Abstract
Background and Objective
Type 2 diabetes risk and its relationship to free fatty acid (FFA) exposure and visceral fat by prediabetes status in minority adolescents has yet to be explored. Therefore, the objective of this study was to examine the association of circulating FFA under varying conditions with prediabetes in Latino adolescents and to determine the relative relationships of FFA and visceral adiposity to insulin sensitivity, secretion, and β-cell function.
Subjects and Outcome Measures
Overweight or obese, but otherwise healthy Latino adolescent males and females (n=164, 14.2±2.5 years) were recruited for assessment of prediabetes, abdominal fat, and FFA levels taken at a fasting state (FFAF), during an OGTT (FFAOGTT), and overnight (FFANOCTURNAL).
Results
Prediabetic adolescents had a higher FFAF than those with normal glucose tolerance when controlling for age, sex, pubertal status, total percent body fat, and visceral fat. FFAOGTT and FFANOCTURNAL did not differ between participants with prediabetes and those with normal glucose tolerance after adjusting for covariates. Visceral fat was independently related to insulin sensitivity and secretion in pubertal adolescents, however in post-pubertal adolescents, FFAF and visceral fat were both independent and negatively related to β-cell function.
Conclusion
These results support a plausible progression of the lipotoxicity theory of diabetes development during the pubertal transition.
Keywords: Prediabetes, Latino, FFA, visceral fat
INTRODUCTION
Studies in prediabetic and diabetic adults suggest that elevated circulating levels of fasting free fatty acids (FFAF) may play a role in the development of type 2 diabetes (T2DM) [1, 2]. Elevated FFAF have been linked to the development of insulin resistance, and defects in insulin secretion [3, 4], and have been shown to be an independent risk factor for progression to T2DM [5]. In addition to imparting diabetes risk, increases in FFAF may be linked to the development of non-alcoholic fatty liver disease [6], metabolic syndrome, [7] and other obesity-related metabolic disorders. While some studies in children have reported relationships between FFAF and T2DM factors [8, 9], others have failed to observe similar relationships [10, 11].
Like adults, T2DM in children and adolescents moves through a pathophysiologic sequence initially characterized by a prediabetic state, which by current definitions includes impaired fasting glucose, impaired glucose tolerance, and/or elevated A1C [12]. We have previously shown that overweight Latino children and adolescents are at high risk for the development of T2DM, demonstrating declining insulin sensitivity and increased visceral fat over time in those with prediabetes compared to those with normal glucose tolerance (NGT) [13–15]. Although studies have shown that insulin sensitivity declines during puberty and recovers post-puberty [16], there is sparse literature on the role of FFAF metabolism and adiposity as it relates to the progression from NGT to prediabetes during the pubertal transition.
Emerging evidence from animal studies suggest that elevated overnight FFA (FFANOCTURNAL) may be relevant in the pathogenesis of diabetes. For example, data from an insulin-resistant canine model demonstrated that after 6 weeks of high fat feeding, these “pre-diabetic” dogs had elevated FFANOCTURNAL levels [17]. Data has shown that FFA is elevated between 4–8 AM in T2DM patients, which may be due to an increased rate of lipolysis and FFA flux [18]. The fluctuations of FFANOCTURNAL in children with prediabetes have not been described; however, one study found that obese children had higher FFANOCTURNAL when compared to their lean counterparts [19]. Together, these studies suggest that obesity and metabolic dysfunction may be associated with elevated FFANOCTURNAL.
Our primary objective was to examine fasting FFAF, FFA across an OGTT (FFAOGTT) and FFANOCTURNAL in prediabetic and NGT overweight Latino adolescents. Our secondary objective was to determine relationships between FFA and visceral adiposity with β-cell function. The specific hypotheses were: (1) FFA would be higher and would remain higher following an oral glucose challenge in those with prediabetes vs. those with NGT; 2) plasma levels of FFANOCTURNAL would be higher in those with prediabetes vs. those with NGT; 3) FFA exposure would be inversely related to insulin sensitivity and β-cell function and; 4) FFA, in addition to visceral fat, would independently contribute to T2DM risk factors.
