Abstract
Disruption of epithelial organization and loss of growth control are universal features of carcinomas, yet how these features are linked during cancer progression remains poorly understood. Cell polarity proteins control cellular and tissue organization and are emerging as important mediators of cancer progression. The Par3 polarity protein is a molecular scaffold that functions to recruit and spatially organize signaling factors, and was recently identified as a suppressor of breast cancer invasion and metastasis. Here we show that loss of Par3 in mammary epithelial cells promotes apoptosis and that oncogenic Notch overcomes the apoptotic signal to reveal an unexpected pro-proliferative role for loss of Par3 in mammary tumors. In this context, loss of Par3 deregulates Rac1 activity to activate Jun N-terminal Kinase (JNK)-dependent proliferation and tumor growth. Thus, we demonstrate a mechanism by which loss of Par3 promotes proliferation and tumorigenesis, which supports a tumor suppressive function for Par3 in the mammary epithelium.
Keywords: Breast cancer, polarity, tumorsphere, Notch, mammary gland
Introduction
Epithelial integrity is coupled to apoptosis and proliferation to control tissue size and organization.1-3 Loss of epithelial homeostasis, characterized by loss of epithelial organization and growth control, promotes development of carcinomas.4 Understanding how these processes are integrated is important to further understand mechanisms of cancer progression.
The Par complex (Par3, Par6, aPKC, and Cdc42) localizes to tight junctions and is a major regulator of epithelial homeostasis.5, 6 The relationship between the Par complex, cell proliferation, and apoptosis is complex and incompletely understood. Over-expression of Par6 causes increased proliferation in epithelial cells, which requires aPKC and Cdc42, but not Par3, and acts through ERK1/2.7 Furthermore, expression of an N-terminally deleted mutant of Par6 also increases proliferation, but this is offset by an aPKC-dependent increase in apoptosis.8 Inhibition of the Par6/aPKC interaction using a pharmacological inhibitor can block transformed cell growth of lung cancer cells,9 and RNAi knockdown of aPKC strongly promotes apoptosis.8, 10 Deletion of Par3 in skin caused reduced ERK-dependent growth and survival and increased apoptosis through the intrinsic mitochondrial pathway.11 Alternatively, loss of Par3 in the mammary epithelium resulted in stunted mammary ductal outgrowths with elevated proliferation that is offset by a parallel increase in apoptosis.12 Interestingly, increased proliferation is also noted in Par3-depleted MCF10A mammary cells.13 Therefore, there is an important relationship between Par3, proliferation, and apoptosis that is incompletely understood.
A key function of Par3 is to recruit and spatially organize signaling factors that regulate downstream signaling pathways, such as Rho-family GTPases. Independent of Par6/aPKC, Par3 binds and sequesters the Rac1 guanine nucleotide exchange factor (Rac-GEF) Tiam1 to locally regulate Rac1 activity, and loss of Par3 deregulates Rac1 activity in several cell types.14-18 There is emerging evidence linking Rac1 activation to cancer progression in multiple cancer types. A constitutively active Rac1 variant (Rac1b) is over-expressed in lung and breast cancers, and Rac1 activity correlates with lung tumor growth and more aggressive breast cancers.19, 20 Furthermore, activating Rac1 mutations identified in breast cancer cell lines have transforming properties.21 Despite that some mutations in Rac1 have been identified across cancers, the most common mode of deregulated Rac1 activity in tumors results from disrupted upstream signaling.22 Ablation of the Rac1-GEF, Tiam1, reduces metastasis in an ErbB2 mouse model of breast cancer,23 and loss of Par3 in ErbB2 mouse mammary tumors also promotes invasion and metastasis through Tiam1-mediated Rac1 activation.13
Par3 is down-regulated in breast and other cancers and we, and others, have recently reported that Par3 is an important mediator of tumorigenesis, invasion and metastasis in vivo.11, 13, 24 However, how loss of Par3 promotes mammary tumorigenesis, despite inducing apoptosis, is not understood. Here we demonstrate that oncogenic suppression of apoptosis enables loss of Par3 to promote mammary tumor growth through a Tiam1/Rac1/JNK proliferation pathway.
