Abstract
Objective
To understand independent pathways linking emotional distress, medication adherence and glycemic control in adults with type 2 diabetes, as well as the potential mediating effects of perceived control over illness and self-efficacy.
Methods
Adults with type 2 diabetes (N = 142) were recruited for an intervention study evaluating cognitive behavioral therapy for adherence and depression. Depressive symptom severity was assessed via semi-structured interview. Validated self-reports assessed diabetes-related distress, perceived control over diabetes (perceived control), self-efficacy for diabetes self-management and medication adherence. Glycemic control was evaluated by hemoglobin A1c (A1C). Only baseline data were included in correlational and linear regression analyses.
Results
Perceived control was an important mediator for both medication adherence and A1C outcomes. Specifically, regression analyses demonstrated that diabetes distress, but not depression severity, was significantly related to medication adherence and A1C. Self-efficacy and perceived control were also independently associated with medication adherence and A1C. Mediation analyses demonstrated a significant indirect effect for diabetes distress and medication adherence, through perceived control and self-efficacy. The relationship between distress and A1C was accounted for by an indirect effect through perceived control.
Conclusion
Results demonstrate that diabetes-related emotional distress is associated with poorer treatment adherence and glycemic control among adults with type 2 diabetes; these relationships were partially mediated through perceived control over diabetes. Perceptions of one’s personal ability to influence diabetes may be important in understanding the pathway between emotional distress and poor diabetes treatment outcomes.
Keywords: diabetes, depression, diabetes-related distress, perceived control, self-efficacy
The rising prevalence of type 2 diabetes represents a significant challenge for population health in the US and internationally. Although it is well established that reductions in hemoglobin A1c (A1C), a key index of glycemic control in diabetes, can substantially reduce the risk of diabetes complications (United Kingdom Prospective Diabetes Study, 1998; Nathan et al., 2005), about half of US adults with diabetes are not at goal for A1C (Stark Casagrande et al., 2013) and many report nonadherence to prescribed medications (DiMatteo, 2004; Rubin, 2005). Nonadherence is associated with poor control of A1C, blood pressure and lipids, and increased risk of hospitalization and mortality (e.g., Ho et al., 2006); it is also linked to less effective care – providers are less likely to intensify treatment when indicated for nonadherent patients (Grant et al., 2007). Thus, identification of factors associated with diabetes treatment nonadherence is important and could guide interventions to improve health outcomes.
Among patient-level factors, depressive symptoms have been consistently related to treatment nonadherence across a variety of chronic illnesses (DiMatteo, Lepper, Croghan, 2000). A meta-analysis of 47 independent samples showed that higher levels of depressive symptom severity are consistently associated with problematic diabetes self-management across various behavioral domains, including medication adherence (Gonzalez et al., 2008a). Beyond their consistent association with poorer diabetes self-management, depressive symptoms are also related to important diabetes health outcomes over time, such as development of complications (Lin et al., 2010; Black, Markides & Ray, 2003) and mortality (Park, Katon & Wolf, 2013). However, it is important to note that these relationships do not appear to be limited to cases of clinical depression, such as major depressive disorder (MDD). Indeed, depressive symptoms that fall well below MDD diagnostic thresholds are associated with worse self-management both cross-sectionally (Gonzalez et al., 2007) and longitudinally (Gonzalez et al. 2008b); they also predict complications and mortality (Black et al., 2003). Evidence suggests that these ‘subclinical’ depressive symptoms may often represent emotional distress specific to the burdens of living with diabetes rather than a co-morbid depressive mood disorder (Fisher et al., 2007). The need to differentiate diabetes-related emotional distress from depression has led to the development of widely used measures of ‘diabetes distress’ (Polonsky et al., 1995; Polonsky et al., 2005) and of behavioral interventions to directly target diabetes distress (Fisher et al., 2013). Greater precision in distinguishing between depression and diabetes distress could guide the selection of appropriate interventions for patients (Gonzalez, Fisher & Polonsky, 2011).
Both diabetes distress and depressive symptoms have been independently associated with medication nonadherence, cross-sectionally and longitudinally, in adults treated for type 2 diabetes (Fisher et al., 2010; Gonzalez et al., 2007; Gonzalez et al., 2008b; Gonzalez, Delahanty, Safren, Meigs & Grant, 2008c; Aikens, 2012). However, the conceptual and measurement overlap between these constructs contributes to inconsistencies in the literature (Gonzalez, Fisher & Polonsky, 2011; Fisher, Gonzalez, & Polonsky, 2014). For example, although early studies showed consistent associations between depressive symptoms and glycemic control (Lustman et al., 2000), more recent studies examining change over time have failed to demonstrate this relationship (e.g., Georgiades et al., 2007; Fisher et al., 2010; Aikens, Perkins, Lipton & Piette, 2009; Aikens, 2012) and suggest that diabetes distress is more closely associated with glycemic control (Aikens, 2012; Fisher et al., 2010). Thus, although emotional distress is clearly implicated in sub-optimal diabetes treatment adherence and outcomes, there is inconsistency in these relationships. Some of this inconsistency may result from an over-reliance on self-report measures of depressive symptoms, which are often more reflective of general distress than clinical depression (Coyne, 1994) and may be particularly vulnerable to overlap with diabetes distress (Gonzalez et al., 2011).
Despite the size of the literature on the relationship between emotional distress and diabetes self-management, little research is available to shed light on how distress is linked to diabetes self-management. Social-cognitive variables may play an important role. Negative mood states are known to have a direct influence on self-efficacy, generally reducing perceived ability to carry out activities across various domains (e.g., Kavanagh & Bower, 1985; Salovey & Birnbaum, 1989). Thus, one mechanism linking emotional distress with poor diabetes treatment adherence and health outcomes may involve self-efficacy. Several studies have reported evidence to support self-efficacy as a mediator of the relationship between depressive symptoms and type 2 diabetes self-management (Chao, Nau, Aikens, Taylor, 2005;Wagner, Tennen, Osborn, 2010). Evidence has also been reported for self-efficacy for diabetes management as a mediator of the relationship between depressive symptoms and glycemic control among males with type 2 diabetes (Cherrington, Wallston, Rothman, 2010).