METHODS
Participants
One hundred sixty-four overweight or obese, but otherwise healthy Latino adolescent males and females were recruited from greater Los Angeles (LA) County through community health clinics, health fairs, and word of mouth for participation in either the DREAM (Diabetes Risk and Ectopic Adiposity in Minority youth) or the SOLAR-2 (Study of Overweight Latino Adolescents at Risk-2) study. Participants met the following inclusion criteria: 1) Latino ethnicity (all four grandparents of Latin-American descent); 2) age 8–17 years; 3) age and sex-adjusted BMI ≥85th percentile. SOLAR-2 criteria additionally required that participants be in Tanner stage 2, 3 or 4, and have a family history of T2DM (at least one immediate family member was diagnosed with T2DM). In DREAM, a family history of T2DM was not an inclusion criterion; however, 54.1% (n=55) of participants reported such family history. Exclusions included previous major illness, medications or conditions known to influence body composition, insulin action, or insulin secretion. Participants and their parents provided written informed consent. Studies were approved by the Institutional Review Board of University of Southern California (USC) at the Health Sciences Campus, LA.
Procedures
All participants attended two visits at the USC General Clinical Research Center at the LA County General Hospital or, after 2008, at the Clinical Trials Unit at the USC University Hospital. On the first visit, participants received a comprehensive physical examination by a licensed health care provider. Pubertal stage was determined as breast stage for girls and pubic hair stage for boys [20, 21]. Blood was drawn at the fasting state and then at 30, 60 and 120min post oral glucose challenge (1.75 g oral glucose solution/kg body weight, maximum 75g).
Within approximately 2 months following the first visit, participants were admitted for their inpatient visit and served dinner and a snack before 2000hr, after which only water was permitted. The following morning, subjects underwent a 13-sample insulin-modified frequently sampled intravenous glucose tolerance test (FSIVGTT) beginning shortly after 0700hr as previously described [14, 15, 22]. FSIVGTT glucose and insulin were entered into MINMOD software (version 6.02; RN Bergman, Los Angeles, CA) for calculation of whole body insulin sensitivity, the acute insulin response (AIR), and the disposition index (DI) [23]. All subjects underwent abdominal MRI scanning to determine volume of visceral fat on a commercial 3-Tesla MRI system (Excite HD, GE Healthcare, Waukesha, WI) as previously described [22].
Participants from the SOLAR-2 study underwent nocturnal blood sampling (n=65). An intravenous (IV) catheter was placed on the antecubital fossae of the either the right or left arm. Immediately after, participants were served 1 of 2 standardized dinner options and were allowed 30min to complete their meal. Thereafter, only water was permitted. Blood samples were drawn hourly overnight from 0000hr to 0600hr. At approximately 0530hr, participants were awakened to have a second IV catheter placed on the antecubital fossae of the non-catheterized arm and underwent the FSIVGTT procedure as described above.
Assays
Hemoglobin A1c was measured by HPLC (Tosoh 11c 2.2 HLC-723, Tokyo, Japan), an assay approved by the International Federation of Clinical Chemistry Working Group (IFCC-WG) on A1C standardization (14). Glucose was assayed using a Yellow Springs Instruments analyzer (YSI INC., Yellow Springs, OH) that uses a membrane bound glucose oxidase technique. Insulin was assayed using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer, Tosoh Bioscience, Inc., South San Francisco, CA). An in vitro enzymatic colormetric method assay was used for the quantitative determination of FFA (Wako NEFA-HR (2) series; Wako Diagnostics, USA).
Statistical Analysis
Prediabetes (n=57) was defined as: fasting glucose ≥100mg/dL and/or 2hr glucose 140–199mg/dL and/or A1C 6.0–6.4% (42–46mmol.mol)] and NGT (n=107) as: fasting glucose <100mg/dL, 2hr glucose <140mg/L, and A1C <6.0% (42mmol/mol)]. The A1C criteria is based on the recommendations of the International Expert Committee [12], and our prior work demonstrating compromised β-cell function in overweight Latino adolescents with A1C between 6.0–6.4% (42–46 mmol/mol) [14]. Insulin and FFA data from the OGTT were used to calculate insulin and FFA area under the curve (insulin AUCOGTT, and FFA AUCOGTT) using the trapezoidal rule. The nocturnal blood sampling data from 0000h to 0600h was used to calculate insulin AUCNOCTURNAL and FFA AUCNOCTURNAL.
