Abstract
Osteoblasts are sensitive to surface microtopography and chemistry. Osteoblast differentiation and maturation are higher in vitro and bone formation and osseointegration enhanced in vivo on microstructured titanium (Ti) compared to smooth surfaces. Cells increased BMP2 expression on microtextured Ti alloy, suggesting a paracrine role in regulating osteoblast maturation. However, recent studies show that exogenous BMP2 inhibits osteoblast production of anti-inflammatory cytokines and osteocalcin, indicating that control of BMP-signaling may be involved. This study examined whether cells modulate BMP ligands, receptors, and inhibitors during osteoblast maturation on Ti, specifically focusing on the roles of BMP2 and Noggin. mRNA and protein for BMP2, BMP4, and BMP7 and receptors BMPR1A, BMPR1B, and BMPR2, and BMP inhibitors were upregulated on microtextured surfaces in comparison to smooth surfaces. Maturation on microstructured Ti was slightly enhanced with exogenous BMP2 while Noggin addition inhibited osteoblast maturation. Cells with Noggin knocked down significantly increased osteoblast maturation. These results demonstrate that BMP-related molecules are controlled during osteoblast maturation on microstructured Ti surfaces and that endogenous Noggin is an important regulator of the process. Modifying paracrine BMP signaling may yield more robust bone formation than application of exogenous BMPs.
Keywords: Osteoblasts, Bone morphogenetic protein, Titanium, Surface roughness, Cell signaling
INTRODUCTION
In vitro studies have shown that osteoblasts are sensitive to surface microtopography and chemistry. When grown on microstructured titanium (Ti) substrates, cells exhibit increased osteoblast differentiation and maturation (1, 2). In addition, osteoblasts on microstructured Ti substrates produce paracrine factors that induce differentiation of mesenchymal stem cells (MSCs) distal to the implant surface (3), resulting in bone formation (4) and angiogenesis (5). These include growth factors as such as transforming growth factor beta-1 (TGF-β1), vascular endothelial growth factor (VEGF), and fibroblast growth factor-2 (FGF2), as well as factors involved in autocrine modulation of osteoblast differentiation, such as bone morphogenetic proteins (BMPs) (6, 7) and Wnt signaling molecules (8, 9).
Recently, we showed that osteoblasts grown on microtextured Ti substrates produce increased levels of endogenous BMP2 and anti-inflammatory cytokines and reduced levels of pro-inflammatory cytokines than osteoblasts grown on smooth Ti substrates (10). Interestingly, addition of BMP2 to these cultures resulted in a dose-dependent decrease in anti-inflammatory cytokines concomitant with an increase in pro-inflammatory cytokines as well as a decrease in osteocalcin, a marker of osteoblast differentiation. This observation suggested that endogenous BMP signaling might be a critical regulatory determinant in modulating osteoblast differentiation, but the specific sites of regulation were not determined.
BMPs are members of the transforming growth factor beta (TGF-β) super family (11) and some of them, particularly BMP2, BMP4, and BMP7, can induce bone and cartilage formation (12-14). BMP2 is used clinically to promote spine fusion and for sinus lift procedures, and recently, it has been investigated as a mechanism to regenerate bone around implants (15, 16). BMPs dimerize either as homodimers or as heterodimers to activate downstream signaling. The dimer then binds to a complex of four transmembrane receptors, consisting of two BMP type I receptors and two BMP type II receptors. The transmembrane receptors then phosphorylate Smad1/5/8 (17) or activate ERK1/2, JNK, or p38 pathways through Smad-independent signaling (18). One method by which BMP signaling is modulated extracellularly is through ligand antagonists (e.g. Follistatin, Coco, DAN, Sclerostin, Chordin, Gremlin, Cerberus, and Noggin (NOG) (18)) that bind to BMP molecules to prevent receptor-ligand interactions (19). Results from a microarray analysis showed that BMPs, their receptors, and their various binding proteins and inhibitors are differentially regulated during osteoblast maturation in vitro and during osseointegration in vivo (7, 20), but it is not known if they participate in a coordinated manner in osteoblast differentiation and maturation on the surface of titanium implants.
Therefore, the aim of this study was to determine whether BMP ligands, receptors, and inhibitors are regulated during osteoblast maturation on microstructured titanium surfaces. To determine whether endogenous regulation is a critical factor, we examined whether addition of BMP2 or modulation of Noggin can enhance or inhibit this process.
MATERIALS AND METHODS
Disk Characterization
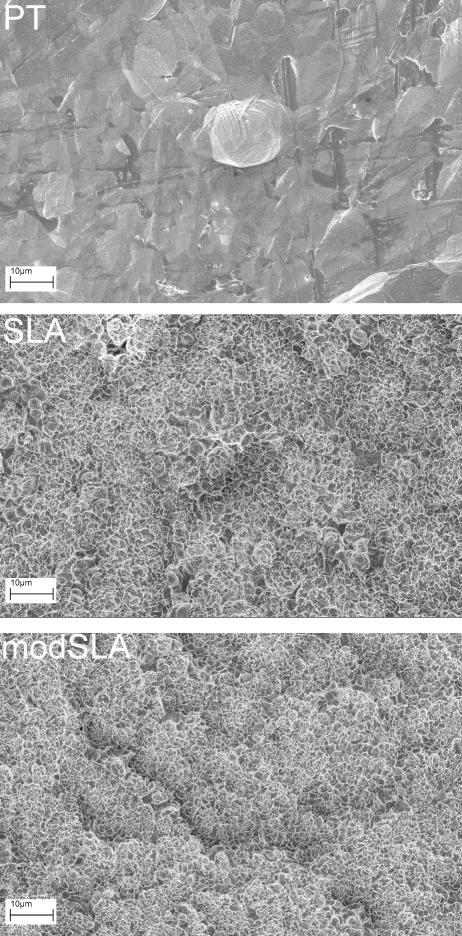
Ti disks were donated by the Institut Straumann AG (Basel, Switzerland). Disks were prepared from 1mm thick sheets of grade 2 unalloyed Ti (ASTM F67 “Unalloyed Ti for surgical implant applications”) and punched to 15 mm in diameter to fit snugly into the well of a 24-well tissue culture plate. Fabrication method and full characterization of the resulting morphology has been reported previously (1, 21). Scanning electron microscopy (Hitachi ) images were taken at 1kX magnification using a 12 mm working distance (Fig. 1). Briefly, smooth Ti surfaces (PT) have a mean peak-to-valley roughness (Ra) of 0.4 μm. Rough SLA surfaces are created by large grit blasting and acid etching titanium disks to create topography with craters (100μm diameter) overlaid with pits (1-3μm diameter) and coated with spikes (700nm diameter). The resultant surface has a Ra of 3.2μm. modSLA surfaces are created by processing SLA surfaces in a nitrogen environment to minimize exposure to the ambient atmosphere and packaged in a sealed glass tube with isotonic saline, thus retaining the hydrophilic chemistry of a clean TiO2 surface. All disks were sterilized overnight by gamma irradiation before use in cell culture studies.