Although there is a rich literature that links perceptions of self-efficacy for specific activities to the amount of effort and persistence expended on these activities (Bandura, 1982), these perceptions should be distinguished from one’s perceived ability to meaningfully affect an outcome of interest, or perceived control (Bandura & Wood, 1989; Skinner, 1996). Bandura viewed self-efficacy and perceived control as linked through reciprocal causation (Bandura & Wood, 1989) and described perceived control as a precondition to the optimal execution of efficacious behaviors – “if people approach situations as largely uncontrollable, they are likely to exercise their efficacy weakly and abortively,” whereas, “when people believe the environment is controllable…they are motivated to exercise fully their personal efficacy” (Bandura & Wood, 1989, p.806). Individuals with type 2 diabetes and elevated depressive symptoms report less perceived control over diabetes (Egede & Ellis, 2008; Macrodimitris & Endler, 2001) and greater perceived control is consistently associated with lower A1C (Egede & Ellis, 2008; Macrodimitris & Endler, 2001; Sharry, Moss-Morris, Kendrick, 2011). This relationship may be mediated through improved adherence, as perceived control has also been consistently associated with better diabetes self-management (e.g., Hampson Glasgow, & Toobert, 1990; Skinner & Hampson, 2001). Thus, although evidence supports self-efficacy and perceived control as potential mediators of the distress – diabetes self-management relationship, prior studies have not examined their independent effects.
We sought to advance our understanding of the relationships among emotional distress, diabetes treatment adherence, and glycemic control in adults with treated type 2 diabetes by examining direct and indirect pathways linking these variables. We used measures of depressive symptoms and diabetes distress as indicators of emotional distress and evaluated perceived control and self-efficacy as sequential mediators of the relationships between emotional distress, medication adherence and glycemic control.
Methods
Participants, setting and recruitment
As previously described (Gonzalez et al., 2010; Safren et al., 2014), participants were recruited from the Diabetes Center and primary care clinics at Massachusetts General Hospital or were self-referred via hospital emails and radio advertisements for an intervention trial of cognitive behavioral therapy for adherence and depression in patients with type 2 diabetes. Entry criteria for the study included the ability to read and write in English, a diagnosis of type 2 diabetes, a prescription for medication to treat hyperglycemia or a diabetes-related condition (i.e., hypercholesterolemia or hypertension), and age between 18 and 70 years. Prescriptions for diabetes and depression medications were required to have been stable for two months. Participants were also required to have an A1C level ≥ 7.0%.
To qualify for the intervention study (See: Safren et al., 2014), participants were required to meet DSM-IV criteria for major depressive disorder or dysthymia or to exhibit clinically significant depressive symptoms despite the prescription of an antidepressant. Those with active and untreated significant psychiatric conditions (e.g., untreated psychosis), bipolar disorder, eating disorder, mental retardation, dementia, or active suicidality revealed by semi-structured psychiatric interview (Sheehan et al., 1998) were excluded. Participants provided informed consent, and the Partners HealthCare Institutional Review Board approved the study. All participants were evaluated for depression severity, completed all self-report measures, and provided a blood sample to measure A1C levels at the initial baseline evaluation. 209 patients were assessed for eligibility and 87 qualified for the intervention study. Current analyses include 142 screened participants who provided a baseline A1C and had complete data on the self-reported measures.
Measures
Diabetes-related Distress
Emotional distress related to the burdens of diabetes and its treatment was evaluated with the Diabetes Distress Scale (DDS) (Polonsky et al., 2005). The DDS is a 17-item questionnaire assessing the experience of distress associated with diabetes over the past month across four subscales: emotional burden, physician-related distress, regimen-related distress, and interpersonal distress (Polonsky et al., 2005). The DDS measures a range of issues related to distress and some subscales do not measure emotional distress per se. For example, physician-related distress includes items such as, “Feeling that my doctor doesn’t know enough about diabetes and diabetes care.” Regimen distress items share content overlap with measures of treatment adherence (e.g., “Feeling that I am not testing my blood sugars frequently enough”). For the present analysis, we therefore selected the 5-item emotional burden scale, which includes items such as, “Feeling angry, scared, and/or depressed when I think about living with diabetes” and “Feeling overwhelmed by the demands of living with diabetes.” Internal reliability of the emotional burden subscale this sample was .89 and it was strongly correlated with the DDS total score (r = .87, p< .001). Clinical validation of the DDS suggests that the following thresholds of severity should be applied when interpreting scores: little or no distress <2.0, moderate distress = 2.0–2.9, and high distress ≥3.0 (Fisher, Hessler, Polonsky, Mullan, 2012).
Depression Severity
Depressive symptom severity was assessed via clinical interview with the Montgomery Asberg Depression Rating Scale (MADRS) (Montgomery & Asberg, 1979). The MADRS is a semi-structured clinical interview of 10 commonly occurring depressive symptoms over the past week, including reported sadness, inner tension, reduced sleep, reduced appetite, concentration difficulties, lassitude, inability to feel, pessimistic thoughts and suicidal thoughts. Scores range from 0 to 60: 0–6 indicates no depression; 7–19 indicates mild depression; 20–34 indicates moderate depression; 35–60 indicates severe depression (Montgomery & Asberg, 1979). Interviewers were clinical psychology trainees or psychologists who were trained on administration and received regular supervision. They were blinded to participant diabetes self-management and glycemic control. Internal reliability was .84. One advantage of this measure over self-report screening measures for depression (e.g., BDI, CES-D, PHQ-9) is that it involves both standardized assessment and clinical judgment in the evaluation of depressive symptoms, which may reduce confounding with symptoms of diabetes. Use of interview data as an index of depressive symptom severity also reduces the impact of shared method error in linking psychosocial variables and measures of depression.
