Abstract
CD28 costimulation is essential for the development of Thymic-derived CD4+CD25+Foxp3+ regulatory T cells ("tTregs"). E3 ubiquitin ligase Cbl-b has been shown to regulate CD28 dependence of T cell activation. Here, we report that the loss of Cbl-b partially but significantly rescues the defective development of tTregs in Cd28−/− mice. This partial rescue is independent of IL-2. Mechanistically, Cbl-b binds to Foxp3 upon TCR stimulation, and together with Stub1, targets Foxp3 for ubiquitination and subsequently degradation in the proteasome. As Cbl-b self-ubiquitination and proteasomal degradation is impaired in Cd28−/− T cells, the defective development of tTregs in Cd28−/− mice may in part be due to increased Foxp3 ubiquitination and degradation targeted by Stub1 and Cbl-b. Treating Cd28−/− mice with a proteasome inhibitor completely rescues defective tTreg development in these mice. Therefore, Cbl-b, together with Stub1, ubiquitinate Foxp3, and regulate tTreg development.
INTRODUCTION
Thymic-derived CD4+CD25+ regulatory T cells (tTregs) have an important role in the mechanisms of peripheral immune tolerance and in the prevention of pathogenic autoimmunity through the suppression of proliferation and production of pro-inflammatory cytokines in effector immune cells. Similar to other T cells, tTregs develop in the thymus, and are defined by expression of the forkhead family transcription factor Foxp3 (forkhead box p3). Expression of Foxp3 is required for tTreg development and appears to control a genetic program specifying this cell fate (1, 2). Mice deficient for Foxp3, or carrying a loss-of-function mutation in Foxp3 (Scurfy mice), present with fatal autoimmune-like disease caused by hyper-responsive CD4+ T cells (2–4). CD28 is required for tTreg development and peripheral homeostasis since mice deficient for CD28 have very few tTregs in both thymuses and spleens, and this defect cannot be rescued by IL-2 (5, 6). In addition to CD28, it has been documented that IL-2 is essential for tTreg expansion and maintenance, whereas IL-2Rγ is also required for tTreg development (5, 7–9).
Casitas-B-lineage lymphoma protein-b (Cbl-b), an E3 ubiquitin ligase, plays an important role in regulating T cell signaling threshold and T cell differentiation (10–12). Gene targeting in mice has shown that Cbl-b functions a gatekeeper involved in the maintenance of a balance between immunity and tolerance (13, 14). Indeed, we demonstrated that CD28 costimulation potentiates TCR-induced Cbl-b ubiquitination and degradation, whereas CTLA-4-B7 interaction induces Cbl-b expression (15, 16). These observations indicate that CD28 and CTLA-4 tightly regulate Cbl-b expression which is critical for establishing the threshold for T cell activation and tolerance. In strong support of this notion, Cblb−/− T cells are resistant to anergy induction in vitro and in vivo (17, 18), and Cblb−/− mice are highly susceptible to autoimmune/inflammatory diseases (12–14, 19).
T cells from Cblb−/− mice show enhanced proliferation and IL-2 production in response to TCR stimulation (13, 14), as well as uncoupling the requirement of CD28 costimulation to promote T cell proliferation and IL-2 production (13, 14, 20). Based upon these observations, we reasoned that Cbl-b deficiency might rescue the defective development of tTregs in Cd28−/− mice. In this study, we found that Cbl-b deficiency partially rescues impaired tTreg development in Cd28−/− mice, and this rescue is independent of increased IL-2 production. Further analysis showed that Cbl-b binds to ubiquitinated Foxp3 upon TCR stimulation via its ubiquitin-associated (UBA) domain and, together with Stub1, targets Foxp3 for poly-ubiquitination and subsequent degradation in the proteasome. Treating Cd28−/− mice with PS-341, a proteasome inhibitor, completely rescues defective tTregs indicating that Foxp3 ubiquitination and degradation are major mechanisms regulating Foxp3 expression.
Materials and Methods
Mice
WT BALB/c and Cd28−/− mice were purchased from the Jackson Laboratory. Cblb−/− mice were obtained from Dr. Josef M. Penninger (University of Toronto, Toronto, ON, Canada), and have been backcrossed onto a BALB/c background for at least 14 generations. CblbC373A mice were described previously (21). Cblb−/− mice were crossed to Cd28−/− mice to generate Cblb−/−Cd28−/− mice. Foxp3gfp mice were obtained from Dr. Alexander Rudensky (Memorial Sloan Kettering Cancer Center; New, NY). Cd28−/− mice were crossed onto Foxp3gfp mice to generate Cd28−/−Foxp3gfp mice. All experimental protocols followed NIH Guidelines and are approved by the Institutional Animal Care and Use Committees of the Ohio State University and the University of Chicago. All of the mice were used for experiments at ages of 6 to 10 weeks.
Antibodies
Purified anti-mouse CD3 (145-2C11) and anti-mouse CD28 (37.51) mAbs and all the antibodies used in flow cytometry were purchased from BD PharMingen (San Diego, CA). Protein G-Sepharose was purchased from GE Healthcare (Piscataway, NJ). Anti-Cbl-b (G-1), anti-HA (D-8 and Y-11), anti-His (H-3), anti-Flag (D-8), anti-Stub1 (G-2), anti-c-Cbl (A-9), anti-Cul2 (H-300), and anti-ubiquitin (P4D1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG were purchased from Kirkegaard & Perry Laboratories (Gaithersburg, MD).
