuronal memory for the tutor’s song in NCM. This tutoring protocol results in reliable copying of the tutor song in males (Phan et al., 2006; Tchernochovski et al., 1999), and similar protocols have been shown to elicit behavioral preferences for the tutor song in females (Riebel, 2000). In the same subjects, we also test memory for recently heard songs by exposing them in adulthood to song playbacks prior to NCM recording and then testing the neuronal memory 6 hours later using previously established methods (Chew et al., 1996a and b; Yoder et al., 2012). We compare our results in females with those of males tested in the same paradigm to assess sex differences in auditory processing and memory.
The hormone estradiol (E2) has been shown, in some cases, to facilitate learning and memory (Calisi et al., 2013; reviewed in Frick, 2012) and some of these effects may be due to changes in sensory processing. E2 increases within NCM when a bird hears conspecific song, and E2 manipulations alter neural responses to sounds in both sexes (Remage-Healey et al., 2010, 2012, 2013; reviewed in Maney and Pinaud, 2011, Yoder and Vicario, 2012). In females, acquiring and remembering the songs of males presumably contributes to the ability to choose a good mate, and E2 in adulthood could facilitate song memory and/or discrimination at a time when reproduction is more likely to be successful. However, the effects of E2 on song memory in female NCM have not been studied using electrophysiological techniques. Therefore, in a subset of females, we also manipulated E2 and tested memory for the tutor’s song and for more recently heard songs, as well as for the neural response bias to conspecific song, to determine whether E2 affects the processing of this sexually salient signal.
Our results show that there are subtle sex differences in how the brain processes communication signals and suggest that E2 levels can influence the way the songs of potential suitors are remembered and compared in the brain, thus contributing to the process of mate selection.
MATERIALS AND METHODS
Subjects
Female nestling zebra finches (n=46) were obtained from our aviary breeding colony and reared in a box-tutoring paradigm in which they were isolated from male song shortly after hatching and tutored during the sensitive period for song learning (described below). In adulthood, a portion of these females received no further treatment and underwent electrophysiological testing (n=10) in order to assess sex differences in response to song. The remainder received hormonal manipulations prior to electrophysiological testing (n= 36) in order to assess the effects of estradiol on song memory. Birds from the same brood were distributed among treatment groups. In addition, data from males, previously tutored (n=9) and tested in the same conditions (see Phan et al., 2006), were used in order to compare female and male responses to song.
Throughout the course of the experiments, birds remained in their isolation chambers except during surgical and testing procedures. Lights were maintained on a 12L: 12D cycle. Birds had ad libitum access to seed and water, and were given supplemental nestling food daily. All procedures were approved by Institutional Animal Care and Use Committees at Rutgers University
Tutoring Procedure
The tutoring procedure has been described previously (Phan et al., 2006; Tchernichovski et al., 1999). Briefly, hatchling broods, together with their mothers, were placed into isolation boxes at d10±2 (Figure 1), then each juvenile was moved into individual isolation at d30±2. From d45 to d85, birds could elicit playback of a tutor song by pecking either of two keys located on the rear wall of the cage (20 playbacks/day). After tutoring, playbacks were disabled and birds remained in their isolation boxes except during surgical and testing procedures. This tutoring paradigm has been shown to elicit reliable song-copying in male zebra finches (Phan et al., 2006; Tchernichovski et al., 1999). Though we did not test behavioral preferences in our current study, similar tutoring protocols have been used in females in other labs and show that developmental auditory playback of a song elicits behavioral preferences for that tutor song (Riebel, 2000).
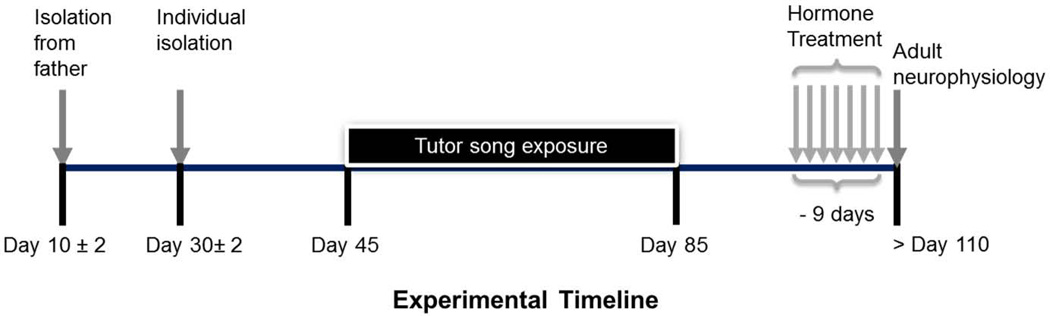
Figure 1.
Experimental timeline. Juvenile female zebra finches (n=46) were exposed to adult male song from d45 to d85. In adulthood, a subset of these females received no further treatment and underwent electrophysiological testing (n=10) in order to assess sex differences in response to song. The remainder (n=36) received hormonal manipulations that began 9 days prior to electrophysiological testing in order to assess the effects of estradiol on song memory. Auditory stimuli included the tutor song and novel and familiar conspecific and heterospecific songs.
Hormone Treatments
For experiments involving hormonal manipulation, birds (n=36) were treated with Estradiol (E2; Steraloids, RI), the aromatase inhibitor Fadrozole (FAD; Novartis, Switzerland), or Saline. Fadrozole was delivered by intramuscular injection and E2 by silastic implant, as described below. Birds were randomly assigned to receive E2 implants + intramuscular saline injections (n= 15), Blank Implants+ FAD injections (n=9), or Blank Implants+ saline injections (n=12).
Treatments began 9 days prior to electrophysiological testing, and the last injection was administered on the evening prior to testing. E2 implants consisted of Silastic tubing (Dow-Corning, NY) filled with 17-beta-estradiol (Steraloids, Newport, RI) according to established procedures (Smith et al., 1977; Svec and Wade, 2009). Estradiol from these implants may be detected in serum samples five days after implantation, is significantly elevated compared to blank-implanted females (Svec and Wade, 2009), and continues to be released for at least 80 days (Maney et al., 2006). The implantation procedure was as follows: under local anesthetic (Lidocaine Hydrochloride, 2%), a small incision was made in the dermis of the breast muscle, the implant was placed underneath the skin and the incision was sealed with cyanoacrylate. The first dose of FAD (100ug dissolved in 10uL 0.75% saline) or saline vehicle was given after implantation and daily doses were given for the next 8 days. This dose of FAD in zebra finches has been shown to decrease aromatase activity to ~33% after six days of treatment (Wade et al., 1994).
We based the duration of our 9d treatment on our best interpretation of the rate of E2 depletion reported in Wade et al.'s (1994) original article which showed that 6d of injections reduced E2 levels to 33%. We extended that time frame by 3d to ensure low E2 levels when testing our neural hypothesis. Thus our procedure was not designed from an ethological perspective.
Surgery
Two days prior to electrophysiological testing, birds were fasted for >=30 minutes, anesthetized under isoflurane (1.5% at 0.3L/min induction, 0.1L/min maintenance) and placed into a stereotaxic apparatus. Marcaine (0.04cc, 0.25%) was injected under the scalp, and the skin incised to expose the underlying skull. The portion of skull overlying the mid-sagittal sinus was removed, and dental cement was used to attach a small metal post to the skull rostral to the opening and to form a chamber around the recording area. Intramuscular injection of Metacam (0.04 cc, 5mg/mL) was administered to relieve post-surgical pain. Birds recovered under a heat lamp and were returned to their isolation boxes where they remained until exposed to song memory stimuli. All procedures were approved by the Rutgers University IACUC.