Perceived Control and Self-Efficacy
An item from the Brief Illness Perception Questionnaire was used to assess participants’ perceived control over diabetes (Broadbent, Petrie, Main, & Weinman, 2006): “How much control do you feel you have over your diabetes?” The visual-analogue response scale ranged from 0 (absolutely no control) to 10 (extreme amount of control). Self-efficacy for diabetes self-management was assessed by an 8-item scale (Sarkar, Fisher, Schillinger, 2006). Questions asked, “At the present time, how sure are you that you can…take care of your health, get medical attention when you need it, take all your diabetes medicines correctly,” etc. The response scale ranged from 1 (not at all sure) to 4 (very sure). The mean of items was taken to compute a total score (α = .79).
Medication adherence
Self-reported medication adherence was measured using a global self-rating previously shown to have close concordance with electronic pill cap data and criterion-validity based on a relationship with glycemic control (Gonzalez et al., 2013). The item was originally validated in HIV via relationships with electronic pill cap and viral load (Lu, Safren, Skolnik, et al 2008). Participants were asked to assess their medication adherence across 11 response categories (0, 10, 20…100%) with the following question: “Thinking about the past month, what percent of the time did you take all your diabetes medications as your doctor prescribed?” This item was selected for use based on its superior relationship to electronic medication monitoring data and A1C and lack of bias associated with depression, age, or education (Gonzalez et al., 2013).
Glycemic control
Blood samples were collected from each participant at baseline and were used to measure Hemoglobin A1C in one of the reference labs for the National Glycohemoglobin Standardization Program (Nathan, Singer, Hurxthal, & Goodson, 1984). A1C provides an index of average glycemic control during the preceding 3 months.
Covariates
To rule out potential confounding between demographic and disease-related variables and the factors of interest for the current study, participant age, sex, years of education, years since diagnosis of diabetes, total number of self-reported diabetes complications (cardiovascular, nephropathy, neuropathy, retinopathy; range = 0–4), and whether or not insulin was prescribed as part of the diabetes treatment regimen were statistically controlled in all multivariate analyses. Although our protocol included the collection of height and weight at the time of the study visit, this information was missing for 15 participants, 14 of which were screen-outs from the intervention. To avoid reducing the analytic sample, BMI was not included as a covariate. However, analyses showed that although higher BMI was significantly associated with increased depressive symptoms on the MADRS (r = .198, p = .026), it was not related to diabetes distress (r = .080, p = .369). Repeating our mediation models in the reduced sample while covarying BMI resulted in the same pattern of findings reported below; significant indirect effects remained significant in these models (data not shown).
Statistical Analysis
Descriptive statistics and bivariate correlations between study variables were first examined. To select the most appropriate measure of emotional distress for mediation analysis, we conducted multiple regression models examining independent effects of depression severity (MADRS total) and diabetes distress (DDS Emotional Burden subscale) when predicting self-rated medication adherence and A1C, in separate models. We followed recommended procedures for using ordinary least squares regression to evaluate statistical mediation using the PROCESS macro for SPSS (Hayes, 2013). These procedures have been shown to be particularly useful for multiple mediator models because they provide significance tests for independent indirect effects and for pair-wise comparisons between indirect effects (Preacher & Hayes, 2004). Rather than relying on significance tests, such as the Sobel test, that require the often-untenable assumption of a normal distribution for indirect effects, the macro uses bootstrapping of the sampling distribution of the indirect effects to derive estimates of the indirect effect, standard error and bias-corrected 95% confidence intervals (Preacher & Hayes, 2004; Hayes, 2013). Bootstrap estimates are based on 10,000 draws with replacement from the current sample. All multivariate analyses controlled for the same set of covariates (see above). We modeled perceived control and self-efficacy as sequential meditators based on the expected relationship between them (Bandura & Wood, 1989), rather than specifying parallel paths. An initial path model examined the indirect effects of distress, through these mediators, on medication adherence. Finally, we added A1C as a distal outcome of these variables.
Results
Participants were 142 men and women with type 2 diabetes with complete data on all study measures. Characteristics of the sample are presented in Table 1. Consistent with the recruitment procedures for our intervention trial, screened participants were likely to be in relatively poor glycemic control, were likely to have major depressive disorder and moderately severe depressive symptoms, and reported a high level of diabetes-related emotional burden. Despite the high levels of emotional distress observed in the sample, overall perceived control over diabetes was moderate and participants were ‘fairly sure’ about their self-efficacy for diabetes self-management. Participants reported taking an average of 79% of their prescribed diabetes-related medications over the prior month. Although those prescribed insulin had significantly (p = .001) higher A1Cs than those on oral medications only, no other significant differences between the treatment regimens were observed.
Table 1.
Participant demographics
| Mean (SD) or Percent | |
|---|---|
| Age (years) | 55.95 (9.24) |
| Education (years) | 14.56 (3.25) |
| Male sex | 55.6% |
| White race | 82.4% |
| Hispanic ethnicity | 3.5% |
| Years since diabetes diagnosis | 10.95 (8.16) |
| On insulin | 51.4% |
| Number of complications | 0.83 (0.99) |
| MDD Diagnosis | 59.9% |
| MADRS Total | 21.95 (9.82) |
| DDS Total | 2.87 (1.06) |
| DDS Emotional Burden | 3.28 (1.40) |
| Self-efficacy | 3.00 (0.55) |
| Perceived control | 5.33 (2.52) |
| Adherence Self-Rating | 79.15 (24.04) |
| A1C | 8.35 (1.65) |
Preliminary Distress Analyses
MADRS depression severity and diabetes distress were significantly bivariately correlated with each other and with medication adherence (Table 2). In a multiple regression controlling for all covariates, only diabetes distress was significantly independently related to medication non-adherence (b = −4.19, se = 1.47, p = .005); MADRS was not related to adherence independent of diabetes distress (b = −.07, se = .20, p = .721). Depression severity and diabetes distress did not have significant bivariate associations with A1C (Table 2). After controlling for all covariates in multiple regression, higher diabetes distress was significantly associated with higher A1C (b = .21, se = .10, p = .040) and remained significant after depression severity was added (b = .21, se = .10, p = .043). Depression severity was not significantly related to A1C in this model (b = −.00, se = .01, p = .840). Thus, only diabetes distress was retained for mediation analyses.