Plasmids and transfection
Cbl-b cDNAs encoding full-length (FL) or different mutant Cbl-b with an HA epitope in pCEFL were described previously (22). His6-tagged ubiquitin plasmid was a gift from Dr. Dirk Bohmann (University of Rochester, Rochester, NY). GFP-tagged Stub1 plasmid was obtained from OriGene (Rockville, MD). HEK293T cells were transfected with HA-tagged Cbl-b, Cbl-b N1/3, C2/3, and UBA together with Flag-tagged Foxp3, lysed in 0.5% NP-40 lysis buffer. HEK293T cells transfected with HA-tagged Cbl-b or Cbl-b C373A mutant, Flag-tagged Foxp3, and His-tagged ubiquitin in the presence or absence of GFP-tagged Stub1, and lysed in RIPA buffer containing 1% SDS.
Knockdown of Stub1 and Cbl-b
For Stub1 and Cbl-b knockdown experiments, naïve CD4+CD25+ T cells from BALB/c mice were nucleofected with Stub1 or Cbl-b siRNAs, or scrambled siRNA according to the manufactory’s instruction (Lonza; Allendale, NJ). The efficiency of knockdown was assessed by immunoblotting. The transfected cells were stimulated with anti-CD3 and anti-CD28 for 15 min, and lysed in RIPA buffer containing 1% SDS.
Isolation of tTregs
The isolation of CD4+CD25+ tTregs was performed using mouse Treg isolation kits from Miltenyl as described (19, 23). For some experiments, CD4+CD25+GFP+ cells were sorted from Foxp3gfp or Cd28−/−Foxp3gfp mice using a FACSAria II cell sorter (BD Biosciences).
Subcellular fractionation
The cytosolic and nuclear fractionations were isolated using a membrane/cytoplasmic and nuclear protein isolation kit (Thermo Scientific; Rockford, IL).
Immunoprecipitation and Western Blot
The CD4+CD25+ T cells from BALB/c mice were isolated as described, and stimulated with anti-CD3 and anti-CD28, and lysed in 0.5% NP-40 or RIPA buffer containing 1% SDS. The lysates from the above cells, or cytoplasmic and nuclear fraction were immunoprecipitated with anti-Foxp3, and Abs indicated, or anti-ubiquitin. Transfected HEK293T cells were immunoprecipitated with anti-HA, blotted with anti-His, anti-Flag, or anti-HA. The fold changes of Foxp3 ubiquitination bands or Cbl-b bands in arbitrary densitometric units were determined by the ImageJ 1.48 (NIH; Bethesda, MD).
In vivo PS-341 treatment
Cd28−/−Foxp3gfp mice (6 wks of age) were i.p. injected with PS-341 (Bortezomib) or vehicle at 0.25 mg/kg every twice a week for two weeks. On day 14, the mice were sacrificed, and the expression of GFP+ cells within CD4+CD25+ population in thymus and spleens was determined by flow cytometry.
Statistical analysis
Two-tailed Student t test was used for calculation of statistical significance unless otherwise stated.
RESULTS
Cbl-b deficiency partially rescues defective development of CD4+Foxp3+ tTregs in Cd28−/− mice
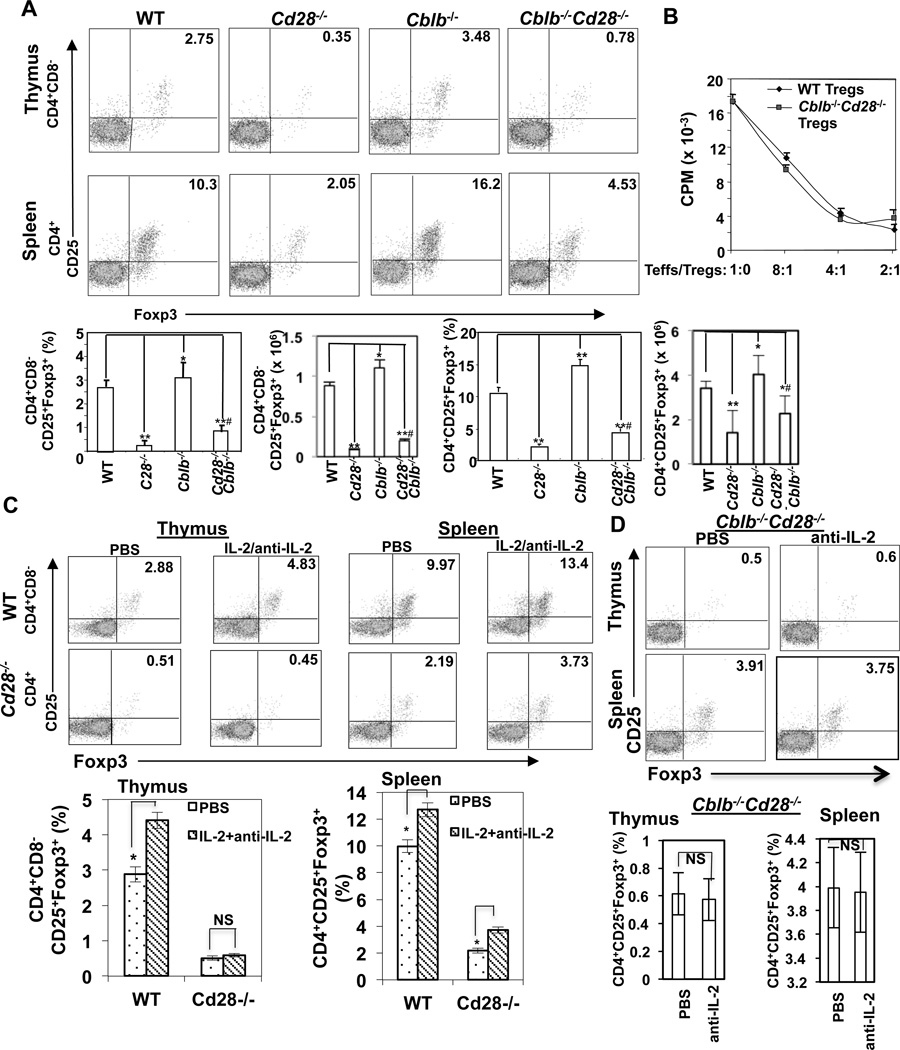