Song Memory Procedure
The evening before testing, each bird in its cage was placed into a soundproof recording booth (Industrial Acoustics, Bronx, NY) in order to acclimate to the testing environment. The next morning at 7:30 am (0.5h after lights on), the bird was exposed to passive song playbacks presented through a speaker in the booth (volume: 65dB SPL). Stimuli included two conspecific (zebra finch; ZF) and 2 heterospecific (canary; CAN) songs (200 repetitions, 8s ISI) that were presented sequentially such that 200 trials of a CAN song were followed by 200 trials of a ZF song (ABAB design). Heterospecific stimuli were partial canary songs similar in duration to zebra finch songs (1.2–1.8s). After hearing these songs, birds remained in the sound booth until the testing procedure began 6 hours later, following a previously established protocol (Chew et al., 1996a).
NCM Recording
For testing, the bird was placed in a restraining tube to minimize movement, and its head was stabilized by clamping the headpost to the stereotaxic apparatus. Using the stereotaxic plane described in Stokes, Leonard and Nottebohm (1974), 7–16 platinum-tungsten micro-electrodes (2–4 MΩ impedance; Thomas Recording, Eckhorn Design, Giessen, Germany) were positioned bilaterally on either side of the bifurcation of the mid-sagittal sinus (0.5 mm rostral, 0.5–1.0 mm lateral) and lowered individually into the brain (1.0–1.5 mm depth) while playing a set of search stimuli from a speaker in front of the bird (0.5m, 65dB SPL). This recording protocol has been used previously in our lab (Chew et al., 1996a; Phan et al., 2006). These stimuli consisted of white noise, shaped with the amplitude envelope of zebra finch songs, presented in randomized order (8s ISI). Physiological responses to stimulus playback were amplified (x19,000; Brownlee Precision, San Jose, CA) and bandpass filtered (0.5 to 5kHz). Once responsive sites typical of NCM were found (robust phasic-tonic responses that are sustained approximately 50–100ms following stimulus offset, as opposed to phasic burst responses seen in nearby Field L2; Terleph et al., 2006), stimulus playbacks began. Stimuli were presented in two sets: the 6-hour memory set contained the 4 previously-heard songs (2 zebra finch, 2 canary) and 6 novel songs (3 zebra finch, 3 canary), and the Tutor song set contained the Tutor song and 3 novel zebra finch songs. Stimuli in each set were presented in randomized order (25 repeats, 8s ISI). During the experiment, each subject was monitored for comfort and wellbeing. Adjustments to the restraint were made if the animal displayed any signs of discomfort.
At the end of recording, electrolytic lesions (20uA for 12 seconds) were made at two sites in each hemisphere. After~48 hours (to ensure that gliosis had occurred, which increases visibility of lesions at the recording sites), birds were sacrificed with a lethal injection of Nembutal (0.15 cc, IM) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. Fixed brains were sliced in 50 uM sagittal sections, mounted on Fisher DuraFrost Slides (Fisher Scientific, Pittsburgh PA), stained with Cresyl-Violet, coverslipped, and visually analyzed for lesions to verify electrode placement in NCM.
Analysis of Electrophysiology Data
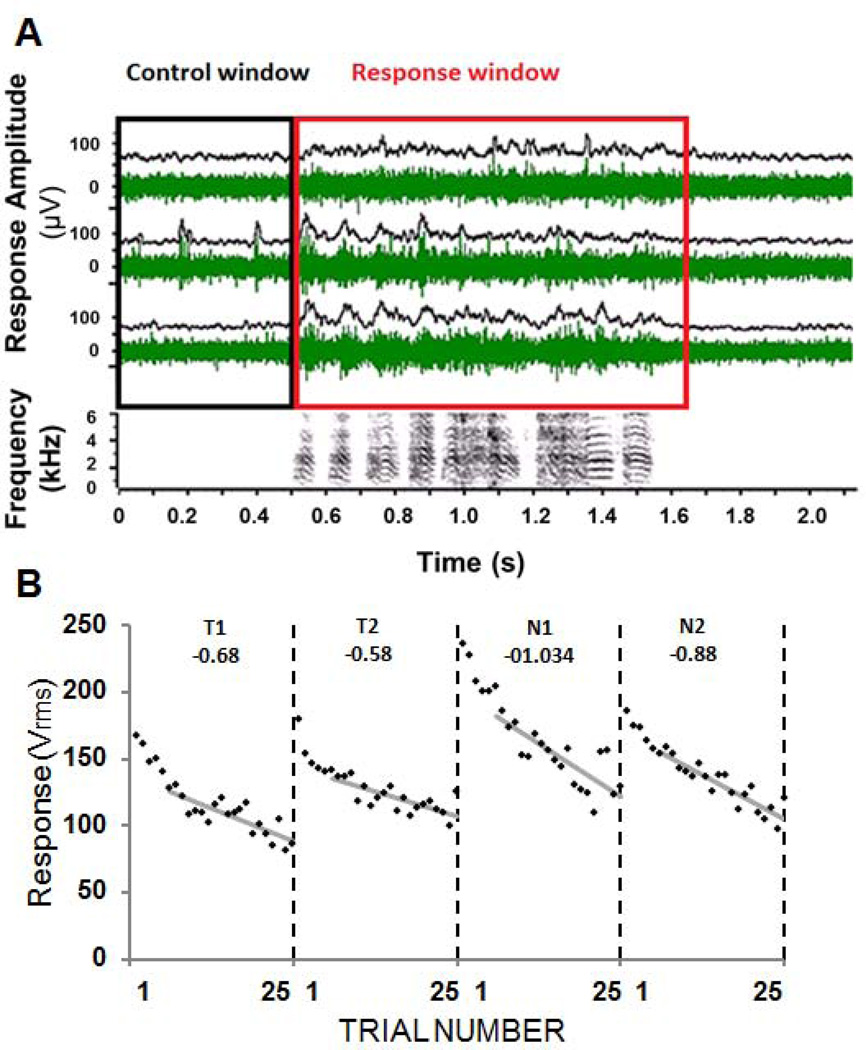
Physiological responses at each site were analyzed to determine 1) the amplitude of NCM multi-unit activity in response to stimulus presentation relative to baseline activity and 2) the adaptation rate of these responses to repetition of each stimulus. The response amplitude (Response-Control, or R-C) was calculated as the root mean squared (RMS) during the Response window (R: duration of stimulus + 100 milliseconds) minus the average RMS during the Control window (C: 500 milliseconds before the onset of the stimulus) across 25 trials (Figure 2A). The R-C was calculated for each stimulus at each electrode. Each recording site’s response magnitude to a stimulus was calculated by averaging R-C across trials 2–6, following established methods (Phan et al., 2006; Yoder et al., 2012). Sites with responses that did not differ significantly from the control level were excluded from the analysis, resulting in exclusion exclusion of <5% of all the data.
Figure 2.
A) Representative responses at 3 recording sites in NCM. Raw multi-unit records (green and black traces) are shown for a single trial. To calculate the response amplitude at a given site, the response during the Control (C) window (black box; 500ms of silence prior to stimulus onset) was subtracted from the response during the Response (R) window (red box; stimulus presentation + 100ms of silence). R-C was calculated for each trial of each stimulus for each recording site. B) The response magnitude of multiunit activity recorded at one site in NCM during presentation of four songs that were previously heard during training (T1, T2) or were novel (N1, N2). Songs were played 25 times each in shuffled order, but responses have been reordered for clarity. Adaptation rates were calculated for trials 6–25 (gray lines) and are shown at the top of each panel.