Table 2.
Bivariate Correlations Between Study Variables
| Depression Severity |
Diabetes Distress |
Perceived Control |
Self- efficacy |
Medication Adherence |
A1C | |
|---|---|---|---|---|---|---|
| Depression Severity | __ | .300*** | −.118 | −.185* | −.176* | .072 |
| Diabetes Distress | __ | −.269** | −.368*** | −.318*** | .138 | |
| Perceived Control | __ | .349*** | .311** | −.306*** | ||
| Self-efficacy | __ | .299*** | −.197* | |||
| Medication Adherence | __ | −.270** | ||||
| A1c | __ |
Note:
p < .05;
p < .01;
p < .001.
Perceived control and self-efficacy were bivariately correlated with each other and with medication adherence. Each was also significantly correlated with diabetes distress (Table 2). In multiple regression, higher levels of perceived control (b = 1.85, se = .79, p = .021) and self-efficacy (b = 8.46, se = 3.59, p = .020) were each independently associated with better medication adherence. Perceived control and self-efficacy were also each significantly bivariately correlated with A1C (Table 2). In a multiple regression model, perceived control remained independently associated with lower A1C (b = −.17, se = .05, p = .002) while self-efficacy was reduced to non-significance (b = −.41, se = .25, p = .096). Each of these variables was retained for mediation modeling.
Mediation Analyses
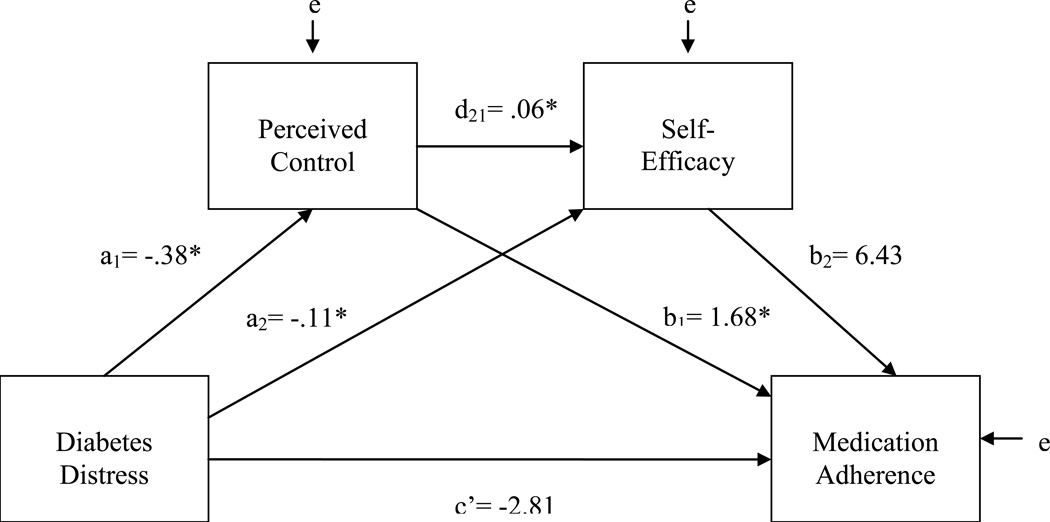
An initial path model evaluated the indirect effects of diabetes distress on medication adherence, sequentially mediated through perceived control and self-efficacy. The total effect of distress on medication adherence was significant and negative (c = −4.32, p = .003). As shown in Figure 1 and Table 3, those reporting higher diabetes-related emotional burden reported significantly lower levels of personal control over diabetes and less self-efficacy for diabetes self-management. Perceived control was, in turn, independently associated with greater self-efficacy. Although perceived control was independently and significantly associated with better medication adherence, self-efficacy was not. A bias-corrected bootstrap confidence interval for the sequential indirect effect of distress through perceived control and self-efficacy (a1d21b2 = −.15) was statistically significant (95%CI: −.673, −.002). The direct effect of distress on adherence fell short of significance, independent of its indirect effects (c' = −2.81, p = .056).
Figure 1.
Preliminary Path Model Predicting Medication Adherence with Perceived Control and Self-efficacy as Sequential Mediators
Table 3.