Results and Discussion
Loss of Par3 triggers both apoptosis and proliferation
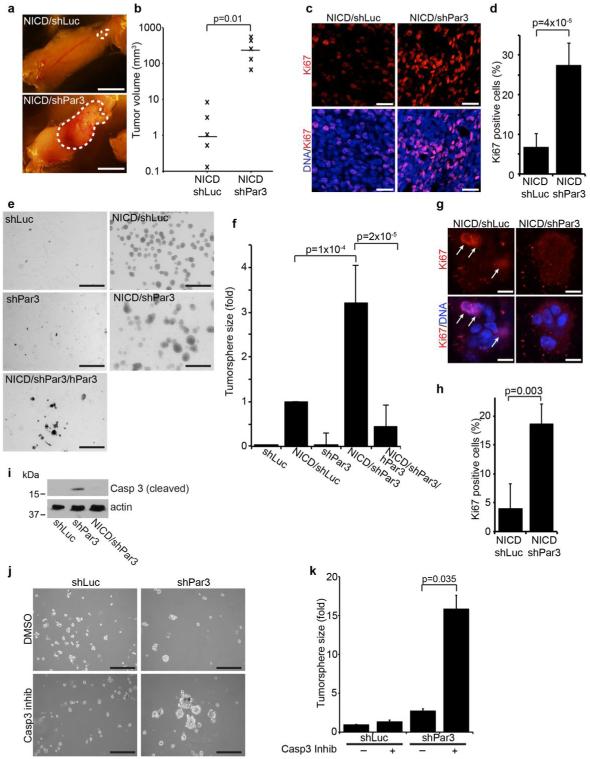
The Notch Intracellular Domain (NICD) induces non-metastatic mammary tumors in mice and is associated with poor prognosis in humans.25, 26 Importantly, NICD-induced tumors retain luminal epithelial characteristics,27, 28 making it an ideal model to dissect the mechanisms of polarity signaling in cancer. To study a role for Par3 in mammary tumor growth, we transduced freshly isolated primary mouse mammary epithelial cells (MECs) with lentivirus to express NICD and control (shLuc) or Par3-directed (shPar3) shRNA and transplanted them into the mammary fat pad of recipient mice.24 After 25 weeks, tumors were removed, imaged, and measured. We found that Par3-depleted tumors were markedly larger than control (Figure 1a, b). Immunostaining tumor sections for Ki67 revealed that Par3-depletion promoted a ~4-fold increase in the proportion of Ki67 positive cells (Figure 1c, d), indicating that loss of Par3 promoted proliferation, which is consistent with the increased tumor size observed.
Figure 1. Loss of Par3 cooperates with NICD to promote mammary tumor growth.
(a) Mammary tumors from mammary epithelial cells expressing NICD/shLuc (control) or NICD/shPar3 (shRNA sequence: shPar3 5’-acaagcgtggcatgatcca). Primary mammary epithelial cells (MECs) were isolated from wild-type mice, infected with lentivirus to express NICD and shRNA, then transplanted into the cleared mammary fat pad. (b) Volumes of NICD/shLuc and NICD/shPar3 tumors were calculated using the formula V=□/6×(length×width2). (c) Tumor sections were immunostained for Ki67 (1:100, Abcam). (d) Quantification of cells with nuclear Ki67 from (c). Two regions from each of 3 tumors were analyzed for each. (e) MECs were infected with lentivirus to express shLuc, NICD/shLuc, shPar3, NICD/shPar3, or NICD/shPar3/hPar3 and were grown in suspension cultures for 9 days to form tumorspheres. hPar3 is resistant to the mouse-specific Par3 shRNA. (f) Quantification of tumorsphere sizes from (d). The area of each tumorsphere was measured using a custom ImageJ macro. Each independent experiment contained at least 30 tumorspheres. (g) Tumorspheres were grown for 4 days, then immunostained for Ki67. Arrows show Ki67 positive cells. (h) Quantification of cells with nuclear Ki67 from (g); n= 4 independent experiments. (i) Cell lysates were prepared from tumorspheres and immunoblotted for cleaved Caspase-3 (1:1000, Cell Signaling Technology) or actin (1:1000, Sigma) as a loading control. (j) MECs expressing shLuc or shPar3 were treated with DMSO (control) or 10μM Caspase-3 inhibitor (Ac-DEVD-CHO, BD Biosciences), and were grown for 9 days in suspension culture. (k) Quantification of tumorspheres in (j). n=3 independent samples, unless otherwise noted. Error bars=sd. p-values were calculated using the student’s t-test (b, d, h) or the ANOVA with Tukey HSD post-hoc test (f, k) using SPSS software. Scale bars=1 cm (a); 20μm (c); 1 mm (e); 200μm (j).