For descriptive purposes, independent t-tests and chi-square tests were used to determine mean physical and metabolic characteristics by prediabetes status. To test our first aim, a repeated measure ANCOVA was used to test for differences in FFA during the OGTT in those with and without prediabetes while adjusting a priori covariates of age, sex, pubertal status [puberty (Tanner 2–4) vs. post-puberty (Tanner 5)] [16], total percent body fat, and visceral fat. To assess potential differences by study, we adjusted for family history of T2DM; however, results remained the same so these variables were not included in the final analyses. Differences in FFA AUCNOCTURNAL and insulin AUCNOCTURNAL by prediabetes status were tested with an ANCOVA.
For our second aim, we used linear regression analysis to determine the contribution and relationship of FFAF and visceral fat to each metabolic outcome (insulin sensitivity, AIRG, and DI). Standardized betas and change in variance are shown in Table 2 by pubertal status. Data were analyzed using SPSS18.0 (IBM Inc., Chicago, IL), with an a priori significance level of p<0.05.
Table 2.
Predictors of SI, AIRG, and DI in overweight and obese Latino adolescents
| Pubertal | Post-Pubertal | |||||
|---|---|---|---|---|---|---|
| Standardized β | p-value | Change in Variance | Standardized β | p-value | Change in Variance | |
| Model 1: SI | ||||||
| Age | −0.092 | 0.405 | 3.5% | 0.142 | 0.107 | 2.9% |
| Sex | −0.133 | 0.243 | 13.8% | −0.048 | 0.724 | 12.4% |
| Total % Fat | −0.124 | 0.239 | 0.2% | −0.030 | 0.811 | 11.4% |
| Visceral Fat | −0.436 | <0.001 | 13.0% | −0.685 | <0.001 | 27.3% |
| Fasting FFA | −0.127 | 0.201 | 1.5% | −0.163 | 0.081 | 2.3% |
| Model 2: AIRg | ||||||
| Age | −0.270 | 0.024 | 1.7% | 0.005 | 0.964 | 0.1% |
| Sex | 0.096 | 0.431 | 8.2% | 0.086 | 0.639 | 6.6% |
| Total % Fat | −0.054 | 0.631 | 1.2% | 0.175 | 0.307 | 0.4% |
| Visceral Fat | 0.378 | 0.004 | 9.8% | 0.390 | 0.011 | 9.9% |
| Fasting FFA | 0.126 | 0.236 | 1.5% | −0.162 | 0.198 | 2.3% |
| Model 3: DI | ||||||
| Age | −0.382 | 0.003 | 10.4% | 0.12 | 0.278 | 1.5% |
| Sex | −0.026 | 0.839 | 0.3% | 0.053 | 0.755 | 2.7% |
| Total % Fat | −0.179 | 0.133 | 2.7% | 0.059 | 0.709 | 7.9% |
| Visceral Fat | −0.013 | 0.924 | 0% | −0.435 | 0.003 | 10.2% |
| Fasting FFA | 0.013 | 0.906 | 0% | −0.290 | 0.016 | 7.3% |
RESULTS
Physical and metabolic characteristics in subjects by prediabetes status are shown in Table 1. BMI and BMI z-score was higher in those with prediabetes than NGT. Total fat mass, total lean tissue, total percent fat, visceral fat and subcutaneous abdominal adipose tissue did not differ by prediabetes status (p>0.05). By definition, those with prediabetes had higher fasting & 2-hour glucoses, and A1C (p<0.01). Those with prediabetes had higher insulin AUCOGTT, insulin AUCNOCTURNAL, FFAF, FFA AUCOGTT, and lower insulin sensitivity and DI than those with NGT (p<0.05).
Table 1.