The adaptation rate to each stimulus was calculated by performing a regression of R-C for trials 6–25, which produces a negative slope, and then dividing this rate by the mean response amplitude over the same trials. This normalization produces a dimensionless quantity (change expressed as a percent of the mean response) that can be used to compare adaptation between sites and birds. Thus, the rate is a percent change that is independent of absolute amplitude, and can be compared across sites and across birds. Earlier work in the laboratory established that adaptation across trials 6–25 is approximately linear and best differentiates familiar from novel songs (see Phan et al., 2006; this measure provides a standard for quantifying memory effects (Figure 2B)). Sites that did not show adaptation to novel song stimuli were excluded from all analyses. The adaptation criteria were set at a conservative level such that recording sites that had an adaptation rate > −0.05 (i.e. were essentially not adapting) were excluded, which resulted in exclusion of less than 5% of all recording sites in each comparison group (males, females and hormone treatment groups).
Since stimuli elicit faster adaptation when they are novel than when they are familiar, the relative familiarity of a stimulus may be determined by comparing the adaptation rates of NCM neurons to “familiar” versus novel stimuli. As in an earlier study (Phan et al., 2006), we calculated a “Familiarity Index” (FI) for the tutor and training songs at each site by dividing the average adaptation rate to novel songs by the adaptation rate to the familiar songs (FI= avgNOVEL/avgFAMILIAR). FIs near 1.0 reflect no difference in adaptation rates to novel and familiar songs and so indicate no memory for the training songs, whereas FIs >1 reflect shallower adaptation to the familiar songs and so indicate memory for the training songs.
Statistical differences between groups in response magnitudes, adaptation rates, and familiarity indices to song stimuli were assessed with appropriate parametric tests (ANOVA, t-test) and, when data were not normally distributed, non-parametric tests (Kruskal-Wallis, Mood’s Median, Kolmogorov-Smirnov). Statistical analyses were carried out in Statistica (StatSoft, Tulsa, OK), with statistical significance set at p < 0.05, two tailed. For multiple comparisons, Bonferroni-corrected or Kruskal-Wallis p-values are shown. These analyses were conducted using data from recording sites as the unit of analysis (as in Phan et al., 2006).
RESULTS
Sex Differences in NCM processing
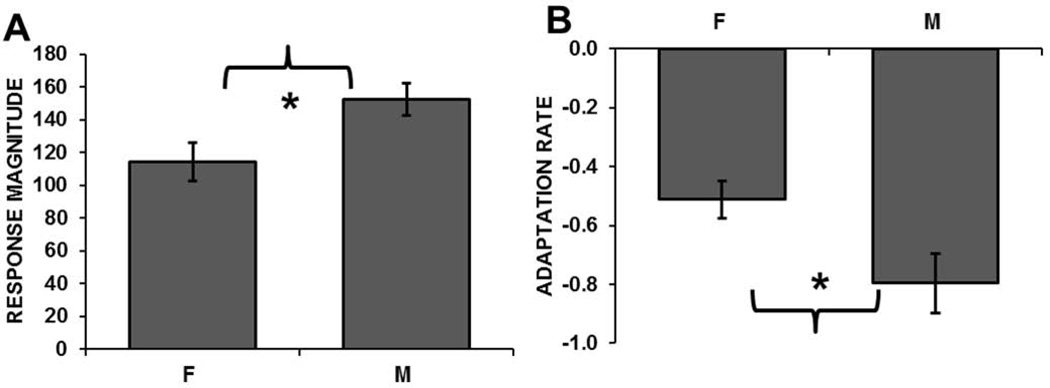
When we directly compared untreated females to males recorded under the same conditions (previously reported in Phan et al., 2006), male and female absolute response magnitudes to novel zebra finch (ZF) songs there was a sex difference in NCM response characteristics. Females (n= 10 birds at 35 sites) had significantly lower response amplitudes than males (n= 9 birds at 41 sites; t = −2.53, p = 0.01; Figure 3a). Females also exhibited shallower adaptation rates than males (t = 2.28; p = 0.03; Figure 3b).
Figure 3.
Sex differences in NCM responses to novel zebra finch songs. A) Recordings in females (F) had lower response magnitudes than in males (M). B) Recordings in females had shallower adaptation rates than in males.
Tutor-song Memory in Intact Females
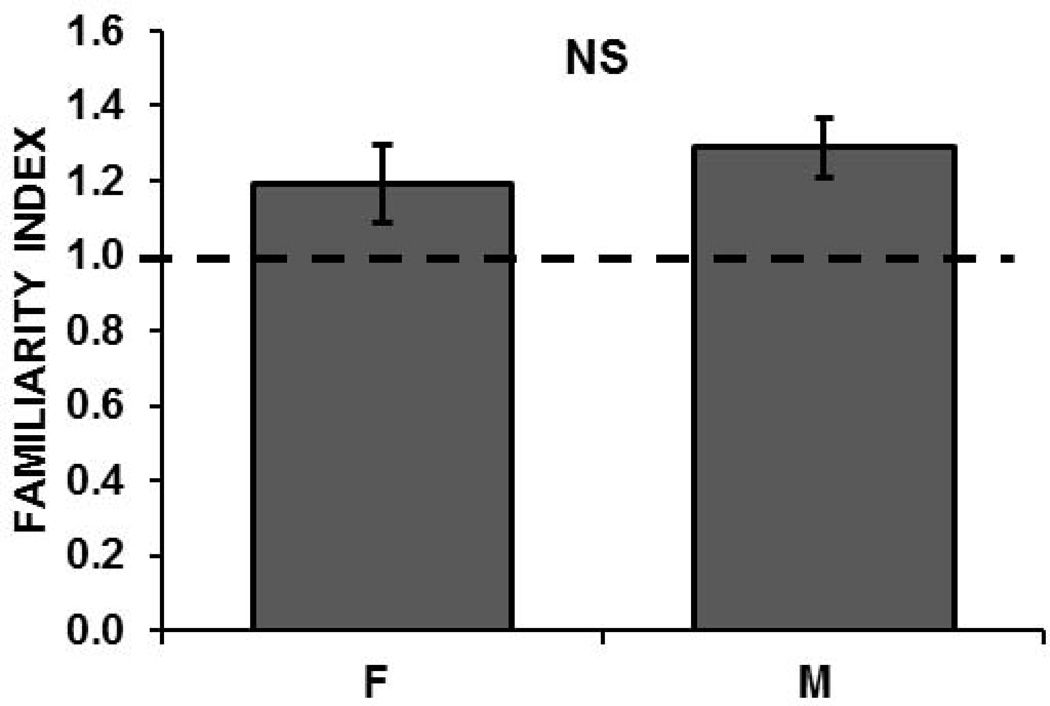
To assess the memory for tutor-song in intact females, familiarity to the tutor song was calculated as the FI (see Methods) at each site in females that had been box-tutored as juveniles. The average FI in females was greater than 1 (tutored females = 1.19, SE= 0.10), indicating that the adaptation rate to the tutor’s song was shallower than the adaptation rate to novel songs and thus that the tutor’s song was remembered. In addition, FIs in these females did not differ significantly from those of males who were reared in the same conditions and showed a tutor song memory (tutored males = 1.29, SE= 0.08; male data published in Phan et al., 2006; t= −0.78, p = 0.44; Figure 4). However, we cannot definitely conclude that females express a tutor song memory that is as strong as that in tutored males, because, 1) the mean FI was slightly lower than in males; and 2) 52% of sites expressed an FI greater than 1 in tutored females, while 70% of sites did so in tutored males. When we compared the distributions of FIs among males versus females using nonparametric statistics, we found no significant difference in the distributions (Kolmogorov-Smirnov test (KS), D = 0.267, Z = 1.16, p= 0.108). The FI index, used to quantify neuronal memory, was further evaluated by determining whether an FI of 1.0 (indicating no memory) lay within the 95% confidence interval (CI) of the distribution of sample FIs. CIs greater than 1.0 thus indicated the presence of a neuronal memory. The FI for tutor song memory in untreated males was significant by this criterion (mean FI=1.29, CI=1.130); however in untreated females, 1.0 was just inside the interval (1.19, CI=0.983), suggesting a strong but non-significant trend for tutor song memory. We interpret these data to indicate the presence of weak neuronal memories for tutor song in females, but the results are not consistent across individuals; thus, FI indicates significant memory in some female samples, but not in others. In the grouped data, the memory thus appears weak, in contrast to the reliable memory measured in males, which confirmed earlier male data (Phan et al, 2006).