Results from initial path models including self-efficacy
| Perceived Control (M1) |
Self-Efficacy (M2) |
Medication Adherence (Y1/M3) |
A1c (Y2) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | ||||
| Female Sex | −.52 | .44 | .242 | −.02 | .09 | .869 | −8.97 | 3.87 | .022 | −.15 | .27 | .567 | ||||
| Age | .03 | .02 | .228 | .00 | .00 | .534 | .44 | .21 | .041 | .02 | .01 | .139 | ||||
| Years of Education | .01 | .07 | .918 | −.00 | .01 | .950 | −1.47 | .58 | .013 | .08 | .04 | .052 | ||||
| Diabetes Duration | −.00 | .03 | .917 | .01 | .01 | .345 | −.32 | .25 | .199 | .01 | .02 | .393 | ||||
| Diabetes Complications | −.04 | .23 | .862 | −.06 | .05 | .229 | −1.18 | 1.98 | .552 | .29 | .14 | .036 | ||||
| Prescribed Insulin | −.63 | .45 | .163 | .08 | .09 | .365 | 1.64 | 3.96 | .679 | .45 | .27 | .104 | ||||
| Antecedent Variables | ||||||||||||||||
| Diabetes Distress (X) | a1 | −.38 | .16 | .017 | a2 | −.11 | .03 | .001 | c’ | −2.81 | 1.46 | .056 | c’ | .07 | .10 | .518 |
| Perceived Control (M1) | --- | --- | --- | d21 | .06 | .02 | .001 | b1 | 1.68 | .79 | .034 | b1 | −.14 | .05 | .010 | |
| Self-Efficacy (M2) | --- | --- | --- | --- | --- | --- | b2 | 6.43 | 3.71 | .085 | b2 | −.27 | .25 | .294 | ||
| Medication Adherence (M3) | --- | --- | --- | --- | --- | --- | --- | --- | --- | b3 | −.01 | .01 | .064 | |||
| R2 = .10 | R2 = .22 | R2 = .26 | R2 = .27 | |||||||||||||
| F(7, 134) = 2.22, p = .037 | F(8, 134) = 4.78, p< .001 | F(9, 132) = 5.15, p< .001 | F(10, 132) = 4.85, p< .001 | |||||||||||||
A subsequent model added A1C as an outcome, with its relationship with diabetes distress sequentially mediated by perceived control, self-efficacy and medication adherence (See Table 3, last column). The total effect of distress on A1C was significant and positive in this model (c = .21, p = .041). Perceived control was the only significant independent predictor of A1C. Self-efficacy was not independently associated with A1C and the independent effect for medication adherence fell short of significance. The only significant indirect path between distress and A1C was via perceived control (a1b1 = .055; 95%CI = .006, .164). There was no evidence that distress had a meaningful relationship with A1C after accounting for indirect effects (c' = .07, p = .518).
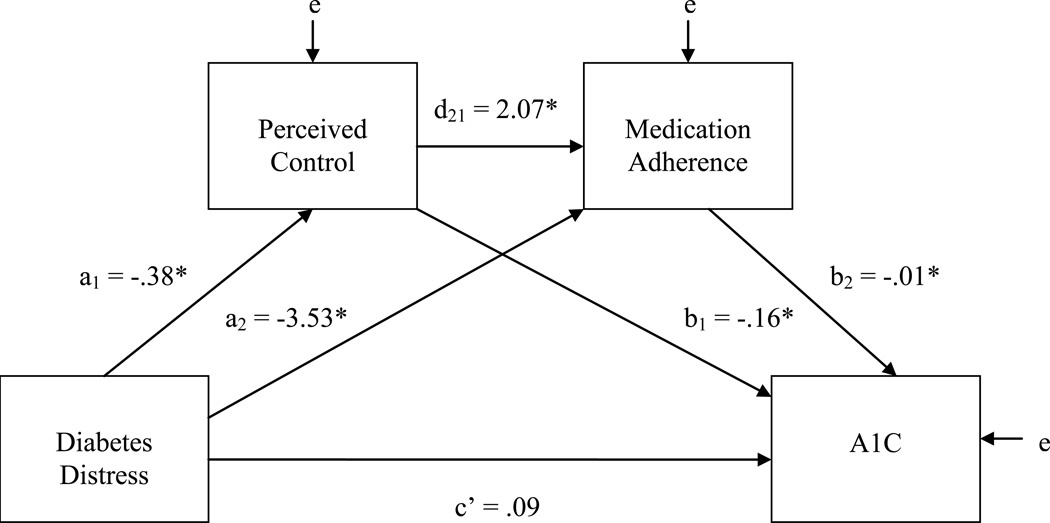
Because self-efficacy did not have significant independent relationships with the outcomes in the above models, we examined a final, more parsimonious model that excluded self-efficacy (See Figure 2 and Table 4). The total variance explained in medication adherence and A1C was not meaningfully reduced in these models (R2 = .243 and R2 = .264, respectively) when compared to models including self-efficacy (R2 = .260 and R2 = .270). Perceived control and medication adherence each had significant independent effects on A1C. Diabetes distress and perceived control were independently associated with increased medication adherence. Bootstrap confidence intervals showed that the indirect effect of diabetes distress through perceived control (a1b1 = .06) was significant (95%CI = .007, .168), as was the indirect effect through medication adherence (a2b2 = .04; 95%CI = .003, .127) and the sequentially mediated path through perceived control and medication adherence (a1d21b2 = .01; 95%CI = .0001, .048). Contrasts showed that the indirect effect through perceived control was significantly larger (95%CI = .002, .156) than the sequential indirect effect and was not significantly different (95%CI = −.069, .129) in magnitude from the indirect effect through medication adherence. There was no evidence of a direct effect of distress on A1C, independent of these indirect effects (c' = .09, p = .347).
Figure 2.
Final Path Model with Perceived Control and Medication Adherence as Sequential Mediators
Table 4.