To test whether Cbl-b deficiency rescues the defective development and/or expansion of tTregs in Cd28−/− mice, we crossed Cblb−/− mice onto a Cd28−/− background and generated Cblb−/−Cd28−/− mice. Although very few CD4+Foxp3+ tTregs were found in Cd28−/− thymuses and spleens, Cblb−/− mice had increased tTregs in their thymuses and spleens compared their WT controls (Fig. 1A). Loss of Cbl-b partially but significantly rescued this defect in both thymuses and spleens of CD28−/− mice (Fig. 1A; p<0.001 compared Cd28−/− tTregs with Cblb−/−Cd28−/− tTregs in both thymuses and spleens). Therefore, Cbl-b deficiency rescues at least in part tTreg development in Cd28−/− mice. Functionally Cblb−/−Cd28−/− CD4+CD25+ tTregs suppressed TCR-induced WT effector T cell proliferation comparable to WT CD4+CD25+ tTregs (Fig. 1B). This partial rescue is likely to be independent of IL-2 because treating Cd28−/− mice with IL-2/anti-IL-2 complex, which has been shown to selectively stimulate tTregs (8), failed to rescue defective development of CD4+CD8−CD25+Foxp3+ tTregs in thymuses of Cd28−/− mice, although this treatment indeed increased splenic tTregs (Fig. 1C). Furthermore, treating Cblb−/−CD28−/− mice with a neutralizing anti-IL-2 antibody failed to abrogate the increased tTregs in thymuses and spleens of Cblb−/−Cd28−/− mice (Fig. 1D).
FIGURE 1.
Loss of Cbl-b partially rescues defective development of CD4+CD25+Foxp3+ Tregs in Cd28−/− mice. (A) Thymocytes and splenocytes from WT, Cblb−/−, Cd28−/−, and Cblb−/−Cd28−/− mice (n=5/group; 8 wks of age) were surface-stained with anti-CD4, anti-CD8, and anti-CD25, and intracellularly stained with anti-Foxp3. The percentage of CD4+CD8−CD25+Foxp3+ cells in thymuses and CD4+CD25+Foxp3+ cells in spleens of different groups of mice were calculated. Statistical significance was calculated using the student t-test. *p<0.05 compared to WT, ** p<0.01 compared to WT; #p<0.001 compared to Cd28−/−. (B) WT CD4+CD25− T cells were incubated with CD4+CD25+ T cells isolated from WT and Cblb−/−Cd28−/− mice at different ratios in the presence of anti-CD3 and T-depleted APCs for 72 hrs. T cell proliferation was determined by [3H]thymidine incorporation. (C) WT and Cd28−/− mice received daily i.v. injections of a combination of mouse IL-2 (1.5 µg/day, eBioscience) and anti-mouse IL-2 mAb (clone JES6-1A12) (50 µg/day; BioXCell) for 7–9 days. Thymuses and spleens were analyzed for Tregs on day 8–10 by flow cytometry as above. (D) Cblb−/−Cd28−/− mice were treated with anti-IL-2 daily by i.v. for 7–9 days, and Tregs in thymuses and spleens were analyzed by flow cytometry. *p<0.05 compared to PBS-treated controls; NS, no significance. The data shown are one representative of three independent experiments.
