Abstract
Orbital meningiomas can be classified as primary optic nerve sheath (ON) meningiomas, primary intraorbital ectopic (Ob) meningiomas and spheno‐orbital (Sph‐Ob) meningiomas based on anatomic site. Single‐nucleotide polymorphism (SNP)‐based array analysis with the Illumina 300K platform was performed on formalin‐fixed, paraffin‐embedded tissue from 19 orbital meningiomas (5 ON, 4 Ob and 10 Sph‐Ob meningiomas). Tumors were World Health Organization (WHO) grade I except for two grade II meningiomas, and one was NF2‐associated. We found genomic alterations in 68% (13 of 19) of orbital meningiomas. Sph‐Ob tumors frequently exhibited monosomy 22/22q loss (70%; 7/10) and deletion of chromosome 1p, 6q and 19p (50% each; 5/10). Among genetic alterations, loss of chromosome 1p and 6q were more frequent in clinically progressive tumors. Chromosome 22q loss also was detected in the majority of Ob meningiomas (75%; 3/4) but was infrequent in ON meningiomas (20%; 1/5). In general, Ob tumors had fewer chromosome alterations than Sph‐Ob and ON tumors. Unlike Sph‐Ob meningiomas, most of the Ob and ON meningiomas did not progress even after incomplete excision, although follow‐up was limited in some cases. Our study suggests that ON, Ob and Sph‐Ob meningiomas are three molecularly distinct entities. Our results also suggest that molecular subclassification may have prognostic implications.
Keywords: chromosome 22, cytogenetics, NF2, orbital meningioma, optic nerve sheath, SNP array
Introduction
Meningiomas account for approximately 4% of intraorbital tumors 37. Based on their origin and location, orbital meningiomas can be subclassified as primary optic nerve sheath (ON) meningiomas 23, primary intraorbital ectopic(Ob) meningiomas 13 and secondary lesions extending from an intracranial sphenoid wing component (Sph‐Ob) 23. One study of orbital tumors showed that 29 of 1264 (2%) cases in their cohort were ON meningiomas, whereas 24 cases (2%) were secondary Sph‐Ob meningiomas 23. Ob meningiomas, on the contrary, are extremely rare 31. Using a PubMed search, we identified approximately a dozen cases in the literature.
ON meningiomas are believed to arise from the arachnoid lining of the intraorbital or intracanalicular segments of the optic nerve 19. They commonly affect middle‐aged women but may occur in adult men and in both male and female children. Most of these tumors are unilateral; however, bilateral tumors may occur in rare instances, a subset of these in patients with neurofibromatosis type 2 (NF2) 4, 10. The characteristic radiologic features of ON meningiomas include calcifications surrounding the nerve, tubular enlargement of the entire nerve within the orbit or bulbous enlargement of the nerve at the apex with a more proximal tubular enlargement. On contrast‐enhanced axial computed tomography (CT) or T1‐weighted magnetic resonance (MR) imaging, the optic nerve usually is hypointense compared with the enhancing meningioma, producing a “tram‐track” appearance 21. The majority of ON meningiomas are World Health Organization (WHO) grade I, with the meningothelial and transitional histologic subtypes being the two most common 19. Current treatment options for ON meningiomas are no longer limited to surgical removal of the affected optic nerve or observation without surgery. Stereotactic fractionated radiotherapy has shown effectiveness in preserving visual function with good short‐ and long‐term prognosis and minimal morbidity 2, 27, 34.
Rarely, an orbital meningioma may arise without any connection with the optic nerve sheath or optic canal, and also lack an intracranial component. These tumors have been classified as ectopic (Ob) meningiomas. They frequently are located at the superonasal orbit rim or in the medial orbital wall 13. Different from ON meningiomas, the majority of Ob meningiomas occur in men 31. Histologic examination of a handful of Ob meningiomas revealed that most cases were WHO grade I, meningothelial type 13, 31, 34, 49. The short‐term prognosis appeared favorable: limited follow‐up of the patients for 0–42 months showed no recurrence after surgical excision 13, but no long‐term assessments have been performed in these patients.
Secondary orbital meningiomas originate intracranially, usually in the region of the planum sphenoidale or tuberculum sellae. These tumors, which gradually extend into the orbit via the optic canal 23, superior orbital fissure or both, and may infiltrate the sphenoid bone and extend into the orbit from the bone itself, are known as spheno‐orbital (Sph‐Ob) meningiomas. For some cases, it can be difficult to determine if the tumor has arisen in the optic canal and then spread intracranially, or involves the orbit from extension from the intracranial cavity. In terms of the histologic subtypes, the overall distribution of Sph‐Ob meningioma is similar to that of ON meningiomas, with the meningothelial and transitional variants accounting for essentially all cases. It should be noted that meningiomas affecting the orbit in particular are very challenging to treat. Extent of resection and surgical expertise represent significant factors affecting prognosis, quality of life and outcomes. Surgery is the standard of care to prevent the tumor from invading adjacent crucial intracranial structures. However, because of frequent extensive involvement of the sphenoid wing, orbit and cavernous sinus, complete resection can be difficult if not impossible. Postoperative radiation therapy often is recommended for incompletely excised tumors to minimize progression and recurrence 23.
ON meningiomas, Ob meningiomas and Sph‐Ob meningiomas are morphologically indistinguishable (Figure 1). Despite their morphologic similarity, these tumors may have different clinical or molecular features. For example, Stafford and colleagues found a higher recurrence rate associated with anterior visual pathway meningiomas 42; however, as the cases in their study were not subclassified based on tumor location, it is difficult to know if all subtypes of orbital meningioma had a similar outcome.
Figure 1.