Figure 1.
Scanning electron microscopy of Ti surfaces. SEM images were taken at 1000x magnification of PT, SLA, and modSLA surfaces used for subsequent cell studies.
Cell Culture
Human osteoblast-like MG63 cells were obtained from the American Type Culture Collection (Manassas, Virginia). These cells are a well-characterized osteoblast model used to examine cell response to titanium materials (22). Osteoblasts from a healthy, 17-year-old male donor tibia were isolated by allowing them to migrate from bone chips as previously described (23). Bone specimens were obtained under Institutional Review Board approval from the Georgia Institute of Technology and Children's Healthcare of Atlanta.
All cells were plated at 10,000 cells/cm2 and cultured in Dulbecco's Modification of Eagle's Medium (DMEM, Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum (Thermo Fisher HyClone, Logan, UT) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA) at 37°C, 5% CO2, and 100% humidity.
Expression of BMP genes
MG63 cells or normal human osteoblasts were plated on Ti disks (PT, SLA, and modSLA), using tissue culture polystyrene (TCPS) as a control. Cells were grown to confluence on TCPS, at which time the cells were incubated with fresh media. After 12 hours, RNA was harvested from cells on surfaces using TriZOL® (Invitrogen) and quantified (NanoDrop 1000, Thermo Scientific, Waltham, MA). To create a cDNA template, 1ug of RNA was reverse transcribed using random primers (Promega, Madison, WI) and Omniscript reverse transcriptase (Qiagen, Valencia, CA). To quantify expression, cDNA was used for real-time qPCR with gene-specific primers using an iQ5 (Bio-Rad, Hercules, CA). Fluorescence values were quantified as starting mRNA quantities using known dilutions of MG63 cells grown on TCPS. mRNA levels of each gene were normalized to mRNA levels of glyceraldehyde-3-phophate dehydrogenase (GAPDH) in each sample. Primers (Table 1) were designed using the Beacon designer software and synthesized by Eurofins MWG Operon (Huntsville, AL); NOG primer was purchased from Qiagen (QuantiTect Primer Assay QT00210833).
Table 1.
Human primers used in Real-time PCR analysis.
| Gene | Primer Sequence | |
|---|---|---|
| BMP2 | F | 5’-GCG TGA AAA GAG AGA CTG C-3’ |
| R | 5’-CCA TTG AAA GAG CGT CCA C-3’ | |
| BMP4 | F | 5’-ACG GTG GGA AAC TTT TGA TGT G-3’ |
| R | 5’-CGA GTC TGA TGG AGG TGA GTC-3’ | |
| BMP7 | F | 5’-AGC AGC AGC GAC CAG AGG-3’ |
| R | 5’-ACA GTA GTA GGC GGC GTA GC-3’ | |
| BMPR1A | F | 5’-CAA GAG GCA TCT CAA GCA GCA G-3’ |
| R | 5’-CAG ACC CAC TAC CAG ACC TTT G-3’ | |
| BMPR1B | F | 5’-AAG GCT CAG ATT TTC AGT GTC G-3’ |
| R | 5’-TTC AAT GGA GGC AGT GTA GGG-3’ | |
| BMPR2 | F | 5’-TCT TTG CCC TCC TGA TTC TTG-3’ |
| R | 5’-CAT AGC CGT TCT TGA TTC TGC-3’ | |
| GREM1 | F | 5’-GCA GGG TGG GTG AAC TTT ATT G-3’ |
| R | 5’-AGG AGG CTG AGA AGA TAC AAG G-3’ | |
| CER1 | F | 5’-TAC CTC CTG CTC TCA CTG TT G-3’ |
| R | 5’-ATG CTC CGT CTT CAC CTT GC-3’ | |
| FST | F | 5’-GGG ATT TCA AGG TTG GGA GAG G-3’ |
| R | 5’-GCT GGC ATA AGT GGC ATT GTC-3’ | |
| GAPDH | F | 5’-GCT CTC CAG AAC ATC ATC C-3’ |
| R | 5’-TGC TTC ACC ACC TTC TTG-3’ | |
Quantification of Secreted BMPs
MG63 cells or normal human osteoblasts were cultured on TCPS or Ti until cells reached confluence on TCPS. At confluence, cells were incubated with fresh media for 24 hours. Total cell number, alkaline phosphatase specific activity in the cell lysate, and secreted Noggin, BMP2, BMP4, and BMP7 were measured in the conditioned media as described below.
Effect of BMP2 and Noggin
MG63 cells were culture on TCPS or Ti. Cells were treated with 50 ng/ml BMP2 (R&D Systems) or 100 ng/ml Noggin (R&D Systems) every 48 hours. At confluence, cells were incubated with fresh media without BMP2 or Noggin for 24 hours, harvested, and assayed as described below.
Noggin Silencing
MG63 cells were transduced with shRNA lentiviral transduction particles (SHCLNVNM_005450, Mission®, Sigma Aldrich, St. Louis, MO) to silence Noggin (NOG). MG63 cells were plated at 20,000 cells/cm2 and cultured overnight as above. Particles were added to the cells at a multiplicity of infection of 7.5 and incubated for 18 hours. After incubation, transduced cells were selected with 0.25 μg/ml puromycin. The resulting stably silenced MG63 cells had a 70% reduction in NOG mRNA levels as determined by real-time qPCR and 72% reduction in secreted Noggin as determined by ELISA (24).