Cbl-b associates with Foxp3 upon TCR stimulation via its UBA domain
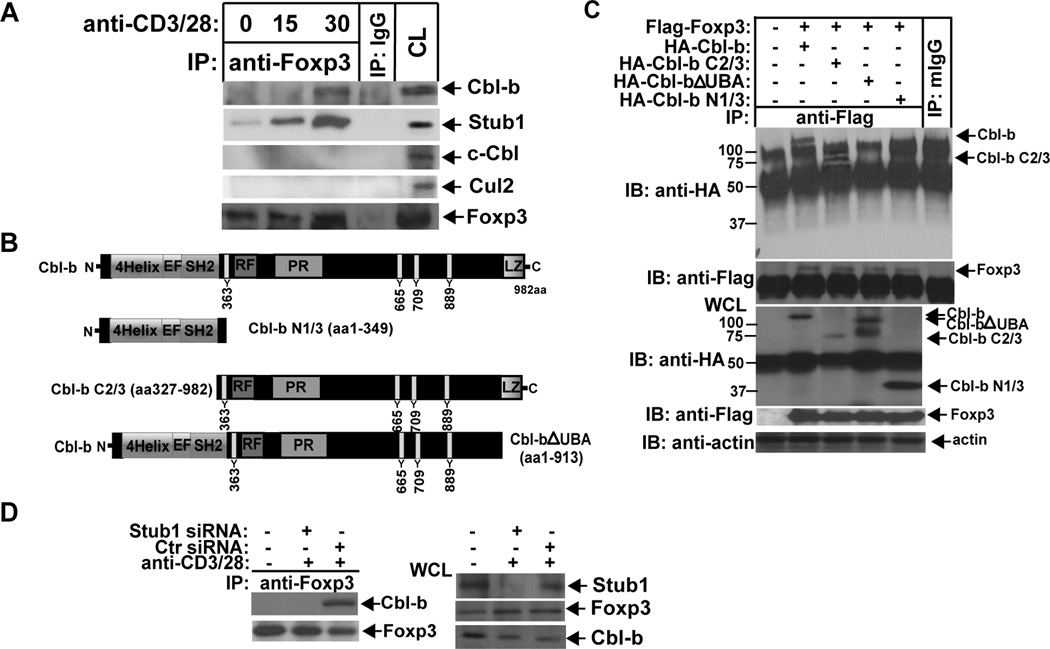
To address how Cbl-b inhibits CD28-dependent tTreg development we investigated whether Cbl-b and Foxp3 form a physical association. We found by co-immunoprecipitation that Cbl-b, but not c-Cbl, forms an inducible association with Foxp3 at 30 min after TCR/CD28 stimulation (Fig. 2A). To assess whether Foxp3 associates with other E3 ubiquitin ligases we also blotted Foxp3 immunoprecipitates with Abs against Cul2 and Stub1. It has been shown that Stub1 ubiquitinates Foxp3 under an inflammatory condition (24), while Cul2 may directly target Foxp3 under hypoxic conditions (25). Stub1 was found to constitutively associate with Foxp3, and this increased upon TCR/CD28 stimulation, but we failed to detect Cul2-Foxp3 interaction (Fig. 2A).
FIGURE 2.
Cbl-b inducibly associates with Foxp3. (A) BALB/c T cells were stimulated with anti-CD3 and anti-CD28 for 15 min or for 15 and 30 min, and lysed in 0.5% NP-40 lysis buffer. The cell lysates were immunoprecipitated with anti-Foxp3, and blotted with anti-Cbl-b (upper panel) or anti-Stub1 (lower panel). The cell lysates from the unstimulated sample were used as a positive control. The cell lysates immunoprecipitated with rabbit IgG (IgG) were used as a negative control. (B) Schematic design of Cbl-b mutant constructs. (C) HEK293T cells were transfected with HA-tagged Cbl-b, Cbl-b N1/3, Cbl-b C2/3, Cbl-b ΔUBA, together with Flag-tagged Foxp3, and lysed. The cell lysates were immunoprecipitated with anti-Flag, and blotted with anti-HA. (D) Naïve CD4+CD25+ T cells from BALB/c mice were nucleofected with siRNA specific for Stub1, stimulated with anti-CD3 and anti-CD28, and lysed. The cell lysates were immunoprecipitated with anti-Foxp3 and blotted with anti-Cbl-b. The data shown are one representative of two independent experiments.
To map the structural requirements for Cbl-b’s association with Foxp3 we generated serial Cbl-b truncated mutants as shown in Figure 2B. Cbl-b-Foxp3 interaction only occurred when the cells were co-transfected with WT Cbl-b and a Cbl-b C2/3 fragment, but not with Cbl-b constructs lacking the UBA domain (Fig. 2C). These data indicate that the Cbl-b UBA domain is critical for the binding of Cbl-b to Foxp3. These findings also raise the possibility that another E3 ubiquitin ligase initiates Foxp3 ubiquitination, which results in the recruitment of Cbl-b via the interaction between ubiquitin chains on Foxp3 and the UBA domain of Cbl-b, thus enhancing Foxp3 ubiquitination. Indeed, knocking down Stub1 by siRNA in WT T cells disrupted Cbl-b-Foxp3 interaction (Fig. 2D).