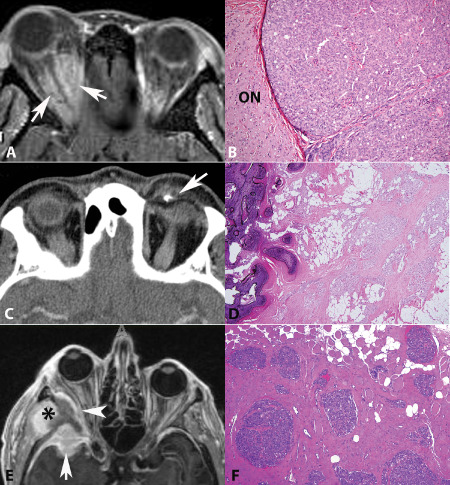
Three subtypes of orbital meningiomas. A. Optic nerve sheath meningiomas form intraorbital masses in close relation to optic nerve (arrows) (axial T1‐weighted MRI post‐contrast). B. Intimate association with optic nerve may be demonstrated on histologic sections (H&E). Intraorbital ectopic meningiomas are unassociated with nerve. A small focus of ossification was present in this example on (C) CT scan (arrow) and (D) H&E. E. Spheno‐orbital meningiomas are characterized by intracranial (arrow) and intraorbital (arrowhead) components. Hyperostosis of the posterior lateral wall and lateral sphenoid, and tumor extension to the subtemporal area is also present (asterisk) (F). This example infiltrated surrounding soft tissues, including skeletal muscle.
To elucidate molecular mechanisms underlying tumorigenesis, several studies have attempted to determine the genetic alterations underlying intracranial meningiomas 9, 12, 29, 30, 33, 36, 44, including high‐resolution single‐nucleotide polymorphism (SNP) arrays 15, 17, 45. Monosomy 22 and deletion of chromosome 22q are the most common cytogenetic abnormalities in these tumors, present in 60%–80% of cases 12, 36. The vast majority of the deleted regions include the NF2 gene 33. These findings, along with the fact that ∼50% of NF2 patients develop meningiomas 40, suggest that NF2 inactivation may lead to meningioma formation. Meningiomas with NF2 gene inactivation have been reported to be most commonly of the fibroblastic and transitional subtypes 48. In addition to NF2 mutation or loss, a number of chromosome alterations including loss of 1p, 6q, 10, 14q and 18q, and gain of 1q, 9q, 12q, 15q, 17q and 20q have been linked to tumor progression and a high‐grade histology 6, 20, 26, 30, 47. Recent whole exome sequencing studies have shown that most non‐NF2 meningiomas carry a different set of genetic alterations, including mutations in TRAF7, KLF4, AKT1 and SMO 5, 8, 32, 35. Multiple meningiomas also arise rarely in families and patients with germline mutations in SMARCB1, SMARCE1 and SUFU 1, 3, 7, 25, 41.
In contrast to the progress made in elucidating the pathogenesis of intracranial meningiomas, the molecular genetics of orbital meningiomas remains largely unknown. Therefore, we performed SNP arrays in archival material obtained from orbital meningiomas in an attempt to detect genomic alterations in these tumors, including copy number changes and copy neutral loss of heterozygosity, which may be associated with clinical features and outcome.
Materials and Methods
Patients and tumor samples
Formalin‐fixed paraffin‐embedded (FFPE) tissue (10 Sph‐Ob, 4 Ob and 5 ON meningiomas) was retrieved from the Surgical Pathology archives of The Johns Hopkins Hospital, Mayo Clinic and University of Manitoba over a 20‐year period (1992–2012). A total of 19 tumors (10 primary and 9 recurrent) (of 29) with sufficient tissue were successfully studied. Eighteen cases were sporadic, whereas one case developed in a patient with well‐documented NF2. The majority of the cases were WHO grade I tumors except for two grade II Sph‐Ob meningiomas. The Sph‐Ob meningioma group included tissue from four men and six women between age 28 and 75 (median = 53). The Ob meningioma group consisted of tissue from two men and two women ranging in age from 53 to 92 (median 70). The ON meningiomas group included tissue from four men and one woman between age 24 and 69 (median = 46). Four patients underwent a gross total resection (GTR) of the tumor, whereas 12 patients had a subtotal resection (STR). Information regarding the surgical procedure was not available for the three remaining patients. The clinical follow‐up ranged from 0 month to 21 years (median = 36 months). Two patients died from their tumor during follow‐up. Detailed demographics and pertinent clinical information are summarized in Table 1. Histologic diagnosis and grading of each tumor were confirmed by at least two neuropathologists (CGE, CH or FJR) before tumor tissue was selected for analysis.
Table 1.
Summary of demographics and clinico‐pathological information of 19 orbital meningioma cases. Abbreviations: GTR = gross total resection; NOS = extent of resection not specified; Ob = intraorbital ectopic meningiomas; ON = optic nerve sheath meningiomas; RFS = recurrence/progression‐free survival; Sph‐Ob = spheno‐orbital meningiomas; STR = subtotal resection
| Case No. | Type | Age/Sex | WHO Grade | Primary (P)/Recurrent (R) | Surgery | Previous treatment | Progression, recurrence or death | RFS (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Sph‐Ob | 42F | I | R | STR | — | + | 25b |
| 2 | Sph‐Ob | 46F | I | P | GTR | — | + | 16b |
| 3 | Sph‐Ob | 64M | I | R | STR | Irradiation | − | 50b |
| 4 | Sph‐Ob | 64F | I | R | STR | — | − | 36b |
| 5 | Sph‐Ob | 49F | II | R | STR | — | + | 6b |
| 6 | Sph‐Ob | 57M | I | R | GTR | Irradiation | + | 36b |
| 7 | Sph‐Oba | 28M | I | R | STR | Gamma Knife | + (Death) | 66b |
| 8 | Sph‐Ob | 69F | I | P | STR | — | − | 85b |
| 9 | Sph‐Ob | 31F | I | P | GTR | — | + | 42b |
| 10 | Sph‐Ob | 75M | II | R | GTR | — | + (Death) | 1 |
| 11 | ON | 46F | I | P | STR | — | − | 60b |
| 12 | ON | 32M | I | R | STR | — | − | 235b |
| 13 | ON | 69M | I | P | NOS | — | − | ? |
| 14 | ON | 24M | I | P | STR | — | − | 3 |
| 15 | ON | 55M | I | P | STR | — | − | 2 |
| 16 | Ob | 53M | I | R | STR | Irradiation | − | 40b |
| 17 | Ob | 80F | I | P | NOS | — | − | ? |
| 18 | Ob | 92M | I | P | Autopsy | — | − | Incidental |
| 19 | Ob | 59M | I | P | STR | — | − | 2 |
Confirmed diagnosis of NF2.
