Abstract
The value of performing follow-up PET/CT imaging more than 6 mo after the conclusion of therapy—either as a routine practice or because of clinically suspected recurrence—is not well established. The purpose of this study was to evaluate the added value of follow-up PET/CT to the clinical assessment and survival outcome of lung cancer patients.
Methods
This was a retrospective study of 261 biopsy-proven lung cancer patients at a single tertiary center. In total, 488 follow-up PET/CT scans done 6 or more months after the completion of initial treatment were included in this study. Median follow-up from the completion of primary treatment was 29.3 mo (range, 6.1–295.1 mo). Overall survival (OS) benefit was measured using Kaplan–Meier plots with a Mantel–Cox log-rank test. A multivariate Cox regression model was provided with clinical covariates.
Results
Of the 488 PET/CT scans, 281 were positive and 207 negative for recurrence. Overall median survival from the time of the PET/CT study was 48.5 mo. The median survival of PET-positive and PET-negative groups was 32.9 and 81.6 mo, respectively (P < 0.0001). A subgroup analysis demonstrated a similar difference in OS for 212 scans completed between 6 and 24 mo after treatment (P = 0.0004) and 276 scans completed after 24 mo (P = 0.0006). In the context of clinical assessment, PET/CT identified recurrence in 43.7% (107/245) of scans without prior clinical suspicion and ruled out recurrence in 15.2% (37/243) of scans with prior clinical suspicion. There was a significant difference in OS when grouped by clinical suspicion (P = 0.0112) or routine follow-up (P < 0.0001). In a multivariate Cox regression model, factors associated with OS were age (P < 0.0001) and PET/CT result (P = 0.0003). An age-stratified subgroup analysis demonstrated a significant difference in OS by PET scan result among patients younger than 60 y and between 60 and 70 y but not in those older than 70 y (P < 0.0001, P = 0.0004, and P = 0.8193, respectively).
Conclusion
18F-FDG PET/CT performed for follow-up more than 6 mo after the completion of primary treatment adds value to clinical judgment and is a prognostic marker of OS in lung cancer patients, regardless of the timing of the follow-up scan, and especially in patients younger than 70 y.
Keywords: survival, lung cancer, PET, surveillance
Lung cancer is the most common cause of cancer-related mortality, accounting for an estimated 1.3 million deaths worldwide per year (1). In the United States, the lifetime risk of lung cancer is 6.9%, with an estimated incidence of 228,190 in 2013 and an estimated mortality of 159,480 in 2013 (2). Overall 5-y survival for individuals diagnosed with lung cancer is 16.6% (2). Lung cancer is most likely to recur in the first 4 y after curative-intent therapy, with 10% of recurrence being after 5 y (3,4).
The recommendations of the National Comprehensive Cancer Network for lung cancer surveillance are chest CT with or without a contrast agent every 6–12 mo for 2 y, followed by annual non–contrast-enhanced chest CT thereafter (5). Similar recommendations for non–small cell lung cancer are made by the European Society for Medical Oncology: follow-up imaging every 3–6 mo for the first 2 y and annually thereafter (1). PET/CT is not included as part of follow-up or surveillance, although the literature provides evidence of its high diagnostic performance, with improved sensitivity and specificity over CT for cancer recurrence (3,5–14). The improved diagnostic accuracy of 18F-FDG PET/CT is necessary but not a sufficient condition in itself to improve outcome, unless the management is altered and therapy is efficacious. Colice et al. completed an analysis of different guidelines on radiologic surveillance of lung cancer; however, a recommendation for routine PET surveillance after therapy was not given because of the limited number of studies showing added value (3). Despite extensive literature on the improved accuracy of 18F-FDG PET and PET/CT over CT, in lung cancer there is little evidence supporting the use of surveillance 18F-FDG PET/CT over current conventional imaging methods for predicting survival, as has previously been established between chest CT and chest radiography (15). However, there is evolving evidence of the value of 18F-FDG PET/CT in follow-up or surveillance for patient outcomes in other cancers (16,17).
The objective of this study was to evaluate the prognostic value of 18F-FDG PET/CT in overall survival (OS) of lung cancer patients when performed between 6 and 295 mo after completion of curative primary treatment, with or without clinical suspicion of recurrence.
MATERIALS AND METHODS
Eligible Patients and Follow-up
This was a retrospective study performed under a waiver of informed consent as approved by the Institutional Review Board. The guidelines of the Health Insurance Portability and Accountability Act were followed. All patients treated for biopsy-proven lung cancer at a single tertiary center with one or more follow-up 18F-FDG PET/CT scans obtained at least 6 mo after completion of primary treatment were included in the study. Research has shown that the accuracy of PET/CT increases when performed at 6 mo or more after treatment (10). This increased accuracy is due in part to reduced inflammation in the treated tissue by that time, and thus follow-up PET/CT is recommended no earlier than 4–5 mo after therapy (10). For this reason, only PET/CT studies conducted at least 6 mo after treatment were included in this study. Between January 2001 and October 2013, a total of 261 lung cancer patients met our study inclusion criteria, providing a total of 488 follow-up PET/CT scans (range, 1–12 per patient). These scans were performed as part of routine clinical follow-up or at the time of clinical concern about suspected recurrence. All patients were followed till death or their last day of clinical follow-up at our center. The median follow-up time among these patients was 29.3 mo (range, 6.1–295.1 mo). Patient demographics, histology, stage, and other therapy details are summarized in Table 1.
TABLE 1.