Stub1 and Cbl-b sequentially induce Foxp3 for ubiquitination and degradation
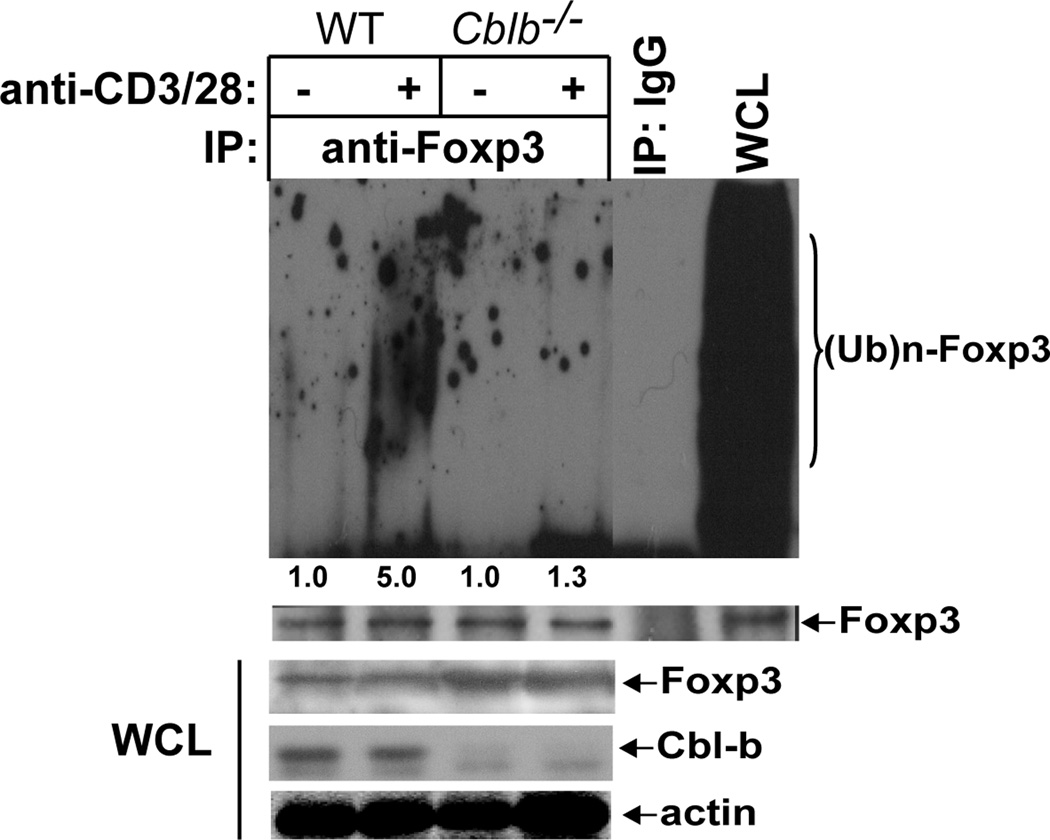
Although Foxp3 undergoes ubiquitination mediated by Stub1 upon inflammatory stimulation (24, 26), it is unknown whether TCR engagement also induces Foxp3 ubiquitination and degradation, which may provide a mechanism of maintaining Foxp3 expression at a steady state. Indeed, Foxp3 underwent ubiquitination upon TCR/CD28 stimulation (Fig. 3A). To test whether Cbl-b is involved in the ubiquitination of Foxp3 we transfected 293T cells with HA-tagged Cbl-b, Flag-tagged Foxp3, and His-tagged ubiquitin. We found that Cbl-b, but not the Cbl-b C373A mutant which lacks E3 ubiquitin ligase activity (27), induced Foxp3 for ubiquitination (Fig. 3B). In support of this finding, CD4+CD25+ tTregs lacking Cbl-b or expressing the C373A mutant displayed defective ubiquitination of Foxp3 (Fig. 3C and D). Interestingly ubiquitinated Foxp3 was only detectable in the nuclei but not the cytoplasm (Fig. 3E). In keeping with these data, Foxp3 expression was significantly decreased at 1 h after TCR/CD28 stimulation, but this down-regulation of Foxp3 expression was completely abrogated in the presence of MG-132 (Fig. 3F), supporting that Foxp3 undergoes proteasome-mediated degradation. To determine whether Stub1 is the initial E3 ubiquitin ligase for Foxp3, we knocked down Cbl-b and Stub1 in CD4+CD25+ T cells from WT mice, respectively. In support of our notion, loss of Cbl-b resulted in a significant decrease in Foxp3 ubiquitination, whereas knocking down Stub1 completely abrogated Foxp3 ubiquitination (Fig. 3G). These data strongly indicate that Stub1 initiates Foxp3 ubiquitination which in turn allows the recruitment Cbl-b via its UBA domain, thus enhancing Foxp3 ubiquitination.
FIGURE 3.
Stub1 and Cbl-b sequentially ubiquitinates Foxp3 upon TCR/CD28 stimulation. (A) CD4+CD25+ T cells from WT mice were stimulated with anti-CD3 and anti-CD28, or left unstimulated, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-Foxp3, and blotted with anti-ubiquitin. (B) HEK293T cells were transfected with HA-tagged Cbl-b or Cbl-b C373A, Flag-tagged Foxp3, and His-tagged ubiquitin. The Cell lysates were immunoprecipitated with anti-Flag, and blotted with anti-HA. (C and D) CD4+CD25+ T cells from WT, Cblb−/− or CblbC373A mice, and stimulated with anti-CD3 and anti-CD28, and lysed. The Foxp3 ubiquitination was determined. (E) Cytoplasmic and nuclear fractions of WT CD4+ T cells stimulated with anti-CD3 and anti-CD28 were separated, and immunoprecipitated with anti-Foxp3, and blotted with anti-ubiquitin. (F) WT CD4+ T cells were stimulated with anti-CD3 and anti-CD28 for 1, 2, and 4 hrs in the presence or absence of MG-132. Foxp3 expression was determined. (G) BALB/c CD4+CD25+ T cells were transfected with siRNAs specific for Stub1, Cbl-b, or a scrambled siRNA, and stimulated with anti-CD3 and anti-CD28, and lysed in RIPA buffer. The cell lysates were immunoprecitated with anti-Foxp3, and blotted with anti-ubiquitin. The fold changes of Foxp3 ubiquitination bands in arbitrary densitometric units were determined by the ImageJ 1.48. The data shown are one representative of three independent experiments.