Duration of follow‐up >36 months.
Molecular analysis
Targeted tissues from 2–10 unstained, 10‐μm‐thick microscopic sections were isolated using Pinpoint reagents (ZymoResearch, Orange, CA, USA). DNA was extracted from the recovered tissue using QIAmp DNA Mini Kit (Qiagen, Valencia, CA, USA), and quantified by OD at 260 nm using the NanoDrop ND‐1000 (NanoDrop Technologies, Inc., Wilmington, DE, USA). To ensure the quality of the DNA for SNP array analysis, the extracted DNA was subjected to the Infinium HD FFPE DNA Restore Kit (Illumina Inc., San Diego, CA, USA). Up to 200 ng DNA per sample was used for the SNP analysis.
The SNP array analysis was performed on the Illumina Infinium II SNP array with 300K markers (HumanCytoSNP_FFPE‐12, Illumina Inc.) as previously described 16. The B allele frequency (BAF) and log R ratio (LRR) data were analyzed using Illumina KaryoStudio software version 2.0 and CNV (copy number variation) partition V2.4.4.0. BAF represents the frequency of B alleles at a given SNP. LRR is the ratio between the observed and the expected probe intensity, thus indicating copy number. Deviation of LRR (log R Dev) was used to assess noise of the SNP array data. The chi‐square test and paired Student's t‐test were used to evaluate statistical significance.
Results
By SNP array analysis, we found chromosomal alterations in 68% (13 of 19) of all orbital meningioma cases included in this study. Chromosomal alterations in the study group are summarized in Figure 2. It should be noted that, in general, data obtained from FFPE specimens are noisier than data from fresh preparations; therefore, it was not always possible for us to unequivocally interpret the observed chromosome abnormalities whether representing copy number change or alternatively copy neutral loss of heterozygosity. Monosomy 22 or partial deletion of chromosome 22q arm were the most common chromosomal abnormalities, seen in 58% (11 of 19) of the cases. The majority of the deleted regions in chromosome 22 were from q11.2 to the end of the long arm (q), accounting for almost the entire long arm and spanning the NF2 gene locus, located at chromosome region 22q12.2. The breakpoint at chromosome 22 in orbital meningiomas was similar to what has been reported for intracranial meningiomas 12, 33, 36. Other frequent cytogenetic abnormalities included partial deletions or CN‐LOH of chromosome 19p, 1p, 4p and 6q, which were detected in 37% (7 of 19), 32% (6 of 19), 26% (5 of 19) and 26% (5 of 19) of the total cases, respectively (Tables 2 and 3).
Figure 2.
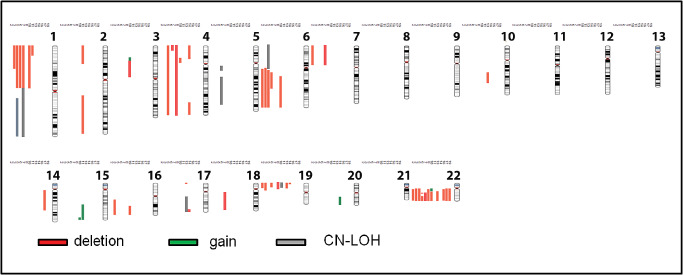
Chromosome map summarizing the genomic alterations in orbital meningiomas by case. Abnormalities are listed sequentially by case number to the left of the respective chromosomes. Only cases with abnormalities are illustrated (2, 3, 5, 6, 7, 8, 10, 11, 13, 14, 16, 17, 19). Gains are illustrated in green, deletions in red and copy neutral loss of heterozygosity (LOH) in gray.
Table 2.