To explore a mechanism by which loss of Par3 promoted tumor growth, we infected MECs at high efficiency with lentivirus to express NICD and shRNAs with a GFP marker, and grew them in suspension culture to form tumorspheres (Figure S1a).27 To verify that tumorspheres resemble primary tumors, we immunostained sections for keratins-8 (K8) and −14 (K14), markers of luminal and basal cells, respectively. Most of the cells in the NICD/shLuc tumorspheres were dual-positive (K8+/K14+), as was true of the primary tumors (Figure S1b). Notably, loss of Par3 increased the heterogeneity both in vivo and in the tumorspheres (Figure S1b). Importantly, the Par3-shRNA phenotype was rescued by expressing human, RNAi-resistant Par3 together with the murine-specific Par3 shRNA, confirming that the changes are specific to silencing of Par3 and are not caused by off-target effects (Figure S1b). Therefore, the primary tumorspheres resemble the in vivo tumor phenotype and represent a valid in vitro tumor model.
NICD expression significantly promoted tumorsphere growth (Figure 1e, f), consistent with previous reports of a role for NICD in tumor proliferation.27 Loss of Par3 alone did not promote growth of primary MEC spheroids, as compared to the shLuc control. Strikingly, however, loss of Par3 in the context of NICD expression increased tumorsphere growth by ~3-fold, and co-expression of RNAi-resistant human Par3 suppressed this increase, confirming the specificity of the effect (Figure 1e, f). Consistent with our in vivo data, loss of Par3 in tumorspheres also increased proliferation ~4-fold (Figure 1g, h), although the absolute proliferation rates were slightly lower in culture than in vivo.
We previously reported that loss of Par3 significantly increased both cell proliferation and apoptosis in the mammary epithelium in vivo.12 We speculated, therefore, that induced apoptosis might mask growth-promoting effects of Par3 depletion. To test this hypothesis we depleted Par3 from MECs and after 5 days in suspension culture blotted cell lysates for cleaved Caspase-3, a marker of apoptosis. Consistent with our previous results,12 Par3 depletion increased levels of cleaved Caspase-3 (Figure 1i). Next, we asked whether inhibiting apoptosis was sufficient to allow growth of Par3-depleted spheroids. Pharmacological inhibition of apoptosis with a Caspase-3 inhibitor (Ac-DEVD-CHO) in control cells had a minimal effect on growth (Figure 1j, k), consistent with the finding that there is negligible apoptosis in these cells (Figure 1i). In contrast, Caspase-3 inhibition caused a 5-fold increase in the growth of Par3-depleted spheroids (Figure 1j, k). This indicates that loss of the Par3 polarity protein triggers two opposing responses in the same cell population, increased apoptosis and increased proliferation. Since NICD can suppress apoptosis,27 we speculated that NICD may enable proliferation in the absence of Par3 by inhibiting apoptosis induced by loss of Par3. Indeed, NICD expression efficiently suppressed Caspase-3 activation in Par3-depleted MECs (Figure 1i).
Tiam1-Rac1 signaling drives proliferation in Par3-depleted tumor cells
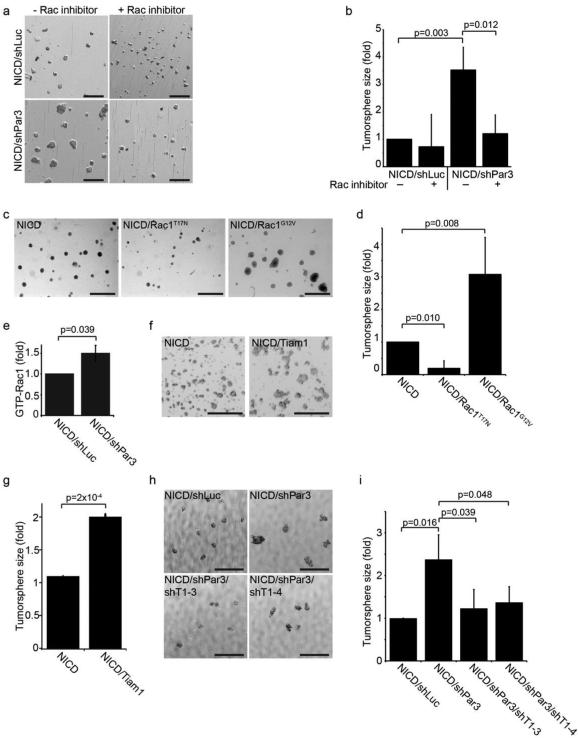
Rac1 can stimulate cell proliferation,22, 29 and loss of Par3 can cause inappropriate activation of Rac1.14, 18 Therefore, to determine whether Rac1 activity was necessary for tumorsphere growth associated with loss of Par3 we first grew tumorspheres in the presence or absence of NSC23766, an inhibitor that blocks Rac1 activation by specific GEFs, Tiam1 and Trio.30 Under these conditions, NICDS/shLuc tumorsphere growth was not affected, but growth of NICD/shPar3 tumorspheres was suppressed (Figure 2a, b).