Biochemical Analysis
At harvest, cells were released from the surface by two sequential 10 minute incubations in 0.25% trypsin-EDTA (Invitrogen) (1). Total cell number was determined using a Z1 cell and particle counter (Beckman Coulter, Brea, CA). Cells were lysed in 0.05% Triton X-100 (Sigma Aldrich, St. Louis, MO) and alkaline phosphatase specific activity measured in the lysate measuring the release of p-nitrophenol from p-nitrophenylphosphate at pH 10.2 (25). Alkaline phosphatase specific activity was normalized to total protein content (Thermo Scientific Pierce BCA Protein Assay Kit, Rockford, IL).
Quantification of Secreted Factors
Secreted factors were measured in the conditioned media by immunoassay. Osteocalcin levels were measured using a commercially available radioimmunoassay (Biomedical Technologies Inc., Stoughton, MA). Levels of osteoprotegerin (OPG, R&D Systems), VEGF (R&D Systems), BMP2 (PeproTech, Rocky Hill, NJ), BMP4 (R&D Systems), and BMP7 (R&D Systems) were measured by enzyme-linked immunosorbent assay (ELISA) following manufacturer's instructions. Total TGF-β1 was measured by acidifying conditioned media and assaying using a commercially available ELISA kit (R&D Systems). In a second aliquot, active TGF-β1 was measured without acidification of the conditioned media. Latent TGF-β1 was defined as total TGF-β1 minus active TGF-β1. Noggin was measured via indirect ELISA using a monoclonal anti-mouse Noggin primary antibody (MAB719, R&D Systems) and Peroxidase-AffiniPure Goat Anti-Rat IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Recombinant human Noggin/Fc chimera (R&D Systems) was used to generate a standard curve to extrapolate concentrations in experimental samples. Results of immunoassays were normalized to total cell number.
Statistical Analysis
Data are mean ± SEM of six independent cultures per variable. Data were first analyzed by analysis of variance (ANOVA). Significant differences between groups were determined using Bonferroni's modification of the Student's t-test. P<0.05 was considered to be significant.
RESULTS
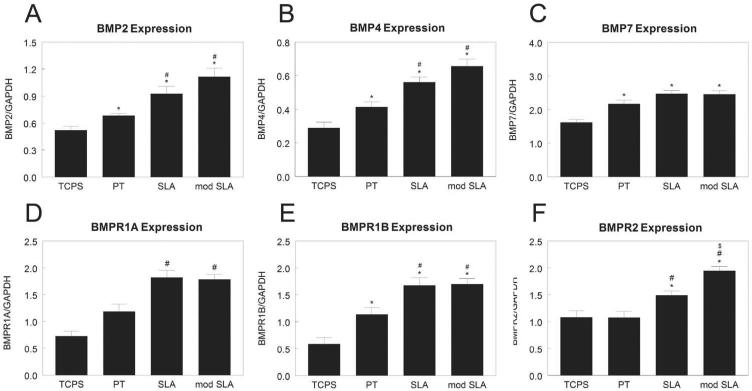
MG63 cells cultured on smooth PT substrates had higher expression of BMP2 (Fig. 2A) and BMP4 (Fig. 2B) than cells cultured on TCPS. BMP2 and BMP4 were further increased when cells were cultured on rough SLA or hydrophilic/rough modSLA surfaces. Cells cultured on Ti had higher expression of BMP7 than cells on TCPS, but there was no enhanced effect due to roughness or surface energy (Fig. 2C). Expression of BMPR1A (Fig. 2D) and BMPR1B (Fig. 2E) were 100% higher on PT and 200% higher on SLA and modSLA than cells cultured on TCPS. Cells on smooth TCPS and PT substrates had similar expression of BMPR2 (Fig. 2F), but expression was upregulated on rough SLA and further increased on modSLA.
Figure 2.
mRNA levels of BMPs and BMP receptors in MG63 cells cultured on microstructured titanium surfaces. Total RNA was extracted from cells cultured on TCPS, PT, SLA, and modSLA 12h after cells reached confluence on TCPS. Expression of BMP2 (A), BMP4 (B), BMP7 (C), BMPR1A (D), BMPR1B (E), and BMPR2 (F) was measured by real-time qPCR and normalized to GAPDH. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA.
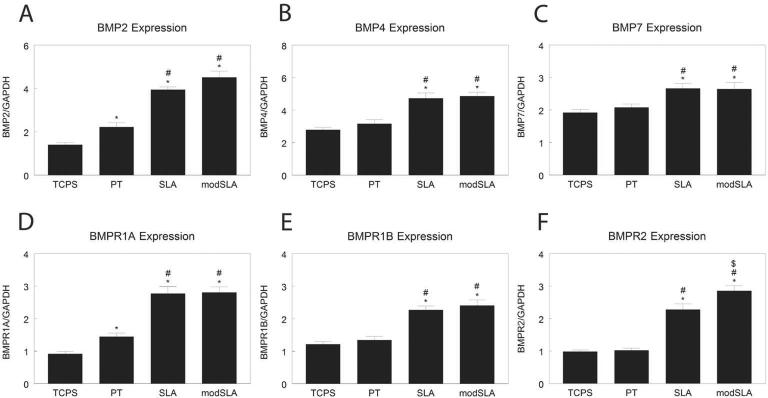
Normal human osteoblasts had higher BMP2 (Fig. 3A) and BMP4 (Fig. 3B) on Ti than TCPS, with highest levels on rough surfaces. BMP7 mRNA levels were increased by culture on rough Ti (Fig. 3C). BMPR1A levels were higher on SLA and modSLA than on PT (Fig. 3D). BMPR1B mRNA levels (Fig. 3E) were higher on Ti than TCPS, with highest levels on rough surfaces. BMPR2 mRNA was higher on SLA than TCPS or PT, and was additionally increased by culture on modSLA (Fig. 3F).
Figure 3.
mRNA levels of BMPs and BMP receptors in normal human osteoblasts cultured on microstructured titanium surfaces. Total RNA was extracted from cells cultured on TCPS, PT, SLA, and modSLA 12h after cells reached confluence on TCPS. Expression of BMP2 (A), BMP4 (B), BMP7 (C), BMPR1A (D), BMPR1B (E), and BMPR2 (F) was measured by real-time qPCR and normalized to GAPDH. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA.