Anthropometric and metabolic parameters of Latino adolescents (n=164) 2-way ANOVA; #sample size =55
| NGT (n=107) | Pre-diabetes (n=57) | P-value | |
|---|---|---|---|
| Age (years) | 14.3 ± 2.4 | 14.0 ± 2.6 | 0.532 |
| Sex (Male/Female) | 62/45 | 33/24 | |
| Tanner Stage | |||
| 2 | 27 | 20 | |
| 3 | 12 | 9 | |
| 4 | 20 | 8 | |
| 5 | 48 | 20 | |
| Weight (kg) | 78.3 ± 20.0 | 83.0 ± 23.8 | 0.186 |
| Height (cm) | 160.5 ± 11.6 | 159.4 ± 12.4 | 0.563 |
| BMI (kg/m2) | 30.1 ± 5.8 | 32.3 ± 6.8 | 0.030 |
| BMI z-score | 1.9 ± 0.5 | 2.1 ± 0.5 | 0.017 |
| Total Fat mass (kg) | 29.2 ± 1.0 | 30.6 ± 1.1 | 0.401 |
| Total Lean mass (kg) | 45.7 ± 1.2 | 47.6 ± 1.3 | 0.340 |
| Total % Fat | 37.5 ± 6.7 | 38.0 ± 7.2 | 0.672 |
| Visceral Adipose Tissue (L) | 1.6 ± 1.0 | 1.9 ± 1.2 | 0.137 |
| Subcutaneous Abdominal Adipose Tissue (L) | 6.5 ± 3.3 | 7.2 ± 3.9 | 0.193 |
| Fasting Glucose (mg/dL) | 86.7 ± 5.6 | 90.2 ± 7.7 | 0.003 |
| 2-hr Glucose (mg/dL) | 115.2 ± 15.1 | 138.6 ± 23.3 | <0.001 |
| HbA1c (%) | 5.5 ± 0.3 | 5.9 ± 0.3 | <0.001 |
| HbA1c (mmol/mol) | <0.001 | ||
| Fasting Insulin (μU/mL) | 13.1 ± 8.9 | 16.0 ± 11.3 | 0.091 |
| Insulin AUCOGTT (μU/mL x min) | 175.1 ± 110.0 | 229.1 ± 163.8 | 0.029 |
| Insulin AUCNOCTURNAL (μU/mL x min) | 52.4 ± 26.0 | 71.7 ± 39.0 | 0.040 |
| Fasting FFA (mmol/L) | 0.68 ± 0.14 | 0.73 ± 0.14 | 0.032 |
| FFA AUCOGTT (mmol/L x min) | 0.61 ± 0.13 | 0.66 ± 0.13 | 0.045 |
| FFA AUCNOCTURNAL (mmol/L x min) | 1.56 ± 0.36 | 1.51 ± 0.28 | 0.542 |
| Insulin sensitivity [(x10−4 min−1)/(μU/mL)] | 2.02 ± 1.19 | 1.45 ± 0.86 | 0.001 |
| Acute Insulin Response (μU/mL x 10min) | 1302 ± 853 | 1448 ± 987 | 0.351 |
| Disposition index | 2188 ± 1087 | 1735 ± 1035 | 0.041 |
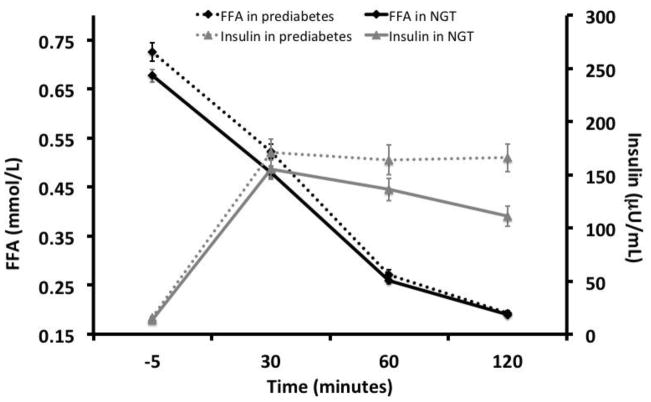
As shown in Figure 1, participants with prediabetes had higher FFAF levels compared to those with NGT (0.725 vs 0.677 mmol/L, p=0.001). FFA AUCOGTT was higher in those with prediabetes than NGT (0.720 vs 0.627 mmol/L × 120min, p=0.001), but after controlling for FFAF, this relationship disappeared (p=0.86). FFA levels were significantly higher in those with prediabetes than NGT at 30min (p<0.05) but not 60min or 120min (p>0.05). Although fasting insulin levels did not differ by prediabetes status (p>0.05), insulin at 30min, 60min, and 2-hours post OGTT were higher in those with prediabetes than NGT (p=0.02–0.05). Lastly, overall exposure of insulin during the OGTT challenge (INS AUCOGTT) was higher in those with prediabetes than NGT participants (255.1 vs 169.1 uU/mL, p=0.01).
Figure 1. FFA and insulin concentration during the OGTT in those with prediabetes vs. NGT.
Repeated measures ANCOVA; covariates: age, sex, pubertal stage, total percent body fat and visceral fat.