Figure 2. Loss of Par3 promotes tumorsphere growth through Rac1 activation by Tiam1.
(a) Tumorsphere cultures of MECs expressing NICD/shLuc or NICD/shPar3 in the presence or absence of 25 μM Rac1 inhibitor (NSC23766, Tocris). (b) Quantification of tumorspheres sizes from (a). (c) Tumorsphere cultures of MECs expressing NICD, NICD/Rac1T17N (dominant negative), or NICD/Rac1G12V (constitutively active). (d) Quantification of tumorsphere sizes from (c). (e) Levels of total active Rac1-GTP were measured in NICD/shLuc and NICD/shPar3 tumorspheres using a GLISA assay kit (Cat. # BK128, Cytoskeleton Inc.), n=5. (f) Tumorsphere cultures of MECs expressing NICD or NICD/Tiam1. (g) Quantification of tumorsphere sizes from (f). (h) Tumorspheres of MECs expressing NICD/shLuc or NICD/shPar3 and one of two Tiam1 shRNA (shRNA sequences: shTiam1-3 5’-cgatgactttatatttata; shTiam1-4 5’-tttcgtgctatgatgaatc). (i) Quantification of tumorsphere sizes in (h), n=4. n=3 independent samples, unless otherwise noted. p-values were calculated using the ANOVA with Tukey HSD post-hoc test (b, d, i) or the student’s t-test (e, g). Error bars represent sd. Scale bars=1mm.
To determine whether activation of Rac1 was able to promote NICD-dependent tumorsphere growth, we transduced MECs with NICD plus GFP, dominant-negative Rac1T17N, or constitutively active Rac1G12V. Notably, expression of Rac1G12V with NICD promoted tumorsphere growth ~3-fold above the level induced by NICD alone (Figure 2c, d). Furthermore, expression of NICD/RacT17N diminished tumorsphere size relative to NICD alone, indicating that Rac1 may have a general role in survival or proliferation of the tumorspheres. Next, to examine whether loss of Par3 alters Rac1 activity in tumorspheres we measured Rac1-GTP levels using a GLISA assay and found a significant 1.5-fold increase in the global activation of Rac1 in Par3-depleted tumorspheres compared to the NICD/shLuc control (Figure 2e). In contrast, NICD expression alone had no effect on Rac1 activity, indicating that NICD does not activate Rac1 to promote tumorsphere growth (Figure S2a). Together, these results support a role for Rac1 in regulating tumorsphere growth in the context of disrupted Par3.
Par3 can repress Rac1 activity through sequestration of the Rac1-GEF, Tiam1,14, 17, 18 suggesting that Tiam1 might mediate the tumorsphere growth driven by Par3 depletion. To examine this possibility we first over-expressed Tiam1 with NICD and found that the resulting tumorspheres were ~2-fold larger than those with NICD alone (Figure 2f, g). Furthermore, Tiam1 expression was sufficient to induce a ~4-fold increase in sphere size in the absence oncogenic NICD (Figure S3a, b). To test if Tiam1 is necessary for tumorsphere growth induction by loss of Par3, we depleted Tiam1 using shRNA. Two independent Tiam1 shRNA constructs (ShT1-3 and shT1-4) were found to efficiently deplete Tiam1 (Figure S2b) and were able to suppress tumorsphere growth induced by loss of Par3 (Fig. 2h, i). Collectively, these results support a role for Tiam1-Rac1 in driving cell proliferation triggered by loss of Par3 in NICD-dependent tumors.