Summary of genomic alterations in orbital meningiomas detected by single‐nucleotide polymorphism arrays. Abbreviations: Ob = intraorbital ectopic meningiomas; ON = optic nerve sheath meningiomas; Sph‐Ob = spheno‐orbital meningiomas
| Case No. | Type | Age | Chromosome abnormalities | Approximate size (Mb) | Total No. of alterations |
|---|---|---|---|---|---|
| 1 | Sph‐Ob | 42 | None | 0 | |
| 2 | Sph‐Ob | 46 | Loss (6)(q12qter) | 106 | 4 |
| 47 | Loss (7)(pterp11.2) | 54 | |||
| Loss (19)(pterp13.11) | 16 | ||||
| Loss (22)(q11.21qter) | 33 | ||||
| 3 | Sph‐Ob | 64 | Loss (1)(pterp31.3) | 64 | 5 |
| Loss (6)(q11.1qter) | 109 | ||||
| Loss (16)(q12qter) | 43 | ||||
| Loss (19)(pterp12) | 22 | ||||
| Loss (22)(q11.1qter) | 35 | ||||
| 4 | Sph‐Ob | 64 | None | 0 | |
| 5 | Sph‐Ob | 49 | Loss (1)(pterp13.1) | 117 | 6 |
| CN‐LOH (1)(q21.1qter) | 105 | ||||
| Monosomy 4 | |||||
| CN‐LOH (6)(pterq12) | 68 | ||||
| Loss (6)(q12qter) | 103 | ||||
| Monosomy 22 | |||||
| 6 | Sph‐Ob | 57 | Loss (1)(pterp13.1) | 117 | 8 |
| Loss (4)(pterp16.1) | 10 | ||||
| CN‐LOH (5)(q11.2q13.1) | 18 | ||||
| CN‐LOH (5)(q14.1q34) | 86 | ||||
| Loss (6)(q13q22.31) | 46 | ||||
| Loss (19)(pterp13.2) | 13 | ||||
| Loss (22)(q12.1q12.2) | 3 | ||||
| Loss (22)(q13.1qter) | 14 | ||||
| 7 | Sph‐Ob | 28 | Loss (1)(pterp12) | 118 | 6 |
| CN‐LOH (1)(p12qter) | 130 | ||||
| Monosomy 4 | |||||
| Loss (7)(pterp12.1) | 53 | ||||
| Loss (18)(q11.2qter) | 59 | ||||
| Loss (22)(q12.1qter) | 22 | ||||
| 8 | Sph‐Ob | 69 | Loss (19)(pterp12) | 25 | 2 |
| Loss (22)(q11.1qter) | 33 | ||||
| 9 | Sph‐Ob | 31 | None | 0 | |
| 10 | Sph‐Ob | 75 | Loss (1)(pterp13.1) | 118 | 10 |
| Gain (3)(p22.3p22.1) | 10 | ||||
| Loss (3)(p22.1p11.1) | 44 | ||||
| Loss (4)(p15.1p11) | 15 | ||||
| Loss (6)(q14.2qter) | 86 | ||||
| Gain (15)(q26.2qter) | 7 | ||||
| Loss (16)(q21qter) | 26 | ||||
| CN‐LOH (19)(pterp13.11) | 18 | ||||
| Gain (22)(q11.1q11.23) | 8 | ||||
| Loss (22)(q11.23qter) | 27 | ||||
| 11 | ON | 46 | Loss (1)(pterp35) | 29 | 4 |
| Loss (2)(pterp16.3) | 51 | ||||
| Loss (2)(q22.1qter) | 106 | ||||
| Gain (15)(q22.2qter) | 43 | ||||
| 12 | ON | 32 | None | 0 | |
| 13 | ON | 69 | Loss (10)(q22.1q24.32) | 30 | 5 |
| Loss or CN‐LOH(17) (pterp13.2) | 4 | ||||
| CN‐LOH(17)(q12qter) | 47 | ||||
| Loss (19)(pterp13.11) | 16 | ||||
| Loss (22)(q11.22qter) | 27 | ||||
| 14 | ON | 24 | Loss (4)(pterp14) | 38 | 5 |
| Loss (4)(q32.1qter) | 35 | ||||
| Loss (17)(q25.1qter) | 7 | ||||
| Loss (19)(pterp13.3) | 4 | ||||
| Gain (20)(q12qter) | 24 | ||||
| 15 | ON | 55 | None | 0 | |
| 16 | Ob | 53 | Loss (22)(q11.21qter) | 33 | 1 |
| 17 | Ob | 80 | Loss (14)(q11.2q24.3) | 56 | 2 |
| Monosomy 22 | |||||
| 18 | Ob | 92 | None | 0 | |
| 19 | Ob | 59 | Monosomy 22 | 51 | 1 |
Table 3.
Common genomic alterations in orbital meningiomas. Abbreviations: Ob = intraorbital ectopic meningiomas; ON = optic nerve sheath meningiomas; Sph‐Ob = spheno‐orbital meningiomas
| Genomic alteration | No. of cases | Percentage (%) | ||||
|---|---|---|---|---|---|---|
| Sph‐Ob | ON | Ob | Sph‐Ob | ON | Ob | |
| del(22q) or −22 | 7/10 | 1/5 | 3/4 | 70 | 20 | 75 |
| del(1p) | 5/10 | 1/5a | 0/4 | 50 | 20a | 0 |
| del(6q) | 5/10 | 0/5 | 0/4 | 50 | 0 | 0 |
| del(19p) | 5/10 | 2/5 | 0/4 | 50 | 40 | 0 |
The deleted region on chromosome 1p in this particular case is different from those detected in Sph‐Ob meningiomas (see Table 2).
Among the various chromosomal alterations detected in orbital meningiomas, loss of 1p and loss of 6q have been shown to correlate with progression, recurrence and poor prognosis in intracranial meningiomas 6, 18, 24, 30, 39, 47. Consistent with the literature, 67% (four of six) of the deleted 1p cases and 80% (four of five) of the deleted 6q cases in our cohort were associated with tumor progression, recurrence or tumor‐related death. Of note, deletion of chromosome arm 6q was present exclusively in the Sph‐Ob meningioma group. Although one case of ON meningioma (case 11) demonstrated partial loss of chromosome 1p, the deleted region was much smaller than the one detected in Sph‐Ob meningiomas. The deletion also did not include the tumor suppressor locus on 1p34 that consistently was deleted in cranial meningiomas with a poor clinical outcome 43.
The majority of our cases carrying the chromosomal aberrations described earlier were WHO grade I tumors, suggesting that these cytogenetic abnormalities may be used as prognostic markers for histologically benign orbital meningiomas. In contrast, loss of 10q and 14q, the allelic abnormalities detected in high‐grade intracranial meningiomas 6, 39, was infrequent in our cohort and irrelevant to disease progression in orbital meningiomas.