P<0.05
FFANOCTURNAL and insulinNOCTURNAL patterns over a 6-hour period are shown in Figure 2. There were no differences in the FFANOCTURNAL patterns or FFA AUCNoc 6HR by prediabetes status (pbetween >0.05), even after adjusting for age, sex, pubertal status, and total percent body fat. Additionally, insulinNOCTURNAL patterns and INS AUCNOCTURNAL did not differ between those with prediabetes and NGT (p=0.10–0.14). Insulin levels from 0300hrs to 0500hrs were significantly higher in participants with prediabetes than NGT (p=0.03- <0.05).
Figure 2.
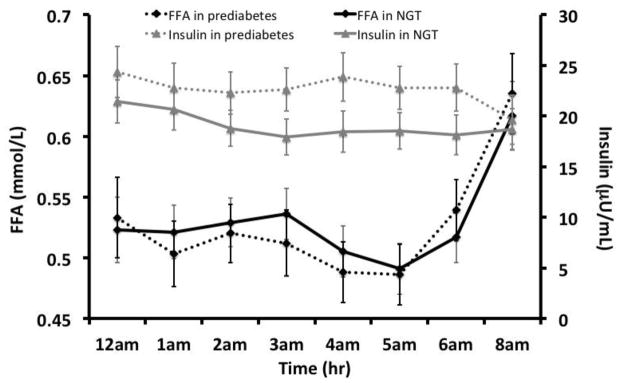
Nocturnal plasma FFA and insulin 8-hour profiles by prediabetes status in pubertal adolescents (n=55)
Given the importance of the pubertal transition and visceral fat in affecting insulin resistance and risk for T2DM, we explored the interaction between pubertal status and visceral fat. Overall, we found that a more advanced pubertal status interacted with visceral fat to predict a lower insulin sensitivity and DI (p<0.05). Therefore, predictors of insulin sensitivity, AIRG, and DI by pubertal status are shown in Table 2. Visceral fat was more strongly related to a lower insulin sensitivity in post-pubertal (βstandardized post-pubertal= −0.69, p<0.001) than pubertal adolescents (βstandardized pubertal = −0.44, p<0.001). Additionally, visceral fat accounted for 27% of the variance explained in insulin sensitivity in post-pubertal adolescents but only 13% in pubertal adolescents. FFAF showed a trend for an inverse association with insulin sensitivity (βstandardized= −0.16, p=0.08) in post-pubertal but not pubertal adolescents (p=0.20). In post-pubertal adolescents, only visceral fat was related to AIRG (βstandardized= 0.39, p=0.01) while age and visceral fat were related to AIRG (βstandardized= −0.27, p=0.02 and βstandardized= 0.38, p=0.004, respectively).
Both visceral fat and FFAF were related to a lower DI in pubertal adolescents (βstandardized= −0.44, p=0.003 and βstandardized= −0.29, p=0.02, respectively), each accounting for 10% and 7% of the variance. Conversely, only age was related to a lower DI in pubertal adolescent (βstandardized= −0.27, p=0.02), accounting for 1.7% of the variance. FFA AUCOGTT and FFA AUCNOCTURNAL were not related to insulin sensitivity, AIRG, DI (data not shown).
DISCUSSION
In overweight and obese Latino adolescents, we found that FFAF, but not FFAOGTT nor FFANOCTURNAL, was independently associated with prediabetes. Visceral fat was negatively related to insulin sensitivity and secretion in all adolescents, while in post-pubertal adolescents, FFAF and visceral fat were both independently and negatively related to β-cell function. Together, these results suggest that although visceral fat may contribute to insulin sensitivity and secretion across the pubertal transition, FFAF may play an additional role in prediabetes and β-cell function after puberty is complete.
Despite data in adults showing an association between elevated FFA and T2DM risk [24, 25], there is little research in children and adolescents. In studies of European Caucasian children, no association was observed between FFAF and insulin resistance using HOMA-IR [10, 11]. In an elegant study among Caucasian and African-American (AA) adolescents, overall plasma FFA and various long and short-chain acylcarnitine species did not differ in those who were NGT, obese, or had T2DM [26]. In contrast, other studies have shown evidence of alterations in FFA and T2DM risk. Cali et al. reported increased fasting plasma FFA in children with prediabetes vs. those with NGT in a group of Caucasian and African-American children [8]. In a group of AA women and girls, Goree et al. showed that increased FFA flux was related to insulin secretion and action [9]. In our study, we found elevated levels of FFAF in prediabetic vs. NGT adolescents. A plausible explanation involves insulin resistance of adipose tissue, leading to increased lipolysis and increased release of FFA into the bloodstream, which results in elevated FFAF. Over time, this process may stress β-cells and impair adequate secretion of insulin post-prandially and therefore results in decreased ability to suppress FFA and increased risk for T2DM. Future longitudinal data are needed to examine this hypothesis.