Cbl-b maintains Foxp3 expression by facilitating CD4+CD25− T cells to express Foxp3 and then targets it for ubiquitination and degradation
We have shown that Cbl-b facilitates Foxp3 expression by naïve CD4+CD25−Foxp3− T cells via down-regulating the threshold for T cell activation (19). However, it is unknown whether Cbl-b also targets this newly-expressed Foxp3 for ubiquitination. To test this, we induced Foxp3 expression by naïve CD4+CD25− T cells of WT and Cblb−/− mice, and then stimulated the cells with anti-CD3 and anti-CD28 antibodies. Stimulation of iTregs with anti-CD3 and anti-CD28 induced Foxp3 ubiquitination and degradation, and this ubiquitination was significantly reduced in the absence of Cbl-b (Fig. 4). These data suggest that Cbl-b facilitates Foxp3 expression by naïve CD4+CD25− T cells, but once Foxp3 is expressed, Cbl-b targets it for ubiquitination and degradation, thus maintaining Foxp3 expression at a steady state.
FIGURE 4.
Cbl-b targets Foxp3 for ubiquitination in iTregs. Naïve CD4+CD25− T cells from WT and Cblb−/− mice were stimulated with plate-coated anti-CD3 (0.05 µg/ml for WT and 0.01 µg/ml for Cblb−/−) and soluble anti-CD28 with 2.5 ng/ml TGF-β1, and 100 U/ml recombinant mouse IL-2 (R&D System) for 72 hrs. The cells were restimulated with anti-CD3 and anti-CD28 followed by crosslinking with rabbit anti-hamster IgG for 15 min, and lysed in RIPA buffer. The cell lysates were immunoprecipitated with anti-Foxp3 and blotted with anti-ubiquitin. The membrane was stripped and reprobed with anti-Foxp3. The whole cell lysates (WCL) were blotted with anti-Foxp3, anti-Cbl-b, and anti-actin, respectively. The data shown are one representative of three independent experiments.
Proteasome inhibitor PS-341 potentiates tTreg development in CD28−/− mice
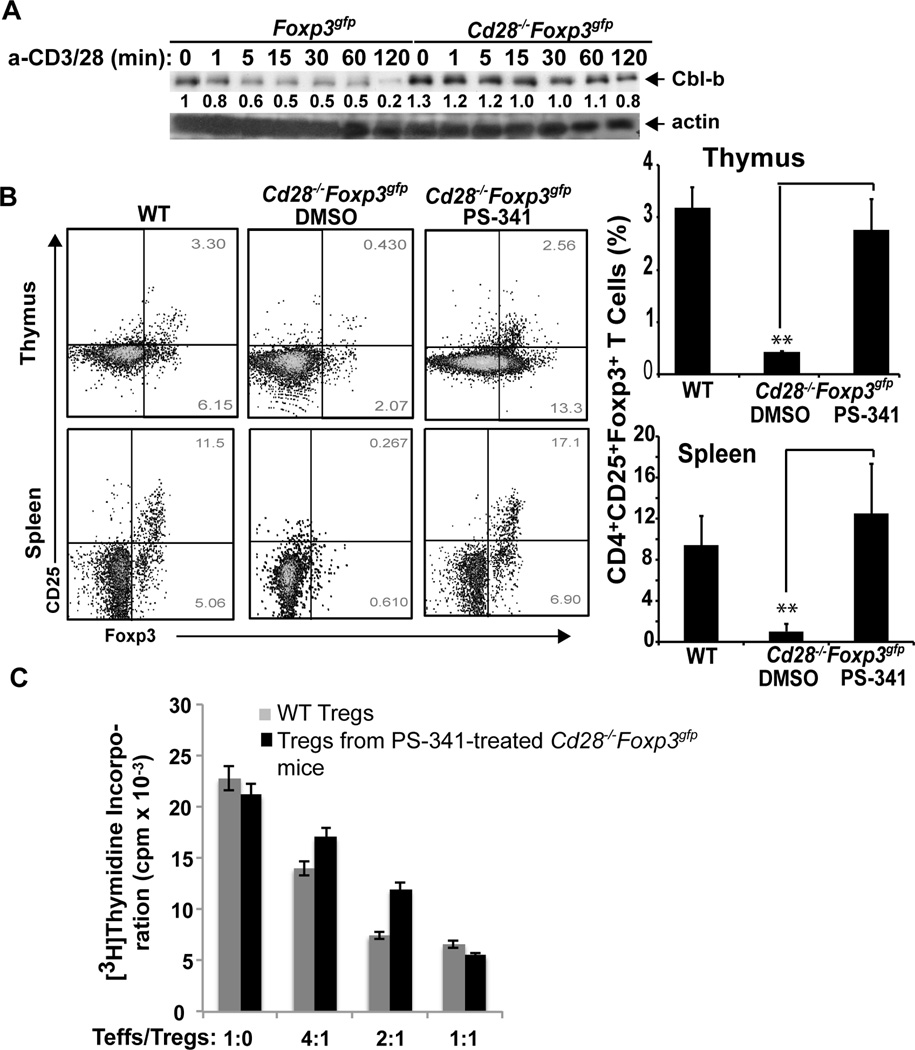
Our previous studies showed that Cbl-b self-ubiquitination and degradation is impaired in Cd28−/− T cells upon TCR stimulation (15). Indeed, Cbl-b degrdataion was impaired in thymic Treg precursors lacking CD28 (Fig. 5A). These observations indicate that higher levels of Cbl-b in Cd28−/− T cells may display higher E3 Ub ligase activity which results in a significantly higher degradation of Foxp3 in these T cells. In support of this notion, treating Cd28−/−Foxp3gfp mice with PS-341 (Bortezomib), a clinically approved drug for cancer therapy, completely rescued defective tTregs in thymuses and spleens of Cd28−/− mice to WT levels (Fig. 5B), and these newly-generated tTregs were able to suppress effector T cell proliferation in vitro (Fig. 5C).