Spheno‐orbital meningiomas
Chromosome abnormalities were detected in 70% (7 of 10) of the Sph‐Ob meningiomas. The majority of the tumors (60%; 6 of 10) had more than three large (>3 Mb) chromosomal numerical alterations (mean = four alterations per case). Sph‐Ob meningiomas frequently demonstrated alterations in chromosome 22 (monosomy 22 or deletion of 22q) (70%; 7 of 10), along with other chromosome anomalies. Partial deletion, or CN‐LOH of chromosomes 1p, 6q or 19p each was present in half (5 of 10) of the cases. Chromosome changes appeared to occur early, based on our observation of one case (case 2) in which identical chromosome abnormalities were detected in both the primary tumor and the recurrent tumor two years later.
There was a NF2 patient in our cohort (case 7) who suffered multiple recurrences of a Sph‐Ob meningioma in 8 years and eventually succumbed to the disease. SNP analysis of the tumor revealed deletion of chromosome arms 1p, 7p, 18q and 22q, CN‐LOH of 1q and monosomy 4.
Primary intraorbital ectopic and optic nerve sheath meningiomas
SNP array analysis was successfully performed on five ON meningiomas and four primary Ob meningiomas. The numbers of ON and Ob meningioma cases included in this study were less than Sph‐Ob meningiomas because of the rarity of these subgroups 13, 31 and the poor quality of DNA recovered from several old archival specimens, which did not allow interpretation of the SNP array data. Similar to Sph‐Ob meningiomas, however, loss of chromosome 22/22q was detected in the majority of the Ob meningioma cases (75%; three of four) (Figure 3A). Compared with Sph‐Ob and ON meningiomas, one characteristic molecular feature of Ob meningiomas was the almost absence of other abnormalities besides monosomy or deletion of chromosome 22 (mean = one alteration per case). Non‐chromosome 22 alterations were substantially less common in Ob meningiomas (mean = 0.25 alteration per case) than in Sph‐Ob and ON meningiomas (mean = 3.2 and 2.6 alterations per case, respectively).
Figure 3.
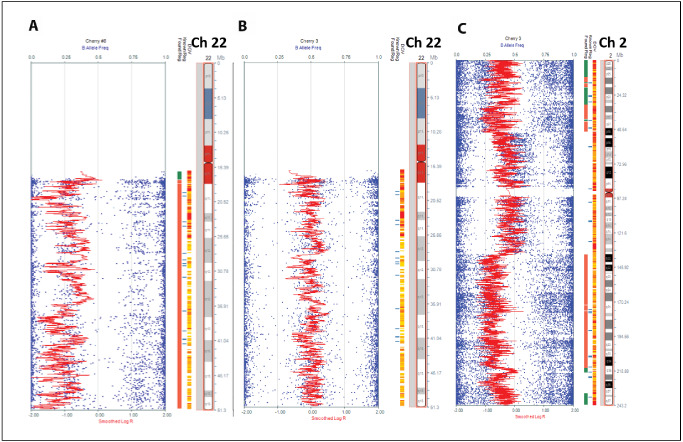
Orbital meningioma subtypes have different cytogenetic abnormalities. Chromosome 22 loss was frequent in ectopic orbital meningiomas (A) but rare in meningiomas of the optic nerve proper which usually had no alterations in Ch 22 (B). Conversely, optic nerve meningiomas contained other less frequent abnormalities. In this example alterations in chromosome 2 (del 2pter‐p16.3, del 2q22.1‐qter) are shown (C).
ON meningiomas, in contrast, had a number of chromosomal alterations (mean = three alterations per case); however, chromosome 22 abnormality, the most common cytogenetic alteration in Sph‐Ob and Ob meningiomas, was infrequent in ON meningiomas (20%; one of five) (Figure 3B). Interestingly, the non‐chromosome 22 alterations detected in ON meningiomas, including chromosome 2, 10, 17 and 20 abnormalities (Table 2), were relatively uncommon in intracranial 29, 30, 47 meningiomas and in our Sph‐Ob meningiomas. Candidate genes present in these regions, some of which encoding for known tumor suppressors, include EML4, MSH2 (Ch 2p); RPRM, GALNT13, KCNJ3 (Ch 2q) and PTEN (Ch10q) 15, 17, 28, 45. Conversely, recurrent alterations in loci previously suspected of participating in meningioma development and/or progression were absent in ON, including MUTYH, PRDX1, FOXD2, FOXE3, PTCH2, RAD54L (1p); CTGF, TREM30A, SESN1 (Ch 6q); and NDRG2, TMEM30B (Ch 14q) 28, 43. These findings suggest that ON meningiomas might be a distinct subset from NF2‐related intracranial and Sph‐Ob meningiomas. Frequent chromosome alterations (genomic instability) also distinguished ON meningiomas from the genetically stable, skull base non‐NF2 meningiomas that have been reported in the literature 8.
Clinical follow‐up and outcome
In terms of relapse rate, 70% (7 of 10) of patients with Sph‐Ob developed progression/recurrent tumors, whereas none of the ON or Ob cases progressed after the time of the current surgery, although clinical follow‐up on the latter two subgroups was particularly limited. Two disease‐related deaths occurred in the Sph‐Ob group during follow‐up. The disease‐free interval or survival for Sph‐Ob meningioma cases with more than 3 years of follow‐up was 36.3 months (10 cases), compared with 142.5 months in ON meningiomas (2 cases). One patient with an ON meningioma has been progression‐free for almost 20 years despite STR and no further treatment of the tumor (case 12).
Unlike Sph‐Ob meningiomas, none of the Ob or ON meningioma cases progressed following STR with no further treatment, although follow‐up >36 months was available for only a total of three patients in these groups. Loss of chromosome 6q, an indicator of progression or recurrence in intracranial 27, 47 and Sph‐Ob meningiomas, was absent in ON and Ob meningiomas.