Elevation in FFA during the night may also be relevant in the pathogenesis of T2DM. FFA have been shown to be elevated between 4–8 AM in those with T2DM [18] and in a canine model for insulin resistant metabolic disease, dogs fed a high fat diet for 6 weeks developed visceral fat and insulin resistance [27]. Studies from the canine model demonstrated that after 6 weeks of high fat feeding, these “pre-diabetic” dogs had elevated FFANOCTURNAL levels when compared to controls and daytime levels between the two groups did not differ [17]. Our current analysis provided a unique opportunity to examine whether FFANOCTURNAL levels might be elevated in Latino adolescents with prediabetes compared to those who were NGT. Contrary to our hypothesis, we found that overall exposure to FFANOCTURNAL did not differ significantly between participants with prediabetes and those with NGT in pubertal adolescents.
Insulin resistance has been also closely associated with an elevation of plasma FFA. The “lipotoxicity theory” of T2DM pathogenesis states that in the presence of insulin resistance, increased lipolysis, and increased fatty acid flux constitute a major pathway of progression to diabetes [28, 29]. Visceral fat has been shown to contribute to elevated FFA in obesity and to be a strong predictor of T2DM [27]. The increased mobilization of FFA contributes to adipose tissue insulin resistance, exacerbating the inability of insulin to suppress lipolysis, and possibly leading to a vicious cycle of increasing levels of plasma FFA. The increase in FFA flux is proposed to lead to β-cell dysfunction and a gradual decrease in insulin secretion, likely due to increased β-cell apoptosis [30]. In prospective studies in Pima Indians, elevated FFAF concentrations were an independent risk factor for the development of T2DM, possibly by reducing insulin secretion [31]. Moreover, Salgin et al [32] reported data from a large longitudinal study where in both children and adults, higher FFAF was associated with lower insulin secretion following a 30min oral glucose challenge in children with NGT. Finally, in a study using an overnight intravenous lipid infusion, Hughan et al. [33] showed declines in insulin sensitivity and β-cell function in AA and Caucasian overweight and obese children, indicating β-cell lipotoxicity. Collectively, these studies show that elevated FFA may lead to alterations in insulin secretion and β-cell function, either independently or in conjunction with obesity. However, none of the aforementioned studies assessed differences in FFA by pubertal status. Given the natural progression of worsening insulin sensitivity during the pubertal transition [16], assessing the role of puberty is key in examining the progression of T2DM, particularly in Latino children who have been shown to have high visceral adiposity and insulin resistance [34] and in whom DI progressively deteriorates across puberty [16]. We showed that in pubertal adolescents, age was the independent predictor of DI whereas in post-pubertal adolescents, FFAF and visceral fat were the independent predictors of DI. Interestingly, visceral fat, but not FFAF, was the primary predictor of insulin sensitivity and AIRG in all adolescents. Our results suggest that whole body insulin sensitivity and insulin secretion may be more strongly related to visceral fat metabolism while β-cell dysfunction may a related to both increased visceral fat and elevated FFA.
To our knowledge, this is the first study to describe FFANOCTURNAL and to examine differences in FFAF and FFAOGTT in an exclusively Latino youth population. Our use of recently recommended A1C cutoffs [34] for defining prediabetes makes this the first study to do so in the examination of FFA levels and T2DM risk. By using the A1C criteria for T2DM risk, we were able to identify more youth with heightened diabetes risk (i.e. prediabetes). Secondly, by using rigorous direct measures of insulin action and secretion were we able to better determine relationships between FFA and insulin sensitivity/secretion dynamics than would not have been possible using coarser measures, such as fasting insulin or HOMA-IR. Despite these strengths, the FSIVGTT method with Minimal Modeling is designed only to provide whole body (ie, predominantly muscle) insulin sensitivity, but is unable to isolate hepatic insulin sensitivity, nor to determine hepatic insulin clearance that can be achieved by C-peptide modeling. Since we did not measure C-peptide during the IVGTT, we cannot determine the degree of contribution to AIR related to hepatic insulin clearance, which is a limitation particularly given the role that FFA released from visceral fat may play on hepatic insulin catabolism [35, 36]. Next, our FFA assay only supplied total concentrations of FFA and it did not provide information on specific FFA (i.e., saturated and unsaturated fatty acids). This may be particularly important since saturated and unsaturated FFA may have differential effects on muscle insulin signaling, as has been shown in vitro [37, 38], and in biopsies from children [39]. Another limitation of this study is that FFANOCTURNAL data was limited to pubertal adolescents, which inhibited our ability to compare differences in pubertal and post-puberty with regard to FFANOCTURNAL and insulinNOCTURNAL exposure. Finally, our studies did not include non-Latino ethnicities, lean, or pre-pubertal children, so our findings cannot be generalized beyond overweight and obese, pubertal and post-pubertal Latino adolescents.