Rac1 promotes proliferation through JNK
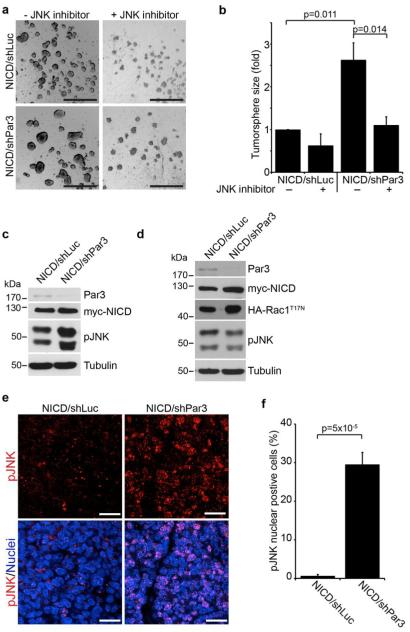
Rac1 can signal through JNK to control cell proliferation,31 and disruption of the Par-complex can regulate JNK activity in Drosophila.32 To test if Par3 acts through JNK in our model, we treated tumorspheres with a JNK inhibitor (SP600125). We found that JNK inhibition blocked tumorsphere growth induced by loss of Par3 (Figure 3a, b). JNK inhibition also suppressed tumorsphere growth induced by Tiam1 or constitutively active RacG12V expression (Figure S3a-d), consistent with a requirement for JNK in proliferation downstream of Tiam1/Rac1 activation induced by loss of Par3. Next we examined whether loss of Par3 induced JNK activation in tumorspheres. Indeed, loss of Par3 increased phospho-JNK levels above basal levels (Figure 3c), and this effect was blocked by co-expression of dominant negative RacT17N (Figure 3d), indicating that Par3 acts through Rac1 to activate JNK. Finally to determine whether loss of Par3 regulates JNK signaling in vivo, we immunostained NICD/shLuc and NICD/shPar3 tumor sections for phospho-JNK (Figure 3e). We observed a robust difference in the proportion of NICD/shPar3 cells positive for nuclear phospho-JNK compared to NICD/shLuc (30% versus 1%) (Figure 3f). Since disruption of polarity can alter growth and survival through MAPK signaling,7, 11 we also examined this pathway. However, we found no differences in phospho-ERK1/2 levels between NICD/shLuc and NICD/shPar3 tumorspheres (Figure S3e), indicating that MAPK signaling is unlikely to be involved in proliferation driven by loss of Par3 in this context. These results demonstrate that depletion of Par3 acts through Tiam1-mediated Rac1 activation to induce JNK-mediated proliferation in mammary tumors.
Figure 3. Par3 regulates tumor growth by activating JNK.
(a) Tumorsphere cultures of MECs expressing NICD/shLuc or NICD/shPar3 in the presence or absence of 25μM JNK inhibitor (SP600125, Sigma). (b) Quantification of tumorsphere sizes from (a), n=3. (c and d) Immunoblots of NICD/shLuc and NICD/shPar3 tumorsphere lysates (c) or co-expressing dominant negative HA-Rac1T17N (d). Antibodies used were rabbit anti-Par3 (Macara lab12), mouse anti-Myc (1:1000, McGill hybridoma facility), rabbit anti-HA (1:1000, McGill hybridoma facility), rabbit anti-pJNK (pThr183/Tyr185, 1:1000, Cell Signaling Technology), mouse anti-a-tubulin as a loading control (1:5000, Sigma). (e) NICD/shLuc and NICD/shPar3 mammary tumor sections were stained for phospho-JNK (1:100). (f) Quantification of cells with nuclear phospho-JNK from (e). Two regions from each of 3 tumors were analyzed for each. p-values were calculated using the ANOVA with Tukey HSD post-hoc test (b) or student’s t-test (f). Error bars represent sd (e) or sem (f). Scale bars = 1mm (a) and 20μm (e).