Discussion
Orbital meningiomas are predominantly indolent neoplasms that can be further classified into primary Ob, ON and Sph‐Ob meningiomas by location and putative origin of the tumors (Figure 4). In contrast to the first two categories that are regarded as primary orbital neoplasms, Sph‐Ob meningiomas are secondary tumors spreading from an intracranial primary component. Regardless of the subtype, the majority of the cases reported were WHO grade I 19. Although GTR is the treatment of choice, surrounding anatomic structures and persistent hyperostosis make it difficult, if not impossible, to completely remove the tumor 11, 22, 38, 46. In addition, aggressive surgical removal or high‐dose radiation therapy may lead to visual deterioration, neurologic morbidity or both 11, 38.
Figure 4.
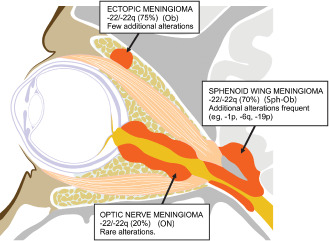
Graphic illustrating the three subtypes of orbital meningiomas at different anatomic sites. Optic nerve sheath (ON) meningioma; intraorbital ectopic (Ob) meningioma; spheno‐orbital (Sph‐Ob) meningioma.
It is unclear from prior studies if subtypes of orbital meningiomas are distinctive clinico‐pathological entities or are merely the same type of tumor occurring at different locations. Recent advances in genomic analysis, including techniques applicable to formalin‐fixed archival material, now make it possible to analyze the genetic makeup of relatively rare tumors to address this issue. Two large‐scale genomic studies of intracranial meningiomas not only identified new molecular players in tumorigenesis 5, 8 but also linked tumor genetics to anatomic locations. Clark and colleagues reported that meningiomas with mutant NF2 and/or chromosome 22 loss were more likely to be atypical and localize to the cerebral surface, cerebellum and lateral skull base, whereas the non‐NF2 meningiomas nearly always were benign and involved the skull base 8. In our study, the tumors arising from the skull base, that is, the Sph‐Ob meningiomas, showed chromosome 22q loss and a more aggressive clinical course despite apparent complete excision in some cases. Genomic instability, a feature of NF2‐related meningiomas reported by Clark et al., was also commonly seen in our Sph‐Ob meningioma group and was manifested by frequent chromosome alterations. Taken together, Sph‐Ob meningiomas in our study are more closely related both clinically and genomically to lateral skull base than to medial skull base meningiomas. One caveat to have in mind is that a large proportion of Sph‐Ob meningiomas in our group were recurrent/progressive tumors and had more extended follow‐up compared with the ON and Ob tumors
Only one WHO grade I meningioma in our study developed in a NF2 patient. Interestingly, in addition to Ch 22q loss, this tumor had additional losses in chromosomes 1, 4, 7 and 18. In a prior study of NF2‐associated meningiomas, such extent of genomic instability was associated with higher grade tumors (II and III) 14. This patient with NF2 in our study received gamma knife therapy for his tumor, which could have played a role in the development of these additional alterations. However, this tumor behaved aggressively, with multiple recurrences and eventually leading to patient's death, raising the possibility that the tumor was intrinsically more aggressive that reflected on its histologic grade.
In contrast to Sph‐Ob meningiomas, the tumors originating from the optic canal, that is, the ON meningiomas, appeared to share genetic and clinical similarities to the non‐NF2, medial skull base meningiomas described in Clark et al.'s report: the majority of the tumors had an intact chromosome 22 and had a favorable clinical course on available follow‐up.
We also included in our study a third group of orbital meningioma—primary Ob meningiomas—that were not connected to the optic nerve sheath and did not have an intracranial component. The majority had chromosome 22q loss, yet did not show genomic instability. We hypothesize that these tumors are either molecularly distinct from typical NF2‐related meningiomas or represent early stage NF2 tumors before genomic instability occurs. Further analysis of tumors in this group may shed light on the initiating events of genomic instability in NF2‐related meningiomas. Given the rarity of these tumors, and the often scant material obtained during surgical procedures at these sites, archival FFPE tissue may be the only material available for study. It is possible that the quality of DNA extracted from FFPE may affect interpretation in individual cases. In this context, small genomic alterations could be missed, and it may not always be possible to distinguish areas of deletion from copy neutral‐LOH. We attempted to minimize these problems by excluding tumors with insufficient or degraded DNA affecting data interpretation, as reflected by noise and waviness in LRR lines (n = 10). Many of these were older samples. However, our study highlights the potential of using archival formalin‐fixed tissue to identify broad genetic changes of rare tumor subtypes, with biologic and/or clinical implications.
In summary, our results indicate that the three subgroups of orbital meningiomas—primary ON, primary Ob and Sph‐Ob meningiomas—all have distinct clinical and molecular features. Our study highlights a potential role of molecular analysis in management of orbital meningiomas. Tumors with intact chromosome 22 (non‐NF2) or a simple loss of chromosome 22q, without many additional alterations, appear to have a more favorable course in our study, with the caveat that follow‐up was necessarily limited given the rarity of these tumors. This question would be addressed by future studies, including larger datasets with exhaustive clinical data. Further prospective studies in particular should clarify the significance of these findings and applicability in clinical decision making may be worthwhile.
Acknowledgments
The authors thank Kerry Morris for helping with the preparation of Figure 2 and Sharon Blackburn for Figure 4. This work was supported in part by the Knights Templar Eye Foundation and the Childhood Brain Tumor Foundation (to FJR).