In conclusion, in a group of overweight and obese Latino adolescents, FFAF, but not FFAOGTT or FFANOCTURNAL, was independently associated with prediabetes. These results suggest that visceral fat independently contributed to insulin sensitivity and secretion across the pubertal transition, but elevated FFAF along with increased visceral fat may play a concomitant role in βcell dysfunction during post-puberty in overweight Latino adolescents. Our findings are supported by the natural progression of worsening insulin sensitivity during the pubertal transition, which may have an impact on the role of circulating FFA and development of T2DM.
Acknowledgments
This study is supported by NCMHD grant R01DK DK59211 and P60MD002254 (M.I. Goran and M.J. Weigensberg). C.M. Toledo-Corral is supported by an American Diabetes Association (ADA) Postdoctoral Fellowship (7-10-MI-04).
We would like to thank the staff of the University of Southern California/LA County GCRC and the dedicated DREAM and SOLAR-2 staff, Quintilia Ávila, MPA, Michelle Muñevar, MPH, Christina Ayala, MPH, and Laura Salguero, MS, and the rest of the staff over the past 5 years. Our gratitude is especially extended to the participants and their families for their participation.
Footnotes
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent
Informed consent was obtained from all patients for being included in the study.
Conflicts of interest: Claudia Toledo-Corral, Tanya Alderete, Joyce Richey, Paola Sequeira, Michael Goran, and Marc Weigensberg have nothing to disclose.
References
- 1.Charles MAEE, Thibult N, Claude JR, Warnet JM, Rosselin GE, Girard J, Balkau B. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40:1101–1106. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N, Stumvoll M, Bogardus C, Tataranni PA. Elevated plasma nonesterified fatty acids are associated with deterioration of acute insulin response in IGT but not NGT. Am J Physiol Endocrinol Metab. 2003;284:E1156–1161. doi: 10.1152/ajpendo.00427.2002. [DOI] [PubMed] [Google Scholar]
- 3.Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 1999;48:2197–2203. doi: 10.2337/diabetes.48.11.2197. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Stern MP, Dunn J, Mobley M, Blackwell J, Bergman RN. Diminished insulin sensitivity and increased insulin response in nonobese, nondiabetic Mexican Americans. Metabolism. 1990;39:842–847. doi: 10.1016/0026-0495(90)90130-5. [DOI] [PubMed] [Google Scholar]
- 5.Paolisso GTP, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 6.Vega GL, Chandalia M, Szczepaniak LS, Grundy SM. Metabolic correlates of nonalcoholic fatty liver in women and men. Hepatology. 2007;46:716–722. doi: 10.1002/hep.21727. [DOI] [PubMed] [Google Scholar]
- 7.Bergman RN, Kim SP, Catalano KJ, et al. Why Visceral Fat is Bad: Mechanisms of the Metabolic Syndrome. Obesity Research. 2006;14:16–19. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 8.Cali AM, Bonadonna RC, Trombetta M, Weiss R, Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab. 2008;93:1767–1773. doi: 10.1210/jc.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goree LL, Darnell BE, Oster RA, Brown MA, Gower BA. Associations of free fatty acids with insulin secretion and action among African-American and European-American girls and women. Obesity (Silver Spring) 2010;18:247–253. doi: 10.1038/oby.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard P, Delvin EE, Paradis G, et al. Distribution of Fasting Plasma Insulin, Free Fatty Acids, and Glucose Concentrations and of Homeostasis Model Assessment of Insulin Resistance in a Representative Sample of Quebec Children and Adolescents. Clin Chem. 2003;49:644–649. doi: 10.1373/49.4.644. [DOI] [PubMed] [Google Scholar]