Results from final path model excluding self-efficacy
| Perceived Control (M1) |
Medication Adherence (M2) |
A1c (Y2) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Coeff. | SE | p | Coeff. | SE | p | Coeff. | SE | p | |||
| Female Sex | −.52 | .44 | .242 | −8.87 | 3.89 | .024 | −.17 | .27 | .536 | |||
| Age | .03 | .02 | .228 | .46 | .21 | .034 | .02 | .01 | .147 | |||
| Years of Education | .01 | .07 | .918 | −1.48 | .59 | .013 | .08 | .04 | .055 | |||
| Diabetes Duration | −.00 | .03 | .917 | −.28 | .25 | .254 | .01 | .02 | .450 | |||
| Diabetes Complications | −.04 | .23 | .862 | −1.53 | 1.98 | .440 | .30 | .14 | .027 | |||
| Prescribed Insulin | −.63 | .45 | .163 | 2.18 | 3.98 | .585 | .42 | .27 | .120 | |||
| Antecedent Variables | ||||||||||||
| Diabetes Distress (X) | a1 | −.38 | .16 | .017 | a2 | −3.53 | 1.41 | .014 | c’ | .09 | .10 | .347 |
| Perceived Control (M1) | --- | --- | --- | d21 | 2.07 | .76 | .007 | b1 | −.16 | .05 | .004 | |
| Medication Adherence (M2) | --- | --- | --- | --- | --- | --- | b3 | −.01 | .01 | .043 | ||
| R2 = .10 | R2 = .24 | R2 = .26 | ||||||||||
| F(7, 134) = 2.22, p = .037 | F(8, 133)= 5.34, p< .001 | F(9, 132) = 4.91, p< .001 | ||||||||||
Discussion
The results of this study add to the small but important literature investigating pathways linking emotional distress to disease self-management and treatment outcomes. Our analyses revealed two main findings. First, emotional distress related to diabetes burden was independently associated with diabetes medication adherence and glycemic control. Depression severity, which was assessed by trained clinicians using a semi-structured interview, was not independently related to either diabetes outcome. Second, much of the relationship between diabetes distress, on the one hand, and self-management and glycemic control, on the other, was explained by perceived control over diabetes, suggesting a possible explanatory mechanism. Although self-efficacy was also evaluated as an independent mediator, based on previously reported evidence (Chao et al., 2005; Cherrington et al., 2010; Wagner et al., 2010), results were less supportive of this independent pathway. We discuss each of these findings in context and consider their implications below.
Although our analyses showed that both depression symptom severity and diabetes-related emotional distress were bivariately associated with medication adherence, only diabetes distress exhibited an independent relationship in multivariate analysis. Though previous studies have reported somewhat contradictory findings regarding these independent relationships, they used self-reports to evaluate depressive symptoms (Gonzalez et al., 2008b; Aikens, 2012; Fisher et al., 2010). Use of semi-structured clinical interviews, which allow for clarifying probes from a trained interviewer, is a strength of the current study. Our finding that diabetes distress was also significantly and independently associated with poorer glycemic control, whereas depressive symptoms were not, is consistent with recent evidence (Fisher et al. 2010, Aikens, 2012). Selection of the DDS emotional burden subscale for analysis, so as to avoid contamination between adherence and distress measures and to avoid the inclusion of items that are not specifically reflective of emotional distress due to diabetes (e.g., dissatisfaction with providers or support from family and friends), also differentiates our study from previous investigations (Fisher et al., 2007; Gonzalez et al., 2008b; Fisher et al., 2010; Aikens et al., 2012). Taken together, these results suggest an independent and robust relationship between diabetes-related emotional distress, treatment adherence and glycemic control among adults with type 2 diabetes.
Although self-efficacy for diabetes self-management and perceived control over diabetes were significantly correlated in our sample, they shared only about 12% of their variance and our results are in line with prior work that views these factors as independent constructs (Bandura & Wood, 1989; Skinner, 1996). Self-efficacy and perceived control were each significantly and independently associated with better medication adherence in multivariable analysis, where distress level was not controlled. However, only perceived control had a significant independent direct relationship with medication adherence and glycemic control in models including distress. A final model dropping self-efficacy as a mediator did not meaningfully reduce the amount of explained variance in A1C values. Taken together, these findings point to perceptions about one’s ability to control diabetes as an important explanatory mechanism between emotional distress and diabetes treatment outcomes. The relative importance of perceived control is consistent with theories of health behavior, such as the Common Sense Model of illness self-regulation, which emphasizes the importance of experiential evidence that health behavior action plans are having the anticipated effects (e.g., “Is this working?”) in shaping beliefs about illness, emotional reactions, and continued motivation for self-management (Leventhal et al., 1997).
Our results must be considered within the context of our cross-sectional design, which does not allow for definitive examinations of directionality or causal ordering. Although we have described direct and indirect effects of distress on adherence and glycemic control, this causal language is a convention of the statistical approach and should not be taken to imply that these cross-sectional analyses can address questions of directionality. Indeed, others have tested a reverse ordering with diabetes self-management influencing depression through self-efficacy (Sacco et al., 2007) and it is plausible that perceived control over diabetes could be influenced by knowledge of one’s actual glycemic control. Thus, although our findings highlight the importance of perceived control in the relationship between emotional distress and diabetes self-management, repeated measures designs and intervention studies are needed to better understand the sequence and causal nature of these relationships. By design, our participants, who were recruited based on their interest in a behavioral intervention for depression and diabetes self-management (Safren et al., 2013), reported higher levels of emotional distress than would be found in the general population of adults with type 2 diabetes. This facilitated our examination of a full range of emotional distress severity but may limit generalizability. Also, perceptions of self-efficacy and perceived control are domain specific (Bandura, 1982; Bandura & Wood, 1989) and though our measure of self-efficacy focused on diabetes self-management, it was not specific to medication taking. Furthermore, our rating of perceived control is useful in tapping into patients’ subjective sense of control but does not allow us to identify exactly what participants were thinking of when they rated their level of control over diabetes. This remains an important area for further investigation. Also, our evaluation of medication adherence with a validated self-rating shown to have an equivalent relationship to glycemic control, as compared to objective electronic medication monitoring data (Gonzalez et al., 2013), did not evaluate other aspects of diabetes self-management. We focused on medication adherence because all participants were prescribed medication for diabetes; other aspects of self-management could vary. However, it is important to acknowledge that diabetes self-management is more complex than what is suggested by our focus on medication adherence. Finally, BMI data was not available on the full sample and was not included as a covariate in the models. Although controlling BMI in the subset with complete data did not change our results, we would have preferred to have data on all participants to fully evaluate potential overlap with distress.