FIGURE 5.
Treating Cd28−/− mice with PS-341 completely rescues tTreg development. (A) CD4+ single positive (SP) thymocytes from Foxp3gfp and Cd28−/−Foxp3gfp mice were purified by CD8 depletion with anti-CD8 microbeads followed by MACS LD columns. CD4+GFP+ Tregs were further isolated by FACS sorting, and stimulated with anti-CD3 and anti-CD28 for 1, 5, 15, 30, 60, and 120 min, and lysed. The cell lysates were blotted with anti-Cbl-b and anti-actin, respectively. The fold changes of Cbl-b bands in arbitrary densitometric units were determined by the ImageJ 1.48. The data shown are one representative of two independent experiments. (B) Cd28−/−Foxp3gfp mice (n=5/group, 6 wks of age) were i.p. injected with PS-341 (0.25 mg/kg) twice a week for two weeks. The expression of GFP+ cells within CD4+CD8−CD25+ population in thymuses and spleens was determined. **p<0.01 compared Cd28−/−Foxp3gfp mice with PS-341-treated Cd28−/−Foxp3gfp mice; Student t test. (C) WT naïve CD4+CD25− T cells were incubated with different ratios of CD4+Foxp3+ T cells isolated from Foxp3gfp and Cd28−/−Foxp3gfp treated with PS-341 in the presence of anti-CD3 and T-depleted APCs for 72 hr. T cell proliferation was determined by [3H]thymidine incorporation. The data shown are one representative of three independent experiments.
DISCUSSION
In this study, we demonstrate that Cbl-b regulates CD28-dependent tTreg development via targeting Foxp3 for ubiquitination. Interestingly, we found that Foxp3 is sequentially ubiquitinated by Stub1 and Cbl-b. Stub1 initiates Foxp3 ubiquitination which results in the recruitment of Cbl-b to the ubiquitin chains that are attached to Foxp3 via the Cbl-b UBA domain. This mechanism is important to maintain Foxp3 at a steady state. Therefore, our study provides a novel molecular mechanism for the regulation of Foxp3 expression in Tregs.
It has been documented that CD28 costimulation is required for tTreg development because Cd28−/− mice have very few tTregs (5, 6). The defective tTreg development in Cd28−/− mice appears to be independent of IL-2 (5), and the molecular mechanism for CD28 in controlling tTreg development remains poorly defined. Since we and others have previously shown that Cbl-b regulates CD28-dependent T cell activation (13–16), we explored the possibility that Cbl-b regulates CD28-dependent Foxp3 expression. By introducing Cbl-b deficiency into Cd28−/− mice we found that loss of Cbl-b partially but significantly increases tTregs, and that this partial rescue of tTreg development is independent of IL-2 (Fig. 1), a finding consistent with a previous report (5). Further analysis revealed that Foxp3 undergoes polyubiquitination and proteasome-mediated degradation, and that Foxp3 ubiquitination is significantly reduced in tTregs lacking Cbl-b or expressing an E3 ubiquitin ligase dead mutation (Fig. 3). These data suggest that Cbl-b is an E3 ubiquitin ligase that promotes Foxp3 ubiquitination. Interestingly, our analysis of the molecular interaction between Cbl-b and Foxp3 reveals that Cbl-b binds to Foxp3 via its UBA domain (Fig. 2), suggesting that Cbl-b interacts with ubiquitinated Foxp3 which is initiated by another E3 ubiquitin ligase. Indeed, Stub1, which was reported to induce Foxp3 under the inflammatory condition (24), is the E3 ubiquitin ligase that initiates Foxp3 ubiquitination which allows Cbl-b to be recruited to the complex, thus synergistically enhancing Foxp3 ubiquitination. As we have shown that Cbl-b self-ubiquitination is impaired in Cd28−/− mice (15), our data suggest that the defective tTreg development in Cd28−/− mice may at least in part be ascribed to heightened expression of Cbl-b. In support of this notion, treating Cd28−/− mice with PS-341 (Bortezomib), a clinically approved drug for cancer therapy, completely rescued defective tTregs in the thymuses and spleens of Cd28−/− mice to WT levels (Fig. 5B). Therefore, we have unveiled a novel molecular mechanism for CD28-dependent tTreg development. Our data also suggest that administration of Bortezomib may provide a useful approach to treat certain autoimmune diseases.