Rac1 is a potent stimulator of mammary cell proliferation
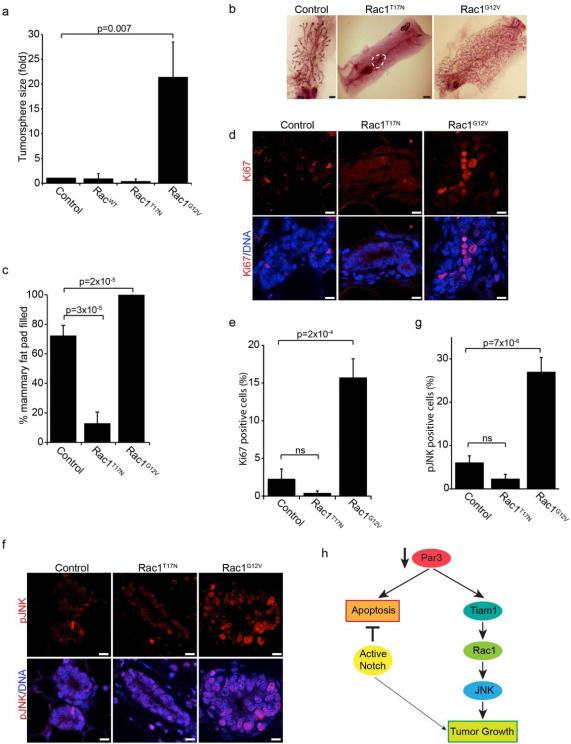
We next asked whether Rac1 activation is sufficient to promote mammary epithelial proliferation in the absence of oncogenic NICD. To this end, we expressed GFP, wild-type Rac1, dominant negative Rac1T17N or constitutively active Rac1G12V in primary MECs in suspension culture. Strikingly, expression of constitutively active Rac1G12V was sufficient to induce a ~20-fold increase in spheroid growth (Figure 4a). To determine whether Rac1 drives growth of mammary epithelial cells in vivo, we performed mammary transplants with control MECs or those expressing dominant-negative Rac1T17N, or constitutively active Rac1G12V. After 8-weeks of regeneration, control outgrowths filled 72% of the mammary fat pad (5/6 transplants had outgrowths), whereas those expressing Rac1T17N filled only 13% (4/6 transplants) (Figure 4b, c). In contrast, mammary outgrowths expressing Rac1G12V filled 100% of the fat pad (6/6 transplants). We examined whether differences in ductal growth reflected differences in proliferation by staining for Ki67 (Figure 4d). We found that mammary glands expressing active Rac1G12V had a ~5-fold increase in the number of Ki67-positive cells (Figure 4e), indicating increased proliferation in these glands. We also consistently found that glands expressing dominant negative Rac1T17N had a reduced number of Ki67-positive cells, however this was not statistically significant (Figure 4e). To confirm a role for JNK downstream of Rac1 in vivo, we stained tissue sections for phospho-JNK (Figure 4f). As expected, glands expressing active Rac1G12V had ~5-fold increased proportion of cells with nuclear phospho-JNK compared to control glands (Figure 4g). As with Ki67 staining, we noted a consistent, but statistically insignificant decrease in the proportion of cells with nuclear phospho-JNK in cells expressing dominant negative Rac1T17N (Figure 4g). Interestingly, JNK1/2 knockout mice do not exhibit defects in mammary epithelial growth in vivo, indicating that JNK1/2 are not essential and that other growth pathways compensate to ensure normal development.33 Notably, no tumors were detected in mice containing MECs transduced with Rac1G12V. Therefore, elevated Rac1 activity promotes mammary proliferation but is not sufficient for oncogenic transformation in this context. This is consistent with a recent report for lung cancer, where expression Rac1b synergized with K-Ras to increase lung tumor growth, but alone did not induce tumorigenesis.20
Figure 4. Rac1/JNK signaling regulates mammary epithelial proliferation.
(a) Quantification of tumorsphere sizes of MECs expressing GFP (control), wild-type (Rac1WT), dominant negative (Rac1T17N) or constitutively active (Rac1G12V) Rac1. (b) Mammary epithelial cells were transduced with control (GFP alone), Rac1T17N, or Rac1G12V lentivirus and then transplanted into a cleared mammary fat pad and allowed to regrow for 8-weeks. (c) Quantification of the percentage of the fat pad filled from (b), n=4. (d) Tissue sections of control (GFP), Rac1T17N, and Rac1G12V mammary glands were immunostained for Ki67. (e) Quantification of cells with nuclear Ki67 from (d). All ducts from 2 tissue sections for each of 3 glands were measured. (f) Tissue sections of control (GFP), Rac1T17N, and Rac1G12V mammary glands were stained for phospho-JNK. (g) Quantification of cells with nuclear phospho-JNK from (f). (h) Model showing that loss of Par3 in the normal mammary epithelium activates both apoptotic and proliferative responses. In the presence of an anti-apoptotic factor (i.e. Notch oncogene), the apoptotic response caused by a loss of Par3 is suppressed and a Tiam1/Rac1/JNK mediated proliferative response is revealed. p-values were calculated using the ANOVA with Tukey HSD post-hoc test. Error bars represent sd (a) or sem (c, e, and (g). Scale bars = 2mm (b) or 10 μm (d and f).