The current study evaluated a sequence where distress influences self-management and A1C through associated perceptions of control and self-efficacy. However, relationships between these factors are likely to be reciprocal over time and may involve other related constructs. Cognitive theory regards negative beliefs about oneself, one’s experiences and one’s future – beliefs arrived at through faulty information processing – as central features of depression (Beck, 1967; 1976). Experimental evidence suggests that even minor elevations in negative mood states can also affect judgments about risk (Johnson & Tversky, 1983), satisfaction with oneself (Schwarz & Clore, 1983), self-efficacy (Salovy & Birbaum, 1989) and standards for performance (Cervone, Kopp, Schaumann & Scott, 1994). Self-efficacy and perceived control, in turn, have long been recognized as important cognitive mediators of the impact of stressful experiences on adaptation and emotional distress (e.g., Litt, 1988).
Diabetes treatment regimens are often burdensome, with strict standards for health behaviors and glycemic targets. Often the feedback involved in monitoring symptoms, blood glucose readings, and A1C is confusing. Consideration of the above cognitive factors may improve our understanding of the vulnerabilities that lead many individuals with diabetes to experience significant emotional distress when facing the demands of diabetes self-management and the uncertainty involved in living with a life-threatening illness. They may also help better understand the processes by which distress is linked to diabetes self-management and treatment outcomes. Should the relationships identified here prove to be causal, they would suggest that interventions that go beyond confidence for implementing self-management behaviors and that specifically target perceptions of control over diabetes outcomes, such as control over blood glucose and risk of complications, may be particularly effective for distressed patients and may lead to improved treatment adherence and health outcomes. This possibility deserves further empirical evaluation in studies using longitudinal and experimental designs.
Acknowledgements
This project was supported by NIH Grant R01MH078571 (Principal Investigator: Steven A. Safren, PhD, ABPP). Dr. Gonzalez is partially supported by NIH Grants P60DK020541 and R18DK098742.
References
- Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care. 2012;35(12):2472–2478. doi: 10.2337/dc12-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycemic control in type 2 diabetes. Diabetes Care. 2009;32(7):1177–1181. doi: 10.2337/dc09-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy mechanism in human agency. American Psychologist. 1982;37:122–147. [Google Scholar]
- Bandura A, Wood R. Effect of perceived controllability and performance standards on self-regulation of complex decision making. Journal of Personality and Social Psychology. 1989;56(5):805–814. doi: 10.1037//0022-3514.56.5.805. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental and theoretical aspects. New York, NY: Harper and Row; 1967. [Google Scholar]
- Beck AT. Cognitive therapy and emotional disorders. Oxford, England: International Universities Press; 1976. [Google Scholar]
- Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26(10):2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. Journal of Psychosomatic Research. 2006;60(6):631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Cervone D, Kopp DA, Schaumann L, Scott WD. Mood, self-efficacy, and performance standards: Lower moods induce higher standards for performance. Journal of Personality and Social Psychology. 1994;67(3):499–512. [Google Scholar]
- Chao J, Nau DP, Aikens JE, Taylor SD. The mediating role of health beliefs in the relationship between depressive symptoms and medication adherence in persons with diabetes. Research in Social and Administrative Pharmacy. 2005;1(4):508–525. doi: 10.1016/j.sapharm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Cherrington A, Wallston KA, Rothman RL. Exploring the relationship between diabetes self-efficacy, depressive symptoms, and glycemic control among men and women with type 2 diabetes. Journal of Behavioral Medicine. 2010;33(1):81–89. doi: 10.1007/s10865-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC. Self-reported distress: analog or Ersatz depression? Psychological bulletin. 1994;116(1):29–45. doi: 10.1037/0033-2909.116.1.29. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, Dallosso HM, Daly H, Doherty Y, Eaton S, Fox C, Oliver L, Rantell K, Rayman G, Khunti K. Effectiveness of the diabetes education and self-management for ongoing and newly diagnosed programme for people with newly diagnosed type 2 diabetes: cluster randomized trial. British Medical Journal. 2008;336:1–11. doi: 10.1136/bmj.39474.922025.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo MR. Variations in Patients' Adherence to Medical Recommendations: A Quantitative Review of 50 Years of Research. Medical Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment—Meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Egede LE, Ellis C. The effects of depression on diabetes knowledge, diabetes self-management, and perceived control in indigent patients with type 2 diabetes. Diabetes Technology & Therapeutics. 2008;10(3):213–219. doi: 10.1089/dia.2007.0278. [DOI] [PubMed] [Google Scholar]
- Fisher L, Hessler D, Glasgow RE, Arean PA, Masharani U, Naranjo D, Strycker LA. REDEEM: A pragmatic trial to reduce diabetes distress. Diabetes care. 2013;36(9):2551–2558. doi: 10.2337/dc12-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Hessler DM, Polonsky WH, Mullan J. When is Diabetes Distress Clinically Meaningful? Establishing cut points for Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–264. doi: 10.2337/dc11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33(1):23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, Glasgow R, Laurencin G. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30(3):542–548. doi: 10.2337/dc06-1614. [DOI] [PubMed] [Google Scholar]