In summary we show that loss of Cbl-b partially uncouples the requirement for tTreg development from CD28 costimulation, and this effect is independent of IL-2. At the molecular level, Stub1 initially induces Foxp3 ubiquitination upon TCR/CD28 stimulation. This ubiquitination event leads to the recruitment of Cbl-b to Foxp3 via the Cbl-b UBA domain and ubiquitin chains attached to Foxp3, thus enhancing Foxp3 ubiquitination. As Cbl-b facilitates Foxp3 expression by naïve CD4+CD25− T cells via inactivating Akt-2 (19), our data reveal a previously unappreciated role for Cbl-b in controlling the development of tTregs and maintaining Foxp3 expression at a steady state.
Acknowledgements
We thank Drs. Josef M. Penninger, Alexander Rudensky, and Stan Lipkowitz for providing Cblb−/− mice, Foxp3gfp mice, and various Cbl-b plasmids which made this study possible.
The project described was supported by grants from the National Institutes of Health (NIH) (R01 AI090901 to JZ) and from the American Heart Association (09GRNT2010084 to JZ).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 2.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 3.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J. Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- 4.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 5.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. of Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 8.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 9.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J. Ubiquitin ligases in T cell activation and autoimmunity. Clin. Immunol. 2004;111:234–240. doi: 10.1016/j.clim.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat. Immunol. 2004;5:883–890. doi: 10.1038/ni1106. [DOI] [PubMed] [Google Scholar]
- 12.Qiao G, Ying H, Zhao Y, Liang Y, Guo H, Shen H, Li Z, Solway J, Tao E, Chiang YJ, Lipkowitz S, Penninger JM, Langdon WY, Zhang J. E3 Ubiquitin Ligase Cbl-b suppresses proallergic T cell development and allergic airway inflammation. Cell Rep. 2014;6:709–723. doi: 10.1016/j.celrep.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, Mariathasan S, Bouchard D, Wakeham A, Itie A, Le J, Ohashi PS, Sarosi I, Nishina H, Lipkowitz S, Penninger JM. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–216. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 14.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Bardos T, Li D, Gal I, Vermes C, Xu J, Mikecz K, Finnegan A, Lipkowitz S, Glant TT. Cutting edge: regulation of T cell activation threshold by CD28 costimulation through targeting Cbl-b for ubiquitination. J. Immunol. 2002;169:2236–2240. doi: 10.4049/jimmunol.169.5.2236. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Gal I, Vermes C, Alegre ML, Chong AS, Chen L, Shao Q, Adarichev V, Xu X, Koreny T, Mikecz K, Finnegan A, Glant TT, Zhang J. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J. Immunol. 2004;173:7135–7139. doi: 10.4049/jimmunol.173.12.7135. [DOI] [PubMed] [Google Scholar]
- 17.Jeon MS, Atfield A, Venuprasad K, Krawczyk C, Sarao R, Elly C, Yang C, Arya S, Bachmaier K, Su L, Bouchard D, Jones R, Gronski M, Ohashi P, Wada T, Bloom D, Fathman CG, Liu YC, Penninger JM. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity. 2004;21:167–177. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 19.Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, Zhang J. T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells. J. Immunol. 2013;191:632–639. doi: 10.4049/jimmunol.1202068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, Zhang J. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol. Cell. Biol. 2008;28:2470–2480. doi: 10.1128/MCB.01505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oksvold MP, Dagger SA, Thien CB, Langdon WY. The Cbl-b RING finger domain has a limited role in regulating inflammatory cytokine production by IgE-activated mast cells. Mol. Immunol. 2008;45:925–936. doi: 10.1016/j.molimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Ettenberg SA, Magnifico A, Cuello M, Nau MM, Rubinstein YR, Yarden Y, Weissman AM, Lipkowitz S. Cbl-b-dependent coordinated degradation of the epidermal growth factor receptor signaling complex. J. Biol. Chem. 2001;276:27677–27684. doi: 10.1074/jbc.M102641200. [DOI] [PubMed] [Google Scholar]
- 23.Qiao G, Yang L, Li Z, Ying H, Hassen Y, Yin F, Zhang J. Program death-1 regulates peripheral T cell tolerance via an anergy-independent mechanism. Clin. Immunol. 2012;143:128–133. doi: 10.1016/j.clim.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, Jinasena D, Fu J, Lin F, Chen C, Zhang J, Yu N, Li X, Shan Z, Nie J, Gao Z, Tian H, Li Y, Yao Z, Zheng Y, Park BV, Pan Z, Zhang J, Dang E, Li Z, Wang H, Luo W, Li L, Semenza GL, Zheng SG, Loser K, Tsun A, Greene MI, Pardoll DM, Pan F, Li B. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39:272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, Meerding J, Berkers CR, Barbi J, Grone A, Sijts AJ, Maurice MM, Kalkhoven E, Prakken BJ, Ovaa H, Pan F, Zaiss DM, Coffer PJ. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity. 2013;39:259–271. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, Yang L, Lipkowitz S, Penninger JM, Langdon WY, Zhang J. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Rep. 2012;1:472–482. doi: 10.1016/j.celrep.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]