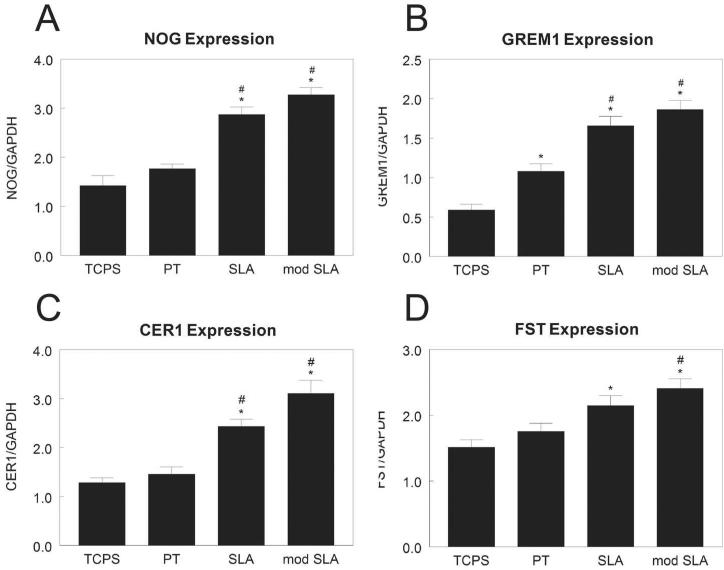
Expression of Noggin (NOG, Fig. 4A) was 100% higher in MG63 cells on SLA and modSLA than cells on TCPS or PT. Gremlin1 (GREM1) was 100% higher on PT and 200% higher on SLA and modSLA than TCPS (Fig. 4B). Surface roughness had a similar effect on expression of cerberus1 (CER1, Fig. 4C). Follistatin was modestly increased on SLA in comparison to TCPS and on modSLA in comparison to TCPS and PT (Fig. 4D).
Figure 4.
BMP pathway inhibitor mRNA levels in MG63 cells grown on microstructured titanium surfaces. mRNA levels of NOG (A), GREM1 (B), CER1 (C), and FST (D) in MG63 cells grown on microstructured titanium surfaces. Total RNA was extracted from cells cultured on TCPS, PT, SLA, and modSLA 12h after cells reached confluence on TCPS. mRNA was measured by real-time PCR and expression normalized to GAPDH. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA.
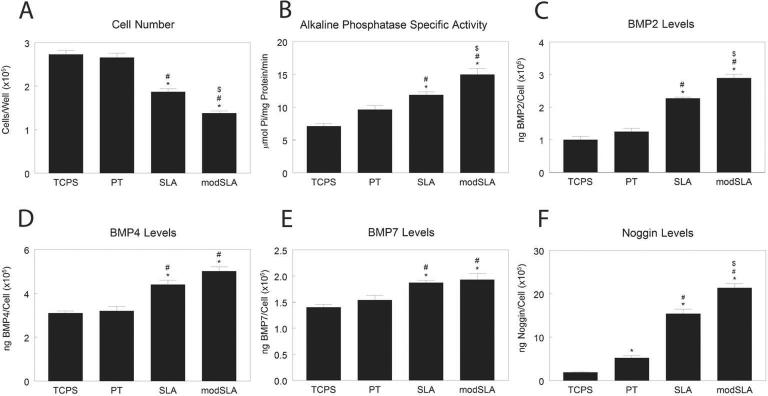
Cell number was similar on TCPS and PT, but was lower on SLA and further decreased on modSLA (Fig. 5A). Alkaline phosphatase specific activity was similar on TCPS and PT but was increased by roughness and surface energy (Fig. 5B). Cells on SLA produced more BMP2 than cells on TCPS or PT, but levels were greatest on modSLA (Fig. 5C). Cells on SLA and modSLA substrates produced more BMP4 than cells on smooth surfaces (Fig. 5D). BMP7 levels were higher on SLA and modSLA than on TCPS (Fig. 5E). Levels of Noggin were higher on Ti substrates than on TCPS, and were increased both by surface roughness and energy (TCPS<PT<SLA<modSLA, Fig. 5F).
Figure 5.
Levels of BMPs in conditioned media of MG63 cells grown on microstructured titanium surfaces. MG63 cells were plated on TCPS, PT, SLA, and modSLA surfaces. Cell number (A) and alkaline phosphatase specific activity (B) of the cell lysates were measured 24h after confluence. Conditioned media was collected and levels of BMP2 (C), BMP4 (D), BMP7 (E), and noggin (F) measured by ELISA. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA.
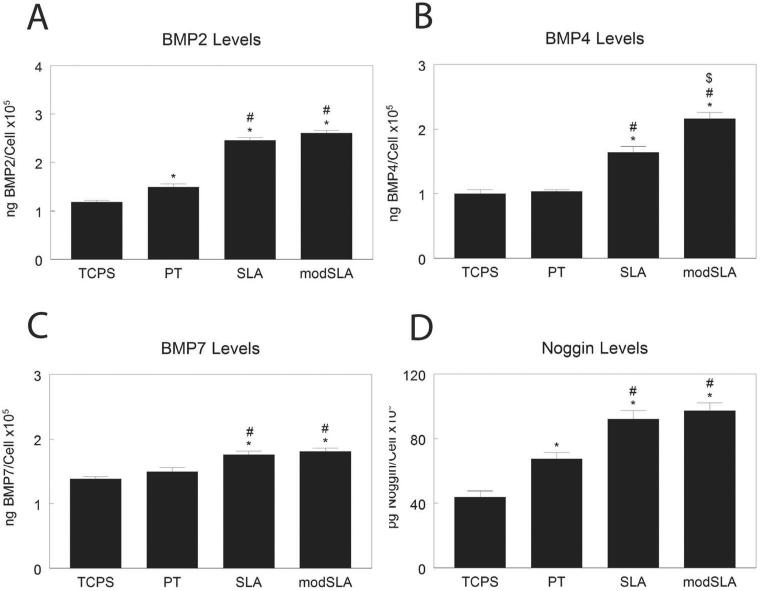
Levels of BMP2 were higher on PT than TCPS, and were additionally increased by surface roughness (Fig. 6A). BMP4 was higher on SLA than TCPS or PT, and additionally increased by modSLA (Fig. 6B). BMP7 was modestly increased by culture on rough SLA and modSLA in comparison to smooth surfaces (Fig. 6C). Secreted Noggin was higher on PT than TCPS, and was additionally increased by surface roughness (Fig. 6D).
Figure 6.