- Georgiades A, Zucker N, Friedman KE, Mosunic CJ, Applegate K, Lane JD, Feinglos MN, Surwit RS. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosomatic medicine. 2007;69(3):235–241. doi: 10.1097/PSY.0b013e318042588d. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Delahanty L, Safren S, Meigs J, Grant RW. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008c;51(10):1822–1825. doi: 10.1007/s00125-008-1113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care. 2011;34(1):236–239. doi: 10.2337/dc10-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, McCarl LA, Wexler DD, Cagliero E, Delahanty L, Soper TD, Goldman V, Knauz R, Safren SA. Cognitive behavioral therapy for adherence and depression (CBT-AD) in type 2 diabetes. Journal of Cognitive Psychotherapy. 2010;24(4):329–343. doi: 10.1891/0889-8391.24.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008a;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Safren S, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, Blais MA, Meigs JB, Grant RW. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30(9):2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Safren SA, Delahanty LM, Cagliero E, Wexler DJ, Meigs JB, Grant RW. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Medicine. 2008b;25(9):1102–1107. doi: 10.1111/j.1464-5491.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Schneider HE, Wexler DJ, Psaros C, Delahanty LM, Cagliero E, Safren SA. Validity of Medication Adherence Self-Reports in Adults With Type 2 Diabetes. Diabetes Care. 2013;36(4):831–837. doi: 10.2337/dc12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R, Adams AS, MahTrinacty C, Zhang F, Kleinman K, Soumerai SB, Meigs JB, Ross-Degnan D. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30(4):807–812. doi: 10.2337/dc06-2170. [DOI] [PubMed] [Google Scholar]
- Hampson SE, Glasgow RE, &Toobert DJ. Personal models of diabetes and their relation to self-care activities. Health Psychology. 1990;9:632–646. doi: 10.1037//0278-6133.9.5.632. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation and Conditional Process Analysis: A Regression Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of Medication Nonadherence on Hospitalization and Mortality Among Patients With Diabetes Mellitus. Archives of Internal Medicine. 2006;66(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Tversky A. Affect, generalization, and the perception of risk. Journal of personality and social psychology. 1983;45(1):20–31. [Google Scholar]
- Kavanagh DJ, Bower GH. Mood and self-efficacy: Impact of joy and sadness on perceived capabilities. Cognitive Therapy and Research. 1985;9(5):507–525. [Google Scholar]
- Keogh KM, Smith SM, White P, McGilloway S, Kelly A, Gibney J, O'Dowd T. Psychological Family Intervention for Poorly Controlled Type 2 Diabetes. American Journal of Managed Care. 2011;17(2):105–113. [PubMed] [Google Scholar]
- Leventhal H, Benyamini Y, Brownlee S, Diefenbach M, Leventhal EA, Patrick-Miller L, Robitaille C. Illness representations: Theoretical foundations. In: Petrie KJ, Weinman J, editors. Perceptions of health and illness, current research and applications. Amsterdam: Harwood Academic; 1997. pp. 19–45. [Google Scholar]
- Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM, Ludman EJ, Young BA, Williams LH, McCulloch DK, Van Korff M. Depression and advanced complications of diabetes: A prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD. Cognitive mediators of stressful experience: self-efficacy and perceived control. Cognitive Therapy and Research. 1988;12(3):241–260. [Google Scholar]
- Lorig K, Holman HR. Self-management education: History, definition, outcomes and mechanisms. Annals of Behavioral Medicine. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, Wilson IB. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behavior. 2008;12(1):86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and Poor Glycemic Control: A Meta-Analytic Review of the Literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Macrodimitris SD, Endler NS. Coping, control, and adjustment in Type 2 diabetes. Health Psychology. 2001;20(3):208–216. [PubMed] [Google Scholar]
- McSharry J, Moss-Morris R, Kendrick T. Illness perceptions and glycaemic control in diabetes: a systematic review with meta-analysis. Diabetic Medicine. 2011;28(11):1300–1310. doi: 10.1111/j.1464-5491.2011.03298.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Singer DE, Hurxthal K, Goodson JD. The Clinical Information Value of the Glycosylated Hemoglobin Assay. New England Journal of Medicine. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- Park M, Katon W, Wolf F. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. General Hospital Psychiatry. 2013;35:217–223. doi: 10.1016/j.genhosppsych.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, Schwartz CE. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments and Computers. 2004;36(4):717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Rubin R. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. The American Journal of Medicine. 2005;18(5S):27–34. doi: 10.1016/j.amjmed.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: The mediating role of diabetes symptoms and self-efficacy. Health Psychology. 2007;26:693–700. doi: 10.1037/0278-6133.26.6.693. [DOI] [PubMed] [Google Scholar]
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, Margolina AI, Cagliero E. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014;37(3):625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salovey P, Birnbaum D. Influence of mood on health-relevant cognitions. Journal of Personality and Social Psychology. 1989;57(3):539–551. doi: 10.1037//0022-3514.57.3.539. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Fisher L, Schillinger D. Is Self-Efficacy Associated with Self-Management Across Race/Ethnicity and Health Literacy? Diabetes Care. 2006;29(4):823–829. doi: 10.2337/diacare.29.04.06.dc05-1615. [DOI] [PubMed] [Google Scholar]
- Schwarz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45(3):513–523. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for the DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Skinner EA. A guide to constructs of control. Journal of Personality and Social Psychology. 1996;71(3):549–570. doi: 10.1037//0022-3514.71.3.549. [DOI] [PubMed] [Google Scholar]
- Skinner TC, Hampson SE. Personal Models of Diabetes in Relation to Self-Care, Well-Being, and Glycemic Control: A prospective study in adolescence. Diabetes Care. 2001;24(5):828–833. doi: 10.2337/diacare.24.5.828. [DOI] [PubMed] [Google Scholar]
- Stark Casagrande S, Ríos Burrows N, Geiss LS, Bainbridge KE, Fradkin JE, Cowie CC. Diabetes knowledge and its relationship with achieving treatment recommendations in a national sample of people with type 2 diabetes. Diabetes Care. 2013;35(7):1556–1565. doi: 10.2337/dc11-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- Wagner JA, Tennen H, Osborn CY. Lifetime depression and diabetes self-management in women with Type 2 diabetes: a case–control study. Diabetic Medicine. 2010;27(6):713–717. doi: 10.1111/j.1464-5491.2010.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WF, Bower GH. Mood effects on subjective probability assessment. Organizational behavior and human decision processes. 1992;52(2):276–291. [Google Scholar]