Figure 4.
Familiarity indices (FIs) for the Tutor Song did not differ significantly between tutored females (F) and tutored males (M). FI is calculated as the average adaptation rate to novel songs/average adaptation rate to Tutor song. When FI = 1 (dashed line), adaptation rates are equal, indicating no familiarity for the Tutor song.
Memory for Recently Heard Songs in Intact Females
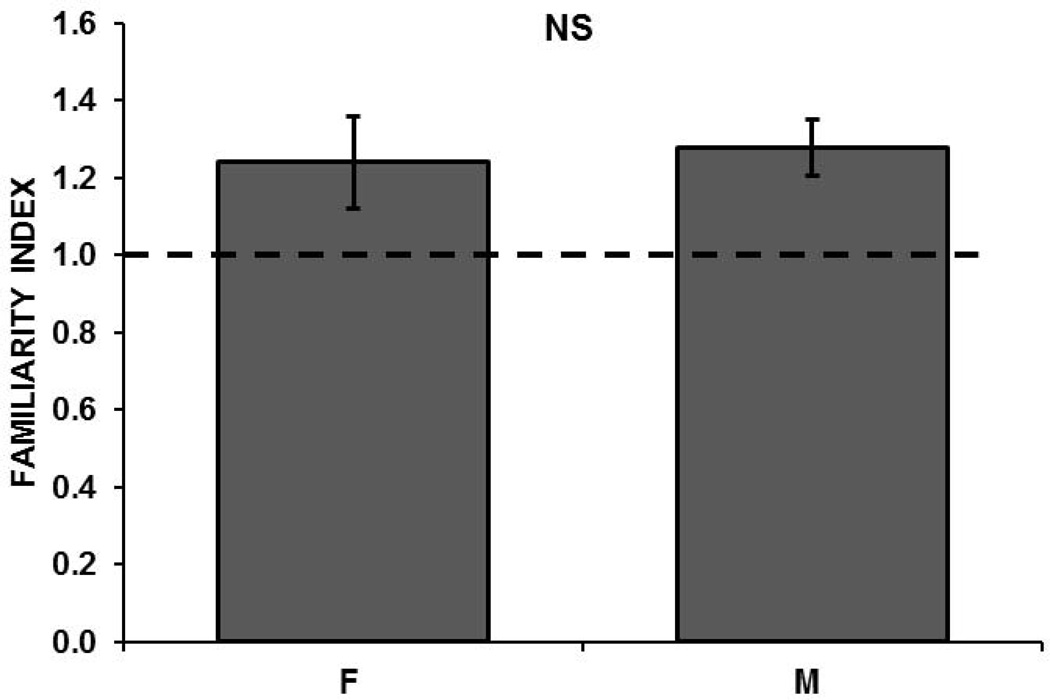
In the same group of females, we assessed memory for recently heard songs by calculating FIs to familiar songs that were presented 6 hours prior to NCM recording. The average FI in females was greater than 1 (1.24, SE= 0.12; n= 10 birds at 48 sites), indicating that adaptation rates were shallower to training than to novel songs and that the training songs were remembered. Female FIs did not differ significantly from those of males tested in the same memory paradigm (1.28, SE=0.07; n=9 birds at 52 sites; t = −0.28, p=0.78; Figure 5). As was observed in the distribution of FIs calculated for the tutor song, there appeared to be a disparity among male and female distributions in the number of sites that showed a memory for the training songs: among females, 58% of sites had FIs greater than 1, compared with 72% of sites in males. Using nonparametric statistics, we compared the distributions of FIs among males versus females, but found no significant difference in the distributions (KS, D = 0.215, Z = 1.08, p= 0.163). In experiments testing recent memory, the CI analysis showed a significant memory in both untreated females (1.24, CI=1.001) and males (1.28, CI=1.136).
Figure 5.
Familiarity indices (FIs) for songs heard 6h prior to NCM recording in adults. FIs for the recently heard songs indicated familiarity that did not differ between females (F) and males (M). When FI = 1 (dashed line), adaptation rates are equal, indicating no familiarity for the training songs.
Tutor-song Memory in Hormone-Manipulated Females
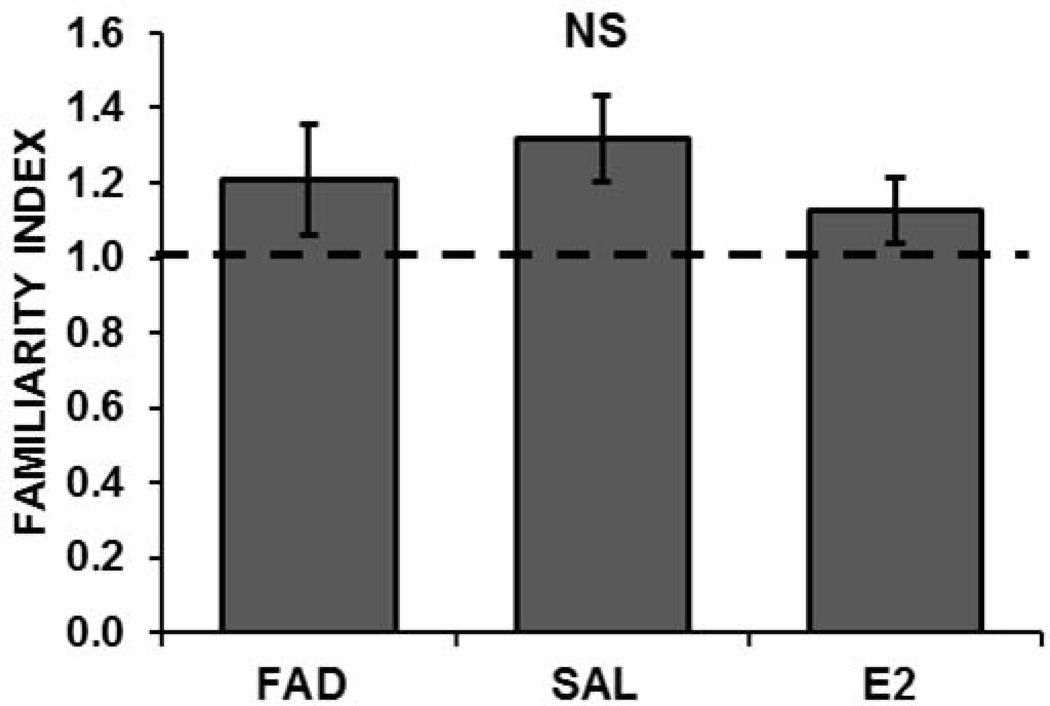
To assess the role of estradiol in memory for the tutor song in females, we manipulated E2 in a separate group of intact females and assessed memory for the tutor’s song. Birds were treated with FAD (n = 9 birds at 35 sites; FI = 1.21, SE=0.15), SALINE (n= 12 birds at 37 sites; FI = 1.32, SE=0.11), or E2 (n=15 birds at 39 sites; FI = 1.13, SE=0.09). In all treatment groups, the average FI to tutor-song was greater than 1, indicating that adaptation rates of NCM neurons were steeper to novel songs than to the tutor’s song and that the tutor’s song was remembered. The FIs did not differ significantly among the treatment conditions (Median test: χ2 square= 0.963, p=0.62; Figure 6 and Table 1), suggesting that this previously formed memory for the tutor song was not affected by E2 manipulation. There was no significant effect of the drug treatment on NCM adaptation rates (F (2, 108) = 2.33, p = 0.10; Table 2) or response magnitudes (F (2, 108) = 2.82, p = 0.06) to the novel songs presented during testing, although there was a trend for responses to be lower in the hormone-manipulated females (Table 3). These results suggest that the processing of novel songs in NCM was not affected by E2 manipulation, although the FIs for the treated birds were lower than those of the saline controls.