Levels of BMPs in conditioned media of normal human osteoblasts grown on microstructured titanium surfaces. Normal human osteoblasts were plated on TCPS, PT, SLA, and modSLA surfaces. Conditioned media was collected and levels of BMP2 (A), BMP4 (B), BMP7 (C), and noggin (D) measured by ELISA. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA.
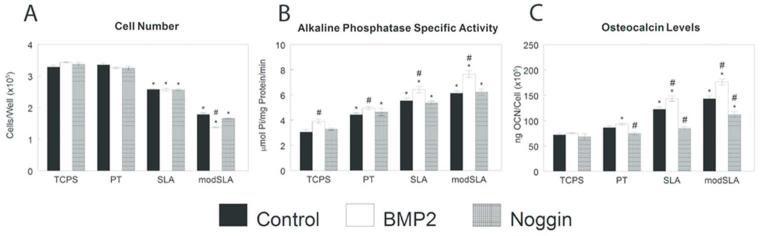
Modulation of BMP molecules affected osteoblastic maturation on microstructured Ti substrates. While on addition of Noggin had no effect on cell number, BMP2 treatment decreased cell number on modSLA surfaces in comparison to control (Fig. 7A). Alkaline phosphatase specific activity was increased by BMP2 treatment in comparison to untreated cells on all substrates (Fig. 7B). Treatments had no effect on osteocalcin on TCPS (Fig. 7C). However, exogenous Noggin decreased osteocalcin on PT and SLA substrates while BMP2 enhanced osteocalcin production only on rough SLA and modSLA substrates.
Figure 7.
Effect of exogenous BMP2 or noggin on osteogenic differentiation of MG63 cells grown on microstructured titanium surfaces. MG63 cells were plated on TCPS, PT, SLA, and modSLA surfaces and treated daily with 40ng/ml BMP2 or 100 ng/ml Noggin. Cell number (A) and alkaline phosphatase specific activity (B) of the cell lysates were measured 24h after confluence. Conditioned media was collected and levels of osteocalcin (C) measured. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA; %p<0.05, treated vs. untreated cell cultures.
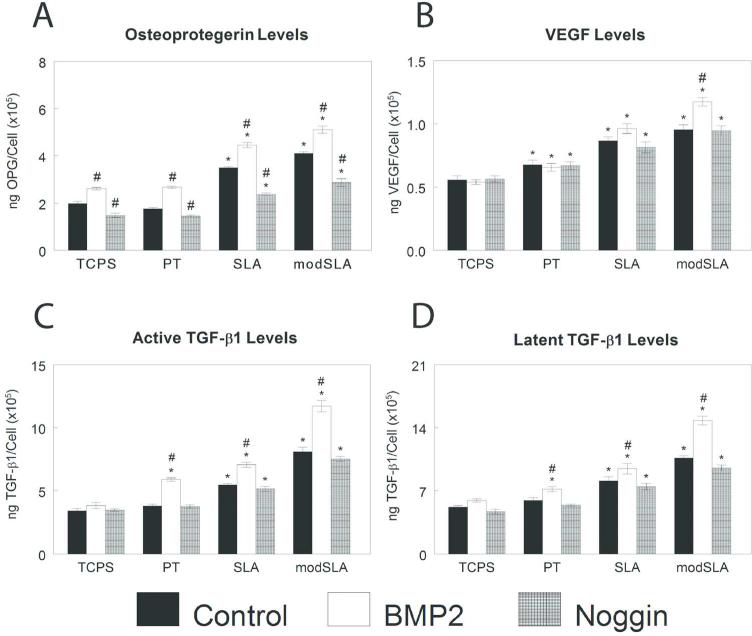
Modulation of BMP signaling also affected local factor production on microstructured Ti substrates. OPG was sensitive to changes in BMP signaling. BMP2 increased and Noggin decreased OPG to a similar extent on all substrates (Fig. 8A). BMP2 treatment increased VEGF modestly only on modSLA; Noggin had no effect (Fig. 8B). Treatment with Noggin had no effect on active (Fig. 8C) or latent (Fig. 8D) TGF-β1, while BMP2 treatment increased TGF-β1 only on Ti.
Figure 8.
Effect of exogenous BMP2 or noggin on local factor production of MG63 cells grown on microstructured titanium surfaces. MG63 cells were plated on TCPS, PT, SLA, and modSLA surfaces and treated daily with 40ng/ml BMP2 or 100 ng/ml Noggin. Conditioned media was collected and levels of OPG (A), VEGF-A (B), active TGF-β1 (C), and latent TGF-β1 (D) measured. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA; %p<0.05, treated vs. untreated cell cultures.
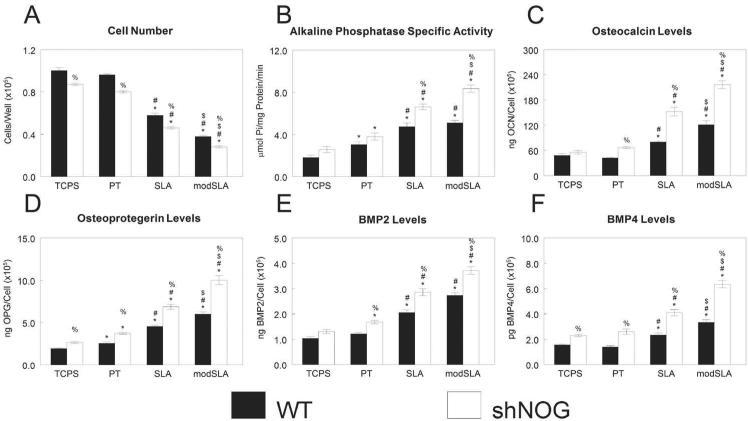
We successfully generated a stably silenced MG63 cell line with 29% expression of NOG as in wild-type (WT) MG63 cells as determined by real-time PCR (NOG/GAPDH: 1.91±0.23 vs. 6.42±2.29). shNOG cells had lower cell number than WT cells both on TCPS and on Ti substrates (Fig. 9A). Alkaline phosphatase specific activity was similar between the two types of cells on TCPS and PT (Fig. 9B). However, shNOG had higher enzyme activity on SLA substrates than WT, an effect enhanced on the high-energy modSLA. WT and shNOG produced similar levels of osteocalcin on TCPS, but shNOG had higher osteocalcin levels than WT on PT; roughness enhanced this effect (Fig. 9C). OPG was higher in shNOG than WT MG63 cells, and increased synergistically with roughness and surface energy (Fig. 9D). On TCPS, there was no difference in BMP2 production between WT and shNOG cells (Fig. 9E). However, shNOG cells on Ti had higher BMP2 levels than WT cells, an effect enhanced by surface roughness. BMP4 levels were higher in shNOG than WT MG63 cells, an effect enhanced by culture on rough and hydrophilic rough surfaces (Fig. 9F).