Most human tumors arise from epithelial tissue, and although they often retain epithelial features, are nonetheless highly disorganized. Disrupted apical-basal polarity frequently results in apoptosis, which may act as a protective mechanism against damaged cells. The switch from apoptosis to uncontrolled proliferation therefore represents an important process for tumor progression. In Drosophila, clonal patches of epithelial cells that lack the Scribble polarity protein are eliminated through JNK-dependent cell death, which is induced by neighboring normal cells.34 Notably, however, expression of oncogenic Ras or Notch blocks apoptosis and converts JNK activation into a proliferative signal that promotes tumor growth and metastasis.35 The underlying process most likely involves a normal, compensatory proliferative response to apoptosis that has been deregulated by oncogenic stimuli. Whether any similar mechanisms occur in mammals has not been established. Our data now reveal a parallel but distinct system operating in mammary cells. Loss of the Par3 polarity protein, which disrupts epithelial integrity, triggers both an apoptotic program and increased proliferation in normal epithelial cells. Oncogene activation blocks apoptosis and uncovers a proliferative response (Figure 4h). The underlying mechanism caused by loss of Par3 is an inappropriate activation of Rac1, which is a sufficient to stimulate proliferation through the activation of JNK. However, contrary to the situation in Drosophila, the two arms of the signaling response to Par3 depletion are unlinked. JNK activation does not stimulate apoptosis, and the proliferative signal does not arise as a compensatory response to the induction of apoptosis.
The regulation of Rac1-dependent signaling by cell polarity proteins may be an important mechanism of tumor progression. Our results are consistent with those of Xue and colleagues showing that loss of Par3 activates Rac1 through Tiam1.13 Surprisingly, however, although they confirmed our previous result that loss of Par3 alone promotes proliferation in non-transformed mammary epithelial cells,12 Par3 depletion did not further enhance tumor growth in the context of the ErbB2 oncogene.13 This outcome may be because ErbB2 itself strongly activates JNK signaling,36, 37 which may mask an effect by loss of Par3. Given that defects in polarity and growth control are common early in cancer progression, we anticipate that the interplay between the two are of widespread importance in malignant transformation of breast and other cancers.
Supplementary Material
Acknowledgements
We thank Didier Trono (Lausanne, Switzerland) for lentivectors. K8 hybridoma, developed by Philippe Brulet and Rolf Kemler, was obtained from the Universtiy of Iowa Developmental Hybridoma Bank. This work was supported by grants from TFRI (Project #1009) and CIRH (MOP-119482) to LM. LM is a FRQS Research Scholar.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011 Dec;21(12):727–35. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Tepass U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol. 2012;28:655–85. doi: 10.1146/annurev-cellbio-092910-154033. [DOI] [PubMed] [Google Scholar]
- 3.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011 Aug;13(8):877–83. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Muthuswamy SK. Polarity protein alterations in carcinoma: a focus on emerging roles for polarity regulators. Curr Opin Genet Dev. 2010 Feb;20(1):41–50. doi: 10.1016/j.gde.2009.12.001. PubMed PMID: 20093003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayase J, Kamakura S, Iwakiri Y, Yamaguchi Y, Izaki T, Ito T, et al. The WD40 protein Morg1 facilitates Par6-aPKC binding to Crb3 for apical identity in epithelial cells. J Cell Biol. 2013 Mar 4;200(5):635–50. doi: 10.1083/jcb.201208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morais-de-Sa E, Mirouse V, St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010 Apr 30;141(3):509–23. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008 Oct 15;68(20):8201–9. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M, Datta A, Brakeman P, Yu W, Mostov KE. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci. 2007 Jul 15;120:2309–17. doi: 10.1242/jcs.007443. Pt 14. [DOI] [PubMed] [Google Scholar]
- 9.Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. A novel small-molecule inhibitor of protein kinase Ciota blocks transformed growth of non-small-cell lung cancer cells. Cancer Res. 2006 Feb 1;66(3):1767–74. doi: 10.1158/0008-5472.CAN-05-3405. [DOI] [PubMed] [Google Scholar]