Figure 6.
Tutor song memory is not affected by hormone treatment. FIs to the tutor-song indicated familiarity that did not differ significantly among females that were treated with Fadrozole (FAD), Estradiol (E2), or Saline (SAL). When FI = 1 (dashed line), adaptation rates are equal, indicating no familiarity for the training songs.
Table 1. Tutor-song Memory in Hormone Manipulated Females.
Mean Familiarity Indices (FI) for the tutor song in females that received hormonal manipulations in adulthood prior to electrophysiological testing. These values are plotted in Figure 6. Fadrozole (FAD), Saline (SAL), Estradiol (E2).
| FAD | SAL | E2 | |
|---|---|---|---|
| Mean | 1.21 | 1.32 | 1.13 |
| SEM | 0.15 | 0.11 | 0.09 |
| Sites | 35 | 37 | 39 |
Table 2. Adaptation Rates in Hormone Manipulated Females.
Mean adaptation rates to novel conspecific song in females that received hormonal manipulations in adulthood prior to electrophysiological testing. Fadrozole (FAD), Saline (SAL), Estradiol (E2).
| FAD | SALINE | E2 | |
|---|---|---|---|
| Mean | −0.60 | −0.46 | −0.44 |
| SEM | 0.07 | 0.06 | 0.04 |
| Sites | 35 | 37 | 39 |
Table 3. Absolute Response Magnitudes in Hormone Manipulated Females.
Mean response magnitudes to novel conspecific song in females that received hormonal manipulations in adulthood prior to electrophysiological testing. Fadrozole (FAD), Saline (SAL), Estradiol (E2).
| FAD | SAL | E2 | |
|---|---|---|---|
| Mean | 91.88 | 105.88 | 78.53 |
| SEM | 8.91 | 9.07 | 6.80 |
| Sites | 35 | 37 | 39 |
Memory for Recently Heard Songs in Hormone-Manipulated Females
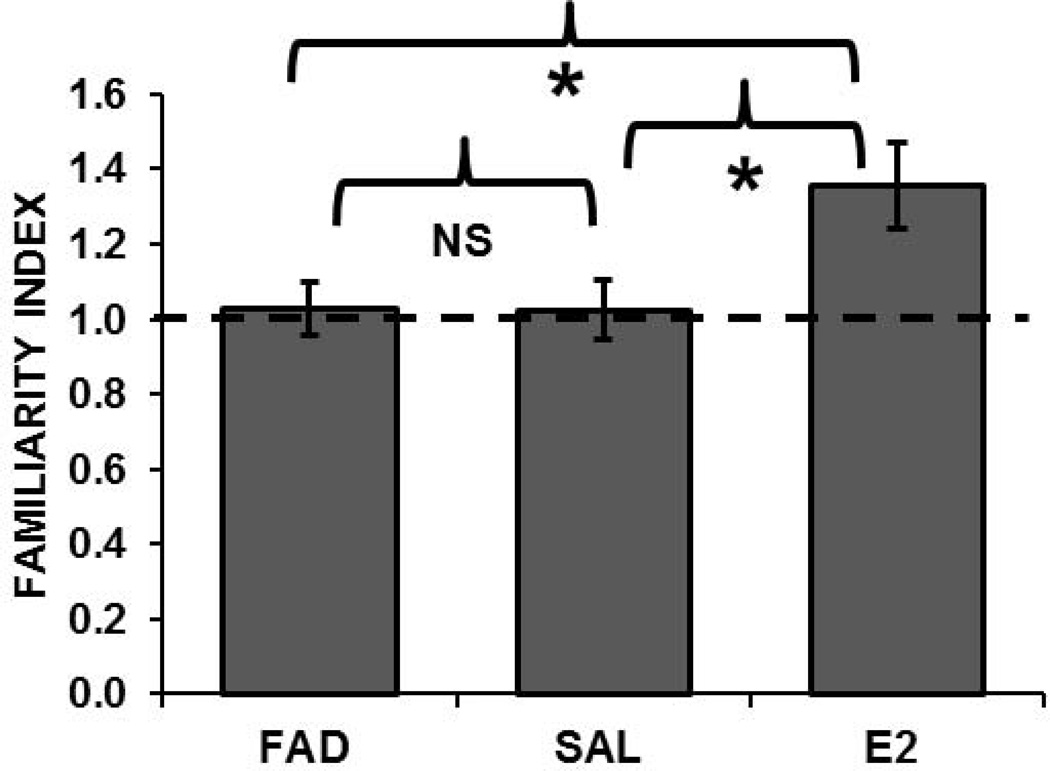
To assess the effects of E2 manipulation on memory for recently heard songs in the same groups of birds, we computed FIs to the training songs that had been presented 6 hours prior to NCM recording and compared them among treatment groups. FIs for the training songs were significantly higher in E2 (FI=1.36) than in FAD females (FI = 1.03; Mood’s Median test; χ2 = 7.69; p = 0.006), and in E2 than in SALINE females (FI = 1.02; Mood’s Median test; χ2 = 5.12; p = 0.024; Figure 7). FIs in FAD-treated females did not differ from those of the SALINE controls, which had lower than expected FIs (compare with untreated females in Fig. 5). In hormone-manipulated females, the FI for song memory was significant in E2-treated birds (1.36, CI=1.121), but not in FAD (1.03, CI=0.886) or saline (1.02, CI=0.863) treated birds.
Figure 7.
Effects of hormone treatment on FIs for songs heard 6h earlier in adult females. FIs for the training songs heard 6 hours prior to NCM recording were significantly greater in estradiol-treated (E2) than in Fadrozole (FAD) or saline-treated (SAL) females. FIs were unexpectedly low in SAL birds (cf. Figure 5 for untreated females).
Because the FI metric represents the ratio of adaptation rates to novel and “familiar” stimuli, we separately tested whether drug treatment had affected adaptation rates to either stimulus type. In addition, we have previously shown in males that the same FAD treatment used in the present study affects the processing of “familiar” songs without affecting the processing of novel songs (Yoder et al., 2012). Specifically, adaptation rates to “familiar” songs were steeper in FAD- versus SALINE-treated males. In our females, there was no significant difference in adaptation rates among treatment groups (repeated measures ANOVA; F (2, 115) = 2.50, p = 0.09), although there was a trend for adaptation rates to be steeper in FAD than in SALINE and E2 females (See Table 2).