- 11.Reinehr T, Kiess W, Andler W. Insulin sensitivity indices of glucose and free fatty acid metabolism in obese children and adolescents in relation to serum lipids. Metabolism. 2005;54:397–402. doi: 10.1016/j.metabol.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goran MI, Bergman RN, Avila Q, et al. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history for type 2 diabetes. J Clin Endocrinol Metab. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 14.Toledo-Corral CM, Vargas LG, Goran MI, Weigensberg MJ. Hemoglobin A1c above Threshold Level is Associated with Decreased beta-Cell Function in Overweight Latino Youth. J Pediatr. 2012;160:751–756. doi: 10.1016/j.jpeds.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta-cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 16.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and beta-cell function in overweight Latino youth. J Pediatr. 2011;158:442–446. doi: 10.1016/j.jpeds.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. American Journal of Physiology-Endocrinology And Metabolism. 2007;292:E1590. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 18.Miles JM, Wooldridge D, Grellner WJ, et al. Nocturnal and Postprandial Free Fatty Acid Kinetics in Normal and Type 2 Diabetic Subjects: Effects of Insulin Sensitization Therapy. Diabetes. 2003;52:675. doi: 10.2337/diabetes.52.3.675. [DOI] [PubMed] [Google Scholar]
- 19.Heptulla R, Smitten A, Teague B, Tamborlane WV, Ma YZ, Caprio S. Temporal patterns of circulating leptin levels in lean and obese adolescents: relationships to insulin, growth hormone, and free fatty acids rhythmicity. J Clin Endocrinol Metab. 2001;86:90–96. doi: 10.1210/jcem.86.1.7136. [DOI] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toledo-Corral CM, Alderete TL, Hu HH, et al. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115–1121. doi: 10.1210/jc.2012-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 24.Reaven GM, Chen YD. Role of abnormal free fatty acid metabolism in the development of non-insulin-dependent diabetes mellitus. Am J Med. 1988;85:106–112. doi: 10.1016/0002-9343(88)90402-0. [DOI] [PubMed] [Google Scholar]
- 25.Boden G. Free fatty acids, insulin resistance, and type 2 diabetes mellitus. Proc Assoc Am Physicians. 1999;111:241–248. doi: 10.1046/j.1525-1381.1999.99220.x. [DOI] [PubMed] [Google Scholar]
- 26.Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18:1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman RN, Kim SP, Hsu IR, et al. Abdominal Obesity: Role in the Pathophysiology of Metabolic Disease and Cardiovascular Risk. The American Journal of Medicine. 2007;120:3–8. doi: 10.1016/j.amjmed.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Bergman RN, Ader M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends in Endocrinology & Metabolism. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 29.McGarry JD. Banting Lecture 2001: Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes. 2002;51:7. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 30.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proceedings of the National Academy of Sciences. 1998;95:2498. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paolisso GTP, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–1217. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- 32.Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. J Clin Endocrinol Metab. 2012;97:3302–3309. doi: 10.1210/jc.2012-1428. [DOI] [PubMed] [Google Scholar]
- 33.Hughan KS, Bonadonna RC, Lee S, Michaliszyn SF, Arslanian SA. beta-Cell lipotoxicity after an overnight intravenous lipid challenge and free fatty acid elevation in African American versus American white overweight/obese adolescents. J Clin Endocrinol Metab. 2013;98:2062–2069. doi: 10.1210/jc.2012-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam TK, van de Werve G, Giacca A. Free fatty acids increase basal hepatic glucose production and induce hepatic insulin resistance at different sites. Am J Physiol Endocrinol Metab. 2003;284:E281–290. doi: 10.1152/ajpendo.00332.2002. [DOI] [PubMed] [Google Scholar]
- 36.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 37.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz-Peiffer C. Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal. 2000;12:583–594. doi: 10.1016/s0898-6568(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 39.Sabin MA, Stewart CE, Crowne EC, et al. Fatty acid-induced defects in insulin signalling, in myotubes derived from children, are related to ceramide production from palmitate rather than the accumulation of intramyocellular lipid. J Cell Physiol. 2007;211:244–252. doi: 10.1002/jcp.20922. [DOI] [PubMed] [Google Scholar]