References
- 1. Aavikko M, Li SP, Saarinen S, Alhopuro P, Kaasinen E, Morgunova E et al (2012) Loss of SUFU function in familial multiple meningioma. Am J Hum Genet 91:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews DW, Faroozan R, Yang BP, Hudes RS, Werner‐Wasik M, Kim SM et al (2002) Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery 51:890–902, discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 3. Bacci C, Sestini R, Provenzano A, Paganini I, Mancini I, Porfirio B et al (2010) Schwannomatosis associated with multiple meningiomas due to a familial SMARCB1 mutation. Neurogenetics 11:73–80. [DOI] [PubMed] [Google Scholar]
- 4. Bosch MM, Wichmann WW, Boltshauser E, Landau K (2006) Optic nerve sheath meningiomas in patients with neurofibromatosis type 2. Arch Ophthalmol 124:379–385. [DOI] [PubMed] [Google Scholar]
- 5. Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G et al (2013) Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai DX, Banerjee R, Scheithauer BW, Lohse CM, Kleinschmidt‐Demasters BK, Perry A (2001) Chromosome 1p and 14q FISH analysis in clinicopathologic subsets of meningioma: diagnostic and prognostic implications. J Neuropathol Exp Neurol 60:628–636. [DOI] [PubMed] [Google Scholar]
- 7. Christiaans I, Kenter SB, Brink HC, van Os TA, Baas F, van den Munckhof P et al (2011) Germline SMARCB1 mutation and somatic NF2 mutations in familial multiple meningiomas. J Med Genet 48:93–97. [DOI] [PubMed] [Google Scholar]
- 8. Clark VE, Erson‐Omay EZ, Serin A, Yin J, Cotney J, Ozduman K et al (2013) Genomic analysis of non‐NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins VP, Nordenskjold M, Dumanski JP (1990) The molecular genetics of meningiomas. Brain Pathology 1:19–24. [DOI] [PubMed] [Google Scholar]
- 10. Cunliffe IA, Moffat DA, Hardy DG, Moore AT (1992) Bilateral optic nerve sheath meningiomas in a patient with neurofibromatosis type 2. Br J Ophthalmol 76:310–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeMonte F, Smith HK, Al‐Mefty O (1994) Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg 81:245–251. [DOI] [PubMed] [Google Scholar]
- 12. Dumanski JP, Rouleau GA, Nordenskjold M, Collins VP (1990) Molecular genetic analysis of chromosome 22 in 81 cases of meningioma. Cancer Res 50:5863–5867. [PubMed] [Google Scholar]
- 13. Farah SE, Konrad H, Huang DT, Geist CE (1999) Ectopic orbital meningioma: a case report and review. Ophthal Plast Reconstr Surg 15:463–466. [DOI] [PubMed] [Google Scholar]
- 14. Goutagny S, Bah AB, Henin D, Parfait B, Grayeli AB, Sterkers O, Kalamarides M (2012) Long‐term follow‐up of 287 meningiomas in neurofibromatosis type 2 patients: clinical, radiological, and molecular features. Neuro‐Oncol 14:1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goutagny S, Yang HW, Zucman‐Rossi J, Chan J, Dreyfuss JM, Park PJ et al (2010) Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin Cancer Res 16:4155–4164. [DOI] [PubMed] [Google Scholar]
- 16. Harada S, Henderson LB, Eshleman JR, Gocke CD, Burger P, Griffin CA, Batista DA (2011) Genomic changes in gliomas detected using single nucleotide polymorphism array in formalin‐fixed, paraffin‐embedded tissue: superior results compared with microsatellite analysis. J Mol Diagn 13:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holland H, Mocker K, Ahnert P, Kirsten H, Hantmann H, Koschny R et al (2011) High resolution genomic profiling and classical cytogenetics in a group of benign and atypical meningiomas. Cancer Genetics 204:541–549. [DOI] [PubMed] [Google Scholar]
- 18. Ishino S, Hashimoto N, Fushiki S, Date K, Mori T, Fujimoto M et al (1998) Loss of material from chromosome arm 1p during malignant progression of meningioma revealed by fluorescent in situ hybridization. Cancer 83:360–366. [PubMed] [Google Scholar]
- 19. Jain D, Ebrahimi KB, Miller NR, Eberhart CG (2010) Intraorbital meningiomas: a pathologic review using current World Health Organization criteria. Arch Pathol Lab Med 134:766–770. [DOI] [PubMed] [Google Scholar]
- 20. Jansen M, Mohapatra G, Betensky RA, Keohane C, Louis DN (2012) Gain of chromosome arm 1q in atypical meningioma correlates with shorter progression‐free survival. Neuropathol Appl Neurobiol 38:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanamalla US (2003) The optic nerve tram‐track sign. Radiology 227:718–719. [DOI] [PubMed] [Google Scholar]
- 22. Maroon JC, Kennerdell JS, Vidovich DV, Abla A, Sternau L (1994) Recurrent spheno‐orbital meningioma. J Neurosurg 80:202–208. [DOI] [PubMed] [Google Scholar]
- 23. Miller NR (2004) Primary tumours of the optic nerve and its sheath. Eye 18:1026–1037. [DOI] [PubMed] [Google Scholar]
- 24. Muller P, Henn W, Niedermayer I, Ketter R, Feiden W, Steudel WI et al (1999) Deletion of chromosome 1p and loss of expression of alkaline phosphatase indicate progression of meningiomas. Clin Cancer Res 5:3569–3577. [PubMed] [Google Scholar]
- 25. van den Munckhof P, Christiaans I, Kenter SB, Baas F, Hulsebos TJ (2012) Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 13:1–7. [DOI] [PubMed] [Google Scholar]
- 26. Ozaki S, Nishizaki T, Ito H, Sasaki K (1999) Comparative genomic hybridization analysis of genetic alterations associated with malignant progression of meningioma. J Neurooncol 41:167–174. [DOI] [PubMed] [Google Scholar]