Figure 9.
Effect of silencing noggin on MG63 cells response to surface roughness and surface energy. WT and noggin-silenced MG63 cells (shNOG) were plated on TCPS, PT, SLA, and modSLA surfaces. Cell number (A) and alkaline phosphatase specific activity (B) of the cell lysates were measured 24h after confluence. Conditioned media was collected and levels of osteocalcin (C), OPG (D), BMP2 (E), BMP4 (F) assayed. Data are the mean±SEM of six independent cultures. *p< 0.05, Ti surface vs. TCPS; #p< 0.05, SLA or modSLA vs. PT; $p< 0.05, modSLA vs. SLA; %p<0.05, shNOG vs. WT cultures.
DISCUSSION
Rough, hydrophilic implants yield increased osteoblast differentiation and maturation, as well as bone formation. However, the factors involved in this process are still unclear. Here we demonstrate that BMP ligands, receptors, and inhibitors are regulated differentially by Ti surface topography and chemistry. Cells grown on microstructured Ti are more mature and produce more BMP-related molecules than cells on smooth Ti. Finally, processes that lead to osseointegration can be regulated by modulating the presence of Noggin, one of the most potent BMP inhibitors.
Several studies have investigated coupling BMP2 with Ti implants to enhance peri-implant bone formation (16, 26, 27). Unfortunately, the results obtained in most of the studies vary from relative success to complete failure. However, it is less studied whether surface modifications alone can enhance endogenous BMP production, thus allowing factors produced in response to the surface characteristics to promote osseointegration in the absence of exogenous factors.
The results of our study demonstrate that mRNA and protein levels of BMP2 and BMP4 are increased in cells on rough surfaces, and that BMP7 mRNA and protein levels are increased in cells cultured on Ti. These results are corroborated by results of several other studies. Increased BMP2 on microrough Ti surfaces has been demonstrated using microarray analysis of human osteoblasts (7) and in mesenchymal stem cells cultured on acid etched surfaces (28). In addition, our group has demonstrated that rough Ti alloy surfaces also stimulated BMP2, BMP4, and BMP7 production in MG63 cells (29).
Our study has also shown that osteoblasts not only regulate secreted BMP molecules but also increase expression of BMP receptors when grown on microstructured Ti surfaces. BMP receptors control signal transduction, and the signaling pathways activated depend on the composition of type I and type II receptors in the complex (30). BMPR1A aggregation is required for transduction of BMP2 signaling (31), and signaling through BMPR1A (32), BMPR1B (33), and BMPR2 (34) are required for osteoblast differentiation and bone formation. Taken together, the results suggest that microstructured Ti surfaces induce osteoblast maturation and production of an osteogenic environment increasing the levels of BMP2, BMP4, and BMP7 that have been demonstrated to be crucial for bone formation and remodeling. Osteoblasts grown on these rough surfaces increase the expression of BMP receptors suggesting that the cells are more sensitive to the BMP stimulus that can lead to more and faster bone formation.
BMP signaling is also regulated by secreted inhibitors of the DAN, twisted gastrulation, and Noggin/Chordin subfamilies (35). Here we demonstrate that BMP inhibitors Noggin, Gremlin-1, Cerberus-1, and Follistatin are strongly regulated by surface roughness. The presence of these BMP inhibitors appears to be important for normal osteoblastogenesis and bone formation, and their perturbation has important effects on skeletal phenotypes. For example, Noggin-null mice have decreased numbers of osteoprogenitor cells, impaired joint formation, and thin calvaria (36). Mice overexpressing Noggin under the osteocalcin promoter have reduced bone formation and lower mineral density than wild type mice (37). Overexpression of Gremlin-1 under the osteocalcin promoter also displayed reduced bone mineral density (38). Follistatin-null mice have cartilage and skeletal defects and die immediately following birth (39). However, mice with deletions in Cerberus do not have a reported bone phenotype (40). The increase of most of the BMP inhibitors in our study at the same time point that the BMP molecules increase can be explained by the fact that most of the BMP inhibitors are regulated by BMPs (41, 42). Our results suggest that surface microstructure may modulate the expression and secretion of BMP molecules and receptors, and these molecules induce the expression and secretion of their own inhibitors providing a mechanism for normal bone formation.
There were fewer cells on the Ti substrates, with the fewest numbers on the microtextured Ti. This was due in part to reduced attachment and enhanced differentiation (43). Differences in maturation state and potentially to fewer cell/cell contacts may have contributed to differences in cell response.
Modulation of BMP signaling, either by enhancing BMP activation with addition of exogenous BMP2 or by reducing BMP signaling with exogenous Noggin, is very attractive from both scientific and clinical perspectives. In recent years, an increase in treatments to enhance bone formation in challenging cases has been achieved by modulating BMP signaling. The most common protocol for manipulating BMP signaling has been the addition of high amounts of exogenous BMP2. The results of these therapies vary from very successful regeneration to lack of activity to unwanted secondary effects. Secondary effects have been reported that include bone resorption, ectopic bone formation, and swelling (44).
In our study, we hypothesized that manipulation of the BMP signaling inhibitor Noggin by RNA silencing technology could lead to an increase in osteoblast maturation without the use of exogenous of BMP2. Exogenous BMP2 had only small effects on osteoblast maturation. BMP2 increased alkaline phosphatase specific activity in MG63 cells, an effect independent of surface roughness or energy, corroborating results seen in both chondrocytes (45) and osteoblasts (46). Unexpectedly, silencing Noggin caused an even greater increase in alkaline phosphatase specific activity than exogenous BMP2. Addition of BMP2 to MG63 cell cultures increased osteocalcin levels in cells cultured on all Ti samples but not on TCPS. However, shNOG cells exhibited a higher increase on microstructured Ti surfaces. Exogenous BMP2 has been demonstrated to increase osteocalcin in mouse osteoblasts cultured on TCPS, but the doses used were higher (100 ng/ml-200 ng/ml) than those used in this study (50 ng/ml) (46, 47). Addition of Noggin reduced osteocalcin secretion on Ti surfaces, suggesting that the exogenous inhibitor was able to prevent osteocalcin production induced by endogenous BMP proteins. Taken together, our data suggest that increasing the availability of endogenous BMP molecules by inhibiting Noggin may have a more potent effect than addition of exogenous BMP molecules. This increase in osteoblast differentiation with a Noggin inhibition strategy has also been demonstrated in vivo (50).