Response Bias for Conspecific Song in Hormone-Manipulated Females
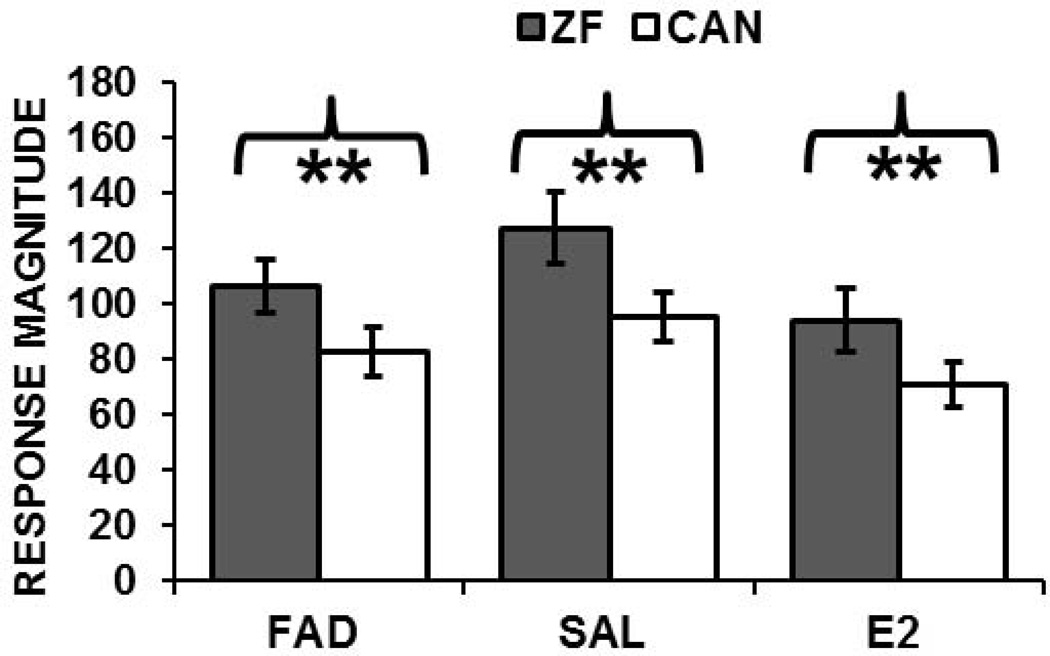
To test whether E2 manipulation affected the response bias for conspecific song in females, we compared absolute responses to novel conspecific and heterospecific songs among treatment groups. There was no effect of drug on response magnitudes (repeated measures ANOVA, F (2, 97) = 1.8, p= 0.17). All birds exhibited significantly higher responses to zebra finch (ZF) than to canary songs (ZF) regardless of treatment condition. This difference in responses to the two stimulus types is represented in Figure 8. A separate ANOVA conducted on adaptation rates showed no significant differences among treatment groups (F (2, 97) = 1.98, p = 0.14).
Figure 8.
Responses to conspecific and heterospecific song. Response magnitudes were significantly higher to novel conspecific (solid bars, zebra finch) than to novel heterospecific (open bars, canary) songs in all treatment groups (FAD, Fadrozole; SAL, saline; E2, estradiol).
DISCUSSION
We compared NCM responses to song playbacks between females and males reared and tested in the same conditions. We also tested whether, in females, manipulating E2 would affect neuronal memory and processing of songs in NCM, since, in males, previous studies have shown that E2 is required for normal song processing (Tremere et al., 2009; Remage-Healey et al., 2010; Pinaud et al., 2012; Yoder et al., 2012). Our results indicate that there are quantitative differences in the way that male and female zebra finches process song: female NCM responses to song playbacks were weaker and adapted more slowly than male responses to the same songs. Despite these sex differences, recordings in females showed neuronal memories for the tutor-song and for recently heard songs that did not differ significantly from those of males, although they are somewhat weaker. E2 manipulation in females did not affect the memory for the tutor’s song (heard during the juvenile period), but did affect the memory for the training songs that the females heard in adulthood. E2 manipulation in females did not disrupt the typical response bias towards conspecific song, similar to what was observed with E2 and FAD treatment in males.
Sex differences in Auditory Processing
Previous experiments (Chew et al., 1995, 1996a and b; Mello et al., 1995) have shown that the responses of neurons in NCM to each novel song adapt as it becomes familiar over repeated presentations, and no sex differences were originally observed for this phenomenon (Chew et al., 1996a), or in the magnitude of responses to song (Tremere et al., 2009). In our current data, we find that females have lower response magnitudes and shallower adaptation rates than males reared and recorded in the same conditions. Our study differs from ones in which no sex differences were observed in that our birds were individually box-tutored with limited exposure to song, whereas previous studies tested birds reared in an aviary (Tremere et al., 2009; Chew et al., 1996a). Furthermore, because zebra finch males sing and females do not, the males had more experience listening to complex song (heard themselves and the tutor) than females (heard the tutor and their own simpler calls). These differences in auditory experience may have affected NCM differently in males and females, although the results of an fMRI study indicate that female experience with song may not be required for NCM responses to develop selectivity, as it is in males (Maul et al., 2010). Further work is required to determine the extent to which auditory and social experience during development underlies male and female NCM responses in adulthood.
If the sex difference we observed persists in aviary-reared birds, the slower adaptation seen in female NCM could reflect sex differences in attention to song. To females, song represents a sexually salient signal that provides a window to a potential mate’s quality (Nowicki and Searcy, 2004) and activates the mesolimbic reward pathway differently from those of males in a similar reproductive condition (Earp and Maney, 2012; Maney, 2013). Neurons in NCM could continue to respond to the same stimulus for many repetitions without adapting, perhaps to allow her to evaluate the song’s characteristics. This sex difference in how song is used could have led us to measure somewhat weaker song memories in females: low adaptation rates may have prevented us from measuring as great a difference between “familiar” (shallower adaptation rates) versus novel (steeper adaptation rates) songs. We assessed this possibility by comparing differences in adaptation rates to novel versus familiar songs but found no significant difference between males and females. This suggests that, even though significantly lower adaptation rates to novel songs are seen in females than in males, familiar songs effectively adapt to the same relative degree with respect to novel songs as in males.
Memory for the Tutor’s Song in Females
Previous work has shown that males store a memory for their tutor’s song in NCM that correlates with quality of learning (Phan et al., 2006; Terpstra et al., 2004) and that NCM is necessary for this learning to occur (London and Clayton, 2008; Gobes and Bolhuis, 2007). We now provide electrophysiological evidence of a tutor song memory in female NCM that is similar to that seen in males reared and tested in the same conditions (Phan et al., 2006), although it appears to be slightly weaker (perhaps to due shallower adaptation rates to song in females). We observed this tutor-song memory in both intact females and in those that received hormone manipulations.
These results complement those in studies that measured expression of the immediate early gene zenk as an indicator of neural response to playback of the birds’ father’s song (Terpstra et al., 2004 and 2006). In males, the father’s song elicited more ZENK in NCM than novel song (2004). In females, the father’s song elicited expression in NCM and another auditory area, CMM, which is reciprocally connected to NCM in males (but has not been tested in females; Vates et al., 1996) and responds selectively to a stimulus based on its behavioral relevance to the listener (Gentner et al., 2004). Expression levels were correlated between the two areas, but were only significantly greater to the tutor’s song than to novel in female CMM, suggesting that the memory is stored in CMM or is stored in parallel by both areas. Our present results now favor the latter since we document a tutor-song memory in female NCM, and the IEG results certainly suggest that CMM is involved in this auditory memory in females. Whether a connection between CMM and NCM is required for female acquisition of memory for the tutor’s song remains to be tested. However, based on evidence from males (London and Clayton, 2008; Gobes and Bolhuis, 2007), it is likely that at least NCM is required for the formation of female tutor-song memory. Future studies could lesion CMM or NCM during tutoring, then test neural and behavioral responses to the tutor’s song and songs of different quality in order to dissociate the roles of these areas in memory acquisition and preference learning.
The IEG expression in both areas could be reflecting two different aspects of a female’s perception of the tutor’s song, both of which contribute to successful mate choices: memory (in NCM) and preference (in CMM). Activity in NCM and CMM are modulated by a stimulus’ familiarity and preference/social context, respectively. Responses tend to be lower in NCM when a stimulus is familiar (Chew et al., 1996 and Mello et al., 1992; Woolley and Doupe, 2008), as is true of a mate’s song, but higher in CMM when a stimulus is preferred (Gentner et al., 2000; Woolley and Doupe, 2008; Bell et al., 2013), as may be true for a directed or complex song. The activation of both areas by the tutor’s song, or by songs of suitor males that sound similar to the tutor’s song, could therefore reflect both the previously stored memory of the song’s identity in NCM, as we observed in our recordings, and the evaluation of the salience of that song in CMM.