- 27. Pacelli R, Cella L, Conson M, Tranfa F, Strianese D, Liuzzi R et al (2011) Fractionated stereotactic radiation therapy for orbital optic nerve sheath meningioma—a single institution experience and a short review of the literature. J Radiat Res (Tokyo) 52:82–87. [DOI] [PubMed] [Google Scholar]
- 28. Perez‐Magan E, Rodriguez de Lope A, Ribalta T, Ruano Y, Campos‐Martin Y, Perez‐Bautista G et al (2010) Differential expression profiling analyses identifies downregulation of 1p, 6q, and 14q genes and overexpression of 6p histone cluster 1 genes as markers of recurrence in meningiomas. Neuro‐Oncol 12:1278–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perry A, Gutmann DH, Reifenberger G (2004) Molecular pathogenesis of meningiomas. J Neurooncol 70:183–202. [DOI] [PubMed] [Google Scholar]
- 30. Perry A, Jenkins RB, Dahl RJ, Moertel CA, Scheithauer BW (1996) Cytogenetic analysis of aggressive meningiomas: possible diagnostic and prognostic implications. Cancer 77:2567–2573. [DOI] [PubMed] [Google Scholar]
- 31. Pushker N, Shrey D, Kashyap S, Sen S, Khurana S, Sharma S (2013) Ectopic meningioma of the orbit. Int Ophthalmol 33:707–710. [DOI] [PubMed] [Google Scholar]
- 32. Reuss DE, Piro RM, Jones DT, Simon M, Ketter R, Kool M et al (2013) Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol 125:351–358. [DOI] [PubMed] [Google Scholar]
- 33. Ruttledge MH, Xie YG, Han FY, Peyrard M, Collins VP, Nordenskjold M, Dumanski JP (1994) Deletions on chromosome 22 in sporadic meningioma. Genes Chromosomes Cancer 10:122–130. [DOI] [PubMed] [Google Scholar]
- 34. Saeed P, Blank L, Selva D, Wolbers JG, Nowak PJ, Geskus RB et al (2010) Primary radiotherapy in progressive optic nerve sheath meningiomas: a long‐term follow‐up study. Br J Ophthalmol 94:564–568. [DOI] [PubMed] [Google Scholar]
- 35. Sahm F, Bissel J, Koelsche C, Schweizer L, Capper D, Reuss D et al (2013) AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol 126:757–762. [DOI] [PubMed] [Google Scholar]
- 36. Seizinger BR, de la Monte S, Atkins L, Gusella JF, Martuza RL (1987) Molecular genetic approach to human meningioma: loss of genes on chromosome 22. Proc Natl Acad Sci USA 84:5419–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shields JA, Shields CL, Scartozzi R (2004) Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology 111:997–1008. [DOI] [PubMed] [Google Scholar]
- 38. Shrivastava RK, Sen C, Costantino PD, Della Rocca R (2005) Sphenoorbital meningiomas: surgical limitations and lessons learned in their long‐term management. J Neurosurg 103:491–497. [DOI] [PubMed] [Google Scholar]
- 39. Simon M, von Deimling A, Larson JJ, Wellenreuther R, Kaskel P, Waha A et al (1995) Allelic losses on chromosomes 14, 10, and 1 in atypical and malignant meningiomas: a genetic model of meningioma progression. Cancer Res 55:4696–4701. [PubMed] [Google Scholar]
- 40. Smith MJ, Higgs JE, Bowers NL, Halliday D, Paterson J, Gillespie J et al (2011) Cranial meningiomas in 411 neurofibromatosis type 2 (NF2) patients with proven gene mutations: clear positional effect of mutations, but absence of female severity effect on age at onset. J Med Genet 48:261–265. [DOI] [PubMed] [Google Scholar]
- 41. Smith MJ, O'Sullivan J, Bhaskar SS, Hadfield KD, Poke G, Caird J et al (2013) Loss‐of‐function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet 45:295–298. [DOI] [PubMed] [Google Scholar]
- 42. Stafford SL, Perry A, Leavitt JA, Garrity JA, Suman VJ, Scheithauer BW et al (1998) Anterior visual pathway meningiomas primarily resected between 1978 and 1988: the Mayo Clinic Rochester experience. J Neuroophthalmol 18:206–210. [PubMed] [Google Scholar]
- 43. Sulman EP, White PS, Brodeur GM (2004) Genomic annotation of the meningioma tumor suppressor locus on chromosome 1p34. Oncogene 23:1014–1020. [DOI] [PubMed] [Google Scholar]
- 44. Tabernero MD, Maillo A, Gil‐Bellosta CJ, Castrillo A, Sousa P, Merino M, Orfao A (2009) Gene expression profiles of meningiomas are associated with tumor cytogenetics and patient outcome. Brain Pathol 19:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tabernero MD, Maillo A, Nieto AB, Diez‐Tascon C, Lara M, Sousa P et al (2012) Delineation of commonly deleted chromosomal regions in meningiomas by high‐density single nucleotide polymorphism genotyping arrays. Genes Chromosomes Cancer 51:606–617. [DOI] [PubMed] [Google Scholar]
- 46. Turbin RE, Pokorny K (2004) Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Control 11:334–341. [DOI] [PubMed] [Google Scholar]
- 47. Weber RG, Bostrom J, Wolter M, Baudis M, Collins VP, Reifenberger G, Lichter P (1997) Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA 94:14719–14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wellenreuther R, Kraus JA, Lenartz D, Menon AG, Schramm J, Louis DN et al (1995) Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol 146:827–832. [PMC free article] [PubMed] [Google Scholar]
- 49. Yokoyama T, Nishizawa S, Sugiyama K, Yokota N, Ohta S, Uemura K et al (1999) Primary intraorbital ectopic meningioma. Skull Base Surg 9:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