- 10.Durgan J, Kaji N, Jin D, Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem. 2011 Apr 8;286(14):12461–74. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iden S, van Riel Wilhelmina E, Schäfer R, Song J-Y, Hirose T, Ohno S, et al. Tumor Type-Dependent Function of the Par3 Polarity Protein in Skin Tumorigenesis. Cancer Cell. 2012;22(3):389–403. doi: 10.1016/j.ccr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 12.McCaffrey LM, Macara IG. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009 Jun 15;23(12):1450–60. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue B, Krishnamurthy K, Allred DC, Muthuswamy SK. Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol. 2013 Feb;15(2):189–200. doi: 10.1038/ncb2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005 Mar;7(3):262–9. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- 15.Georgiou M, Baum B. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci. 2010 Apr 1;123:1089–98. doi: 10.1242/jcs.060772. Pt 7. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, et al. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005 Mar;7(3):270–7. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 17.Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol. 2007 Oct 9;17(19):1623–34. doi: 10.1016/j.cub.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006 Mar;8(3):227–37. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- 19.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000 Jun 15;19(26):3013–20. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 20.Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, et al. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013 Feb 14;32(7):903–9. doi: 10.1038/onc.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K, et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3029–34. doi: 10.1073/pnas.1216141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal. 2012 Feb;24(2):353–62. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strumane K, Rygiel T, van der Valk M, Collard JG. Tiam1-deficiency impairs mammary tumor formation in MMTV-c-neu but not in MMTV-c-myc mice. J Cancer Res Clin Oncol. 2009 Jan;135(1):69–80. doi: 10.1007/s00432-008-0437-8. [DOI] [PubMed] [Google Scholar]
- 24.McCaffrey Luke M, Montalbano J, Mihai C, Macara Ian G. Loss of the Par3 Polarity Protein Promotes Breast Tumorigenesis and Metastasis. Cancer Cell. 2012;22(5):601–14. doi: 10.1016/j.ccr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu C, Dievart A, Lupien M, Calvo E, Tremblay G, Jolicoeur P. Overexpression of activated murine Notch1 and Notch3 in transgenic mice blocks mammary gland development and induces mammary tumors. Am J Pathol. 2006 Mar;168(3):973–90. doi: 10.2353/ajpath.2006.050416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011 Jan;10(1):9–15. doi: 10.1158/1535-7163.MCT-10-0677. [DOI] [PubMed] [Google Scholar]
- 27.Simmons MJ, Serra R, Hermance N, Kelliher MA. NOTCH1 inhibition in vivo results in mammary tumor regression and reduced mammary tumorsphere-forming activity in vitro. Breast Cancer Res. 2012 Sep 19;14(5):R126. doi: 10.1186/bcr3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a "Rac" of all trades. Cell Mol Life Sci. 2009 Feb;66(3):370–4. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004 May 18;101(20):7618–23. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995 Sep 1;269(5228):1270–2. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 32.Warner SJ, Yashiro H, Longmore GD. The Cdc42/Par6/aPKC Polarity Complex Regulates Apoptosis-Induced Compensatory Proliferation in Epithelia. Curr Biol. 2010 Apr;:7. doi: 10.1016/j.cub.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cellurale C, Girnius N, Jiang F, Cavanagh-Kyros J, Lu S, Garlick DS, et al. Role of JNK in mammary gland development and breast cancer. Cancer Res. 2012 Jan 15;72(2):472–81. doi: 10.1158/0008-5472.CAN-11-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. Embo J. 2003 Nov 3;22(21):5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhlirova M, Jasper H, Bohmann D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13123–8. doi: 10.1073/pnas.0504170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A, et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008 May 15;68(10):3697–706. doi: 10.1158/0008-5472.CAN-07-6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han JS, Crowe DL. Jun amino-terminal kinase 1 activation promotes cell survival in ErbB2-positive breast cancer. Anticancer Res. 2010 Sep;30(9):3407–12. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.