Estrogenic Modulation of Female Memory
In males, behavioral responses to the tutor’s song and neural discrimination of familiar songs are affected by both local and peripheral E2 depletion (Remage-Healey et al., 2010; Tremere et al., 2009; Pinaud and Tremere, 2012; Yoder et al., 2012). Based on our current set of results, the memory for tutor-song in female NCM (at least its expression in our paradigm) does not appear to depend on E2; we observed the memory for the tutor’s song in all groups, regardless of hormone treatment.
However, memory for recently heard songs was affected. In males, we have found that FAD treatment results in reduced memory for the training songs (Yoder et al., 2012). In females, we also found reduced memory for the training songs in FAD-treated birds, relative to E2-treated birds. However, our saline-treated females also showed little or no evidence of memory. This was surprising because memory for recently heard songs was present in untreated adult females, and comparable to that of males (Figure 5). It may be that the treatment conditions of our experiment (daily injections, restraint, isolation from other birds) affected females differently than males and induced a stronger stress response (e.g., Shors, 2006). Stress has been shown to suppress gonadotropin release in several species (e.g., Li et al., 2010; Kageyama, 2013; Dobson and Smith, 2000) and can rapidly suppress aromatase activity in the quail hypothalamus (Dickens et al, 2012). It is possible that a global stress effect that reduced circulating E2 or brain aromatase activity affected memory processes in our saline-treated females. Stress and FAD treatment may have both suppressed E2 and prevented memory for the training songs whereas E2-treatment may have effectively restored hormone levels and thus “rescued” the memory.
An alternative, but not exclusive, possibility is that E2-treatment affected the auditory system indirectly by influencing the animal’s arousal state/attention (Maney, 2013; Goodson, 2005); or the stress response itself (Burgess and Handa, 1992; Wei et al., 2014). Hormones are known to act in an orchestrated manner at many loci in the brain and periphery to modulate behavioral responses to salient stimuli (Goodson, 2005). Therefore, it is likely that our treatments acted at multiple levels, including NCM, to facilitate memory. We were unable to obtain E2 measures within NCM and plasma would be useful in clarifying our results, but were unavailable. Future experiments in which E2 and FAD are administered locally into NCM during training and recording in our paradigm would also elucidate the influence of hormone action specifically within NCM during memory acquisition and expression.
In our FAD-treated birds, new song memories were most likely prevented by a lack of zenk induction during song playback. When E2 action is blocked within NCM, song playback no longer induces zenk expression (Tremere et al., 2009). In addition, zenk has been seen as a component in the process of memory acquisition (Clayton, 2000; Mello et al., 2004; Tischmeyer and Grimm, 1999). Therefore, in our FAD-treated (and possibly in our saline-treated) females, lower E2 probably led to lower zenk induction during song playback and prevented some aspect of memory acquisition and/or expression. Since in these same birds, the previously formed tutor-song memory (acquired during the juvenile period prior long before drug treatment) was not affected, we hypothesize that E2 depletion impaired memory acquisition processes rather than expression. However, we are limited in our conclusion since our treatments spanned both the adult training and testing periods. Follow-up studies in which drug treatment is administered only during training or testing would determine whether hormone manipulation affects memory acquisition, expression, or both. In juveniles, a similar study in which hormones are manipulated during tutoring would elucidate the role of estradiol in tutor song memory formation during male song-learning and female preference-learning.
Despite the effect on adult memories and studies showing that peripheral depletion of E2 can affect responses throughout the auditory system (Caras et al., 2012), there was no significant reduction in song response measures with drug treatment, although there were trends. Furthermore, another aspect of NCM discrimination was left intact: the bias for conspecific song was not reduced by FAD- treatment. Together, these results suggest a role for E2 in some aspects of NCM responses in females (e.g., acquiring new memories for songs heard in adulthood) but not others (e.g. discriminating the tutor song from novel ones and conspecific from heterospecific songs).
Continued study of male and female NCM, its interconnections to other brain areas, and possible sex differences in auditory processing will lead to greater understanding of how communication signals are encoded by the brain. Furthermore, knowing how gonadal and brain-derived hormones affect these structures could lead to understanding of the relationship between changing hormone levels and behavior. For male and female songbirds, early experience underlies effective communication between the singer and the listener in adulthood: males learn to sing the tutor song and females learn what a “good” tutor song sounds like. Our data demonstrate a memory for this song heard in development in both sexes but with subtle differences in the way that novel songs are processed. We found that E2 depletion may affect memory and/or discrimination for recently heard songs, while increases in E2 could facilitate song memories at a time when reproduction is likely to be successful. Though further study on the neural circuitry involved in mate selection is required, it seems probable that NCM (where cells synthesize and respond to E2) and CMM (where E2 synthesis is more limited due to few aromatase-positive cells; Saldanha et al., 2000) both contribute to a female’s ability to memorize and evaluate the songs of potential suitors.
Our initial findings presented here suggest that E2 could facilitate auditory memory processes in birds. Similar results have been observed for other models of learning and memory in mammals such as spatial learning (Frick, 2009; Packard and Teather, 1997) and discrimination learning in the auditory (Banerjee and Liu, 2013) and olfactory (Dillon et al., 2013) systems. If E2 has similar effects in the human brain, which is considered possible (Biegon et al., 2010; Roselli et al., 2009), then E2 deficits could contribute to deficits in verbal memory and communication. A better understanding of the role of steroid hormones in human learning and memory can contribute to the design of treatments for an aging population confronted with the onset of dementia, in post-menopausal women that suffer cognitive disorders thought to result from low E2 (Sherwin, 1998; Philips and Sherwin, 1992; Hogervorst et al., 1999), and for people taking aromatase inhibitors as treatment for estrogen-dependent cancers, such as breast cancer (Philips et al., 2011).
Acknowledgements
Experimental procedures and collection of the data were performed by K.M.Y and M.L.P. Data analysis was performed by K.M.Y., M.L.P., K.L. and D.S.V. Manuscript was written by K.M.Y, M.L.P. and D.S.V. The authors thank Utsav Aiya for help with birdcare and technical assistance, and especially Manda DiRubbo for her support in the running of these experiments, and wish her luck in her future in Nursing. The authors thank Novartis Pharmaceuticals for their generous donation of Fadrozole.
Source of Funding: NIH Grant DC008854 to D.S.V.