BMP signaling has been shown to control osteoclastogenesis in vitro (48) and in vivo (49). Activating BMP signaling either by exogenous BMP2 or silencing Noggin also affected production and release of important soluble factors involved in bone formation, remodeling, and homeostasis. Osteoprotegerin, an inhibitor to osteoclast activation, was modulated in osteoblasts with inhibition or activation of BMP signaling on microstructured Ti surfaces, suggesting that BMP signaling influences the process of bone resorption. Interestingly, our results showed that silencing Noggin strongly increased levels of BMP2 and BMP4, particularly on microstructured surfaces. Taken together, these results suggest that endogenous BMP molecules can modulate osteoblast maturation more efficiently and at a lower dose. It is possible that this modulation could be time-dose-specific and yield in a better result than one sustained exogenous BMP2 dose.
Exogenous BMP2 or Noggin had little effect of VEGF production in our study. Many strategies examining instructive microenvironments for bone regeneration co-deliver BMP2 and VEGF (51-53), indicating that BMP2 enhances bone formation but that other signaling molecules are necessary to induce angiogenesis and complete bone healing. In our study, exogenous BMP2 increased TGF-β1. TGF-β1 is important during embryonic skeleton formation as well as in maintenance of the adult bone (54, 55). Specifically, TGF-β1 acts on osteoblasts to regulate matrix synthesis and differentiation (54). Because BMP2 induces osteoblast maturation in this system, it is likely the increased TGF-β1 levels are due to increased maturation state. However, it is also possible that tight regulation of TGF-β superfamily molecules occurs in response to perturbations in the signaling pathway.
CONCLUSIONS
The results of our study demonstrate that BMP ligands, receptors, and inhibitors are tightly regulated on microstructured Ti surfaces. Exogenous BMP2 or Noggin can minimally enhance or inhibit this process, but the most robust maturation response is seen when the amount of Noggin is reduced. It is likely that modulating exploit the body's regenerative capacity using endogenous BMP signaling may yield more robust bone formation than application of exogenous BMPs.
ACKNOWLEDGEMENTS
Ti disks were provided by Institut Straumann AG (Basel, Switzerland) as a gift. We thank The authors have no conflicts of interest to disclose. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR052102. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821–9. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105:15767–72. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares-Navarrete R, Raines AL, Hyzy SL, Park JH, Hutton DL, Cochran DL, Boyan BD, Schwartz Z. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 2012;27:1773–1783. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31:4909–17. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakki SS, Bozkurt SB, Hakki EE, Korkusuz P, Purali N, Koc N, Timucin M, Ozturk A, Korkusuz F. Osteogenic differentiation of MC3T3-E1 cells on different titanium surfaces. Biomed Mater. 2012;7:045006–6041/7/4/045006. doi: 10.1088/1748-6041/7/4/045006. Epub 2012 May 8. [DOI] [PubMed] [Google Scholar]
- 7.Vlacic-Zischke J, Hamlet SM, Friis T, Tonetti MS, Ivanovski S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFbeta/BMP signalling in osteoblasts. Biomaterials. 2011;32:665–71. doi: 10.1016/j.biomaterials.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta biomaterialia. 2011;7:2740–50. doi: 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, Boyan BD, Schwartz Z. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials. 2011;32:6399–411. doi: 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyzy SL, Olivares-Navarrete R, Hutton DL, Tan C, Boyan BD, Schwartz Z. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous BMP-2. Acta Biomater. 2013;9:5821–5829. doi: 10.1016/j.actbio.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine & Growth Factor Reviews. 2005;16:251–263. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 13.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 14.Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;3:mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 15.Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–484. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 16.Schliephake H, Aref A, Scharnweber D, Bierbaum S, Roessler S, Sewing A. Effect of immobilized bone morphogenic protein 2 coating of titanium implants on peri-implant bone formation. Clin Oral Implants Res. 2005;16:563–569. doi: 10.1111/j.1600-0501.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 17.Towler DA. Bone morphogenetic proteins. Blood. 2009;114:2012–2013. doi: 10.1182/blood-2009-06-228544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 19.Wan M, Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651–7. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 20.Donos N, Hamlet S, Lang NP, Salvi GE, Huynh-Ba G, Bosshardt DD, Ivanovski S. Gene expression profile of osseointegration of a hydrophilic compared with a hydrophobic microrough implant surface. Clin Oral Implants Res. 2011;22:365–372. doi: 10.1111/j.1600-0501.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 21.Rupp F, Scheideler L, Olshanska N, deWild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76A:323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 22.Bachle M, Kohal RJ. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin Oral Implants Res. 2004;15:683–92. doi: 10.1111/j.1600-0501.2004.01054.x. [DOI] [PubMed] [Google Scholar]
- 23.Olivares-Navarrete R, Hyzy SL, Chaudhri RA, Zhao G, Boyan BD, Schwartz Z. Sex dependent regulation of osteoblast response to implant surface properties by systemic hormones. Biol Sex Differ. 2010;1:4. doi: 10.1186/2042-6410-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyzy SL, Olivares-Navarrete R, Schwartz Z, Boyan BD. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J Cell Biochem. 2012;113:3236–3245. doi: 10.1002/jcb.24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JY, Dean DD, Cochran DL, Simpson J, Boyan BD, Schwartz Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin Oral Implants Res. 1996;7:27–37. doi: 10.1034/j.1600-0501.1996.070104.x. [DOI] [PubMed] [Google Scholar]