Footnotes
Conflict of Interests: None declared
REFERENCES
- Banerjee SB, Liu RC. Storing maternal memories: hypothesizing an interaction of experience and estrogen on sensory cortical plasticity to learn infant cues. Frontiers in Neuroendocrinology. 2013;34(4):300–314. doi: 10.1016/j.yfrne.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BA, Phan ML, Vicario DS. Auditory responses in the songbird forebrain reflect acquired salience and individual learning rates in a behavioral discrimination paradigm. Abstract, Society for Neuroscience. 2013 196.20/KKK31. [Google Scholar]
- Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [nN=methyl-11c] vorozole. Synapse. 2010;64(11):8014–8017. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic, estrogen-induced alterations in adrenocoticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Calisi RM, Knudsen DP, Krause JS, Wingfield JC. Estradiol differentially affects auditory recognition and learning according to photoperiodic state in the adult male songbird, European starling (Sternus vulgaris) PeerJ. 2013;1:e150. doi: 10.7717/peerj.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras ML, O’Brien M, Brenowitz EA, Rubel EW. Estradiol selectively enhances auditory function in avian forebrain neurons. Journal of Neuroscience. 2012;32:17597–17611. doi: 10.1523/JNEUROSCI.3938-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proceedings of the National Academy of Sciences. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proceedings of the National Academy of Sciences. 1996a;93(5):1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. Quantal duration of auditory memories. Science. 1996b;274(5294):1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- Clayton NS. Song discrimination learning in zebra finches. Animal Behaviour. 1988;36(4):1016–1024. [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiology of Learning and Memory. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Dickens MJ, Cornil CA, Balthazart J. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology. 2012;152(11):4242–4251. doi: 10.1210/en.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon TS, Fox LC, Han C, Linster C. 17B-estradiol enhances memory duration in the main olfactory bulb in CD-1 mice. Behavioral Neuroscience. 2013;127:923–931. doi: 10.1037/a0034839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson H, Smith RF. What is stress, and how does it affect reproduction? Animal Reproductive Science. 2000:60–61. doi: 10.1016/s0378-4320(00)00080-4. 743-52. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: Common themes and mechanisms. Annual Review of Neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Earp SE, Maney DL. Birdsong: Is it music to their ears? Frontiers in Evolutionary Neuroscience. 2012;4:14. doi: 10.3389/fnevo.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick K. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Hormones and Behavior. 2009;55(1):2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Building a better hormone therapy? How understanding the rapid effects f sex steroid hormones could lead to new therapeutics for age-related memory decline. Behavioral Neuroscience. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. Journal of Neurobiology. 2001;46(1):48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Ball GF. Functional differences in forebrain auditory regions during learned vocal recognition in songbirds. Journal of Comparative Physiology. 2004;190(12):1001–1010. doi: 10.1007/s00359-004-0556-x. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: A neural dissociation between song recognition and production. Current Biology. 2007;17(9):789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Recent advances in behavioral neuroendocrinology: Insights from studies on birds. Hormones and Behavior. 2005;48(4):461–473. doi: 10.1016/j.yhbeh.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber ME, Cambell DL, Woolley SM. The functional role and female perception of male song in zebra finches. Emu. 2010;110:209–218. [Google Scholar]
- Hernandez AM, Phillmore LS, MacDougall-Shackleton SA. Effects of learning on song preferences and Zenk expression in female songbirds. Behavioural Processes. 2008;77(2):278–284. doi: 10.1016/j.beproc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Boshuisen M, Riedel W, Willeken C, Jolles J. The effect of hormone replacement therapy on cognitive function in elderly women. Psychoneuroendocrinology. 1999;24:43–68. doi: 10.1016/s0306-4530(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Kageyama Regulation of gonadotropins by corticotopin-releasing factor and urocortin. Frontiers in Endocrinology. 2013;4:12. doi: 10.3389/fendo.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ. Homology and evolutionary origins of the neocortex. Brain behavior and Evolution. 1991;38:264–272. doi: 10.1159/000114393. [DOI] [PubMed] [Google Scholar]
- Lauay C, Gerlach NM, Adkins-Regan E, DeVoogd TJ. Female zebra finches require early song exposure to prefer high-quality song as adults. Animal Behaviour. 2003;68:1249–1255. [Google Scholar]
- Li XF, Knox AM, O’Byrne KT. Corticotrophin-releasing factor and stress-induced inhibition of the gonodotrophin-releasing hormone pulse generator in the female. Brain research. 2010;1364:153–163. doi: 10.1016/j.brainres.2010.08.036. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL. The incentive salience of courtship vocalizations: Hormone-mediated ‘wanting’ in the auditory system. Hearing Research. 2013;305:19–30. doi: 10.1016/j.heares.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. European Journal of Neuroscience. 2006;23(6):1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud RL. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Frontiers in Neuroendocrinology. 2011;32(3):287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul KK, Voss HU, Parra LC, Salgado-Commissariat D, Ballon D, Tchernichovski O, Helekar SA. Developmental Neurology. 2010;70(1):28–40. doi: 10.1002/dneu.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proceedings of the National Academy of Sciences. 1992;89(15):6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent declie in an immediate early gene’s response to that song in zebra finch telencephalon. Journal of Neuroscience. 1995;15(10):6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Annals of the New York Academy of Sciences. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979;280:389–391. [Google Scholar]
- Nowicki S, Searcy WA, Peters S. Quality of song learning affects female response to male bird song. Proceedings of the Royal Society of the Biological Sciences. 2002;269(1503):1949–1954. doi: 10.1098/rspb.2002.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA. Song function and the evolution of female preferences: why birds sing, why brains matter. Annals of the New York Academy of Sciences. 2004;1016:704–723. doi: 10.1196/annals.1298.012. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport. 1997;29(8):3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proceedings of the National Academy of Sciences. 2006;103(4):1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Ribi K, Fisher R. Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Research. 2011;13:203. doi: 10.1186/bcr2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Tremere L. Control of central auditory processing by a brain-generated oestrogen. Nature Reviews Neuroscience. 2012;13:521–527. doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger B. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience. 2008;11(11):1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman ME, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proceedings of the National Academy of Sciences. 2010;107(8):3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. Journal of Neurophysiology. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and actions of oestrogens during auditory processing in a songbird. Journal of Neuroendocrinology. 2013;25:1024–1031. doi: 10.1111/jne.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebel K. Early exposure leads to repeatable preferences for male song in female zebra finches. Proceedings of the Royal Society of Biological Sciences. 2000;267(1461):2553–2558. doi: 10.1098/rspb.2000.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD. Brain aromatization: classical roles and new perspectives. Seminars in Reproductive Medicine. 2010;27(3):207–217. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. Journal of Comparative Neurology. 2000;423(4):619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: Steroid synthesis and action at the synapse. Endocrinology Reviews. 2011;32:532–549. doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annual Review of Psychology. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone administration: Peripheral and intracranial implants. In: Meyers RD, editor. Methods in psychobiology. New York: Academic; 1977. pp. 259–279. [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. Journal of Comparative Neurology. 1974;156(3):337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. Journal of Neuroscience. 1997;17(10):3883–3893. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svec LA, Wade J. Estradiol induces region-specific inihibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behavioural Brain Research. 2009;199(2):298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernikovski O, Lints T, Mitra PP, Nottebohm F. Vocal imitation in zebra finches in inversely related to model abundance. Proceedings of the National Academy of Sciences. 1999;96:12901–12904. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleph T, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS One. 2006;3:e2854. doi: 10.1371/journal.pone.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, den Boer-Visser AM. An analysis of the neural representation of birdsong memory. Journal of Neuroscience. 2004;24(21):4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. Journal of Comparative Neurology. 2006;494(5):784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cellular and Molecular Life Sciences. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. Journal of Neuroscience. 2009;29(18):5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. Journal of Neuroscience. 2011;31(9):271–289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. Journal of Comparative Neurology. 1996;366(4):613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Hodges L, Arnold AP. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. General and Comparative Endocrinology. 1994;94(1):53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in in avian brain. Proceedings of the National Academy of Sciences. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Yuen EY, Liu W, Li X, Zhong P, Karatsoreos IN, McEwen BS, Yan Z. Estrogen protects against the detrimental effects of repeated stress on glutamatergic transmission and cognition. Molecular Psychiatry. 2014;19:588–598. doi: 10.1038/mp.2013.83. [DOI] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. Annals of the New York Academy of Sciences. 2004;1016:1–30. doi: 10.1196/annals.1298.029. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biology. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Vicario DS. To modulate and be modulated: Estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. 2012 doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Lu K, Vicario DS. Blocking estradiol synthesis affects memory for songs in auditory forebrain of male zebra finches. Neuroreport. 2012;23:922–926. doi: 10.1097/WNR.0b013e3283588b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann R. The zebra finch: A synthesis of field and laboratory studies. New York: Oxford Press; 1996. [Google Scholar]