- 26.Wikesjo UM, Polimeni G, Qahash M. Tissue engineering with recombinant human bone morphogenetic protein-2 for alveolar augmentation and oral implant osseointegration: experimental observations and clinical perspectives. Clin Implant Dent Relat Res. 2005;7:112–119. doi: 10.1111/j.1708-8208.2005.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Huse RO, de Groot K, Buser D, Hunziker EB. Delivery mode and efficacy of BMP-2 in association with implants. J Dent Res. 2007;86:84–89. doi: 10.1177/154405910708600114. [DOI] [PubMed] [Google Scholar]
- 28.Balloni S, Calvi EM, Damiani F, Bistoni G, Calvitti M, Locci P, Becchetti E, Marinucci L. Effects of titanium surface roughness on mesenchymal stem cell commitment and differentiation signaling. Int J Oral Maxillofac Implants. 2009;24:627–635. [PubMed] [Google Scholar]
- 29.Olivares-Navarrete R, Gittens RA, Schneider JM, Hyzy SL, Haithcock DA, Ullrich PF, Schwartz Z, Boyan BD. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12:265–272. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinecke K, Seher A, Schmitz W, Mueller TD, Sebald W, Nickel J. Receptor oligomerization and beyond: a case study in bone morphogenetic proteins. BMC Biol. 2009;7:59–7007-7-59. doi: 10.1186/1741-7007-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Effect of the distribution and clustering of the type I A BMP receptor (ALK3) with the type II BMP receptor on the activation of signalling pathways. J Cell Sci. 2003;116:3277–3284. doi: 10.1242/jcs.00519. [DOI] [PubMed] [Google Scholar]
- 32.Baek JA, Lan Y, Liu H, Maltby KM, Mishina Y, Jiang R. Bmpr1a signaling plays critical roles in palatal shelf growth and palatal bone formation. Dev Biol. 2011;350:520–531. doi: 10.1016/j.ydbio.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singhatanadgit W, Olsen I. Endogenous BMPR-IB signaling is required for early osteoblast differentiation of human bone cells. In Vitro Cell Dev Biol Anim. 2011;47:251–259. doi: 10.1007/s11626-010-9378-z. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Zhang R, Chen D, Oyajobi BO, Zhao M. Functional redundancy of type II BMP receptor and type IIB activin receptor in BMP2-induced osteoblast differentiation. J Cell Physiol. 2012;227:952–963. doi: 10.1002/jcp.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe E. Function of BMPs and BMP antagonists in adult bone. Ann N Y Acad Sci. 2006;1068:41–53. doi: 10.1196/annals.1346.007. [DOI] [PubMed] [Google Scholar]
- 36.Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 37.Wu XB, Li Y, Schneider A, Yu W, Rajendren G, Iqbal J, Yamamoto M, Alam M, Brunet LJ, Blair HC, Zaidi M, Abe E. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest. 2003;112:924–934. doi: 10.1172/JCI15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzerro E, Pereira RC, Jorgetti V, Olson S, Economides AN, Canalis E. Skeletal overexpression of gremlin impairs bone formation and causes osteopenia. Endocrinology. 2005;146:655–665. doi: 10.1210/en.2004-0766. [DOI] [PubMed] [Google Scholar]
- 39.Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- 40.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 41.Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest. 1998;102:2106–2114. doi: 10.1172/JCI3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimelman D, Szeto DP. Chordin cleavage is sizzling. Nat Cell Biol. 2006;8:305–307. doi: 10.1038/ncb0406-305. [DOI] [PubMed] [Google Scholar]
- 43.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258–64. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 44.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62:ONS423–31. doi: 10.1227/01.neu.0000326030.24220.d8. discussion ONS431. [DOI] [PubMed] [Google Scholar]
- 45.Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, Rupp F. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem Biophys Res Commun. 2004;315:272–280. doi: 10.1016/j.bbrc.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 46.Jang WG, Kim EJ, Kim DK, Ryoo HM, Lee KB, Kim SH, Choi HS, Koh JT. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. J Biol Chem. 2012;287:905–915. doi: 10.1074/jbc.M111.253187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes-Fulford M, Li CF. The role of FGF-2 and BMP-2 in regulation of gene induction, cell proliferation and mineralization. J Orthop Surg Res. 2011;6:8–799X-6-8. doi: 10.1186/1749-799X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai Y, Bai Y, Matsuzaka K, Hashimoto S, Kokubu E, Wang X, Inoue T. Formation of bone-like tissue by dental follicle cells co-cultured with dental papilla cells. Cell Tissue Res. 2010;342:221–231. doi: 10.1007/s00441-010-1046-9. [DOI] [PubMed] [Google Scholar]
- 49.Sotillo Rodriguez JE, Mansky KC, Jensen ED, Carlson AE, Schwarz T, Pham L, MacKenzie B, Prasad H, Rohrer MD, Petryk A, Gopalakrishnan R. Enhanced osteoclastogenesis causes osteopenia in twisted gastrulation-deficient mice through increased BMP signaling. J Bone Miner Res. 2009;24:1917–1926. doi: 10.1359/JBMR.090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takayama K, Suzuki A, Manaka T, Taguchi S, Hashimoto Y, Imai Y, Wakitani S, Takaoka K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab. 2009;27:402–11. doi: 10.1007/s00774-009-0054-x. [DOI] [PubMed] [Google Scholar]
- 51.Jiang J, Fan CY, Zeng BF. Experimental Construction of BMP2 and VEGF Gene Modified Tissue Engineering Bone in Vitro. Int J Mol Sci. 2011;12:1744–1755. doi: 10.3390/ijms12031744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao C, Zhou H, Liu G, Zhang P, Fu Y, Gu P, Hou H, Tang T, Fan X. Bone marrow stromal cells with a combined expression of BMP-2 and VEGF-165 enhanced bone regeneration. Biomed Mater. 2011;6:015013–6041/6/1/015013. doi: 10.1088/1748-6041/6/1/015013. Epub 2011 Jan 21. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Yang Z, Sun S, Huang H, Sun X, Wang Z, Zhang Y, Zhang B. Adipose-derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy. 2010;12:831–840. doi: 10.3109/14653249.2010.495980. [DOI] [PubMed] [Google Scholar]
- 54.Kanaan RA, Kanaan LA. Transforming growth factor beta1, bone connection. Med Sci Monit. 2006;12:RA164–9. [PubMed] [Google Scholar]
- 55.Bonewald LF, Dallas SL. Role of active and latent transforming growth factor beta in bone formation. J Cell Biochem. 1994;55:350–7. doi: 10.1002/jcb.240550312. [DOI] [PubMed] [Google Scholar]