Characteristics of the 261 Patients
| Characteristic | n | % |
|---|---|---|
| Age (mean ± SD, 66 ± 12 y) | ||
| <60 y | 71 | 27.2 |
| 60–70 y | 97 | 37.2 |
| >70 y | 93 | 35.6 |
| Sex | ||
| Female | 143 | 54.8 |
| Male | 118 | 45.2 |
| Race | ||
| White | 185 | 70.9 |
| Black | 62 | 23.7 |
| Other | 14 | 5.4 |
| Smoking | ||
| Yes | 221 | 84.7 |
| No | 33 | 12.6 |
| Unknown | 7 | 2.7 |
| Histology | ||
| Adenocarcinoma | 79 | 30.3 |
| Bronchioalveolar carcinoma | 7 | 2.7 |
| Bronchogenic carcinoid | 1 | 0.4 |
| Carcinoid | 7 | 2.7 |
| Epithelioid neoplasm | 1 | 0.4 |
| Mesothelioma | 8 | 3.1 |
| NSCLC | 100 | 38.7 |
| SCC | 55 | 21.1 |
| Unknown | 2 | 0.8 |
| Stage | ||
| I | 90 | 34.5 |
| II | 23 | 8.8 |
| III | 81 | 31.0 |
| IV | 17 | 6.5 |
| Unknown | 50 | 19.2 |
| Last treatment | ||
| Surgery | 111 | 42.5 |
| Radiation | 74 | 28.4 |
| Chemotherapy | 76 | 29.1 |
| PET/CT outcome | ||
| Negative | 63 | 24.1 |
| Positive | 198 | 75.9 |
NSCLC = non–small cell lung cancer; SCC = squamous cell carcinoma.
18F-FDG PET/CT Protocol
PET/CT studies were performed according to institutional clinical protocol. Patients were injected with a standard 73.4 MBq/kg (0.9 mCi/lb) dose of 18F-FDG, with a maximum of 925 MBq (25 mCi), and PET/CT imaging was initiated 1 h after 18F-FDG administration using 2 different PET/CT systems: Discovery LS (2-dimensional) or Discovery VCT (3-dimensional) (GE Healthcare). Whole-body scanning was performed, with images obtained on supine patients with their arms above their head, using a 128 × 128 matrix and a rate of 5 min per bed position. PET images were reconstructed with a 2-dimensional and 3-dimensional ordered-subsets expectation maximization algorithm. All PET data were reconstructed with and without CT-based attenuation correction. Helical CT (120 kV; 20–2,000 mAs; 8.0 noise index) images were obtained with a matrix of 512 × 512. Beam collimation was 10 mm, with a pitch of 0.984. Slice thickness was 3.75 mm, and field of view was 50 cm.
Image Analysis
Board-certified nuclear medicine physicians provided clinical readings of all 18F-FDG PET/CT images at the time the imaging was performed. The clinical reports were retrospectively read and categorized as positive, indeterminate, or negative by a nuclear medicine resident and a nuclear medicine postdoctoral fellow. Positive reports clearly stated that there was recurrence of primary disease or evidence of 18F-FDG–avid metastasis. Indeterminate reports did not confirm or deny recurrence, and their impression section included language such as “indeterminate,” “suspicious for recurrence,” or “cannot exclude recurrence.” Negative reports clearly concluded that there was no evidence of recurrence. The first scan positive for recurrence was used in the analysis of OS, and all subsequent scans were excluded from the study. Follow-up studies were further grouped as having been performed for routine surveillance or secondary to clinical suspicion of recurrence or metastases. The last clinical note of the requesting physician and the indication for the study in the clinical report were analyzed to establish clinical suspicion, which was clearly stated as a concern about recurrence or metastasis because of patient symptoms, physical examination findings, or suggestive findings on a recent CT scan. An 18F-FDG PET/CT scan was established as having been obtained for routine surveillance if no concerns about lung cancer recurrence or metastasis were noted in the patient’s electronic medical records or in the request form for the 18F-FDG PET/CT scan.
Outcome Measures
The primary clinical endpoint of the analyses was OS, which was defined as the time between the follow-up 18F-FDG PET/CT scan and death. For each patient, the date of the PET/CT scan was recorded. Additionally, the date of death was recorded from a review of medical records and a public registry of death (18). Surviving patients were censored on their last day of clinic follow-up at our center.
Statistical Analysis
We present data as mean ± SD for central tendencies, as median followed by range in parentheses for skewed data, and as frequency and percentage for categoric variables. Between groups, analyses were performed using independent-samples t testing, but when data were skewed, the Mann–Whitney U test was used. Our analysis was undertaken to determine whether there was an association between the follow-up PET/CT result and OS. Survival probabilities were calculated using Kaplan–Meier survival curves and compared using the Mantel–Cox log-rank test. The association of clinical variables with OS was evaluated using univariate and multivariate Cox regression models. To establish the effect of PET/CT results on survival outcome, a hierarchical regression analysis was also performed using the significant clinical predictors before including the PET/CT result. Statistical significance was set at a 2-tailed P value of 0.05 for all tests. All statistical analysis was performed using the JMP statistical package (version 11.0; SAS Institute Inc.).
RESULTS
Categorization of PET/CT Result
In total, 488 18F-FDG PET/CT scans were obtained from 261 lung cancer patients (118 male, 143 female). Of these patients, 88.9% (232/261) had 1–3 scans, 10.3% (27/261) had 4–6 scans, and 0.8% (2/261) had 7–12 scans. For clinical utility purposes, the negative and indeterminate reports were grouped as “negative for tumor recurrence or metastasis” and positive reports were grouped as “positive for tumor recurrence or metastasis.” PET/CT scans for recurrence or metastasis had negative results in 207 cases and positive in 281 cases. Of the negative scans, 41.5% (86/207) were obtained 6–24 mo after the completion of primary treatment and 58.4% (121/207) were obtained at 24 mo or more. Of those, 39.1% (81/207) were indeterminate scans, with 43.2% (35/81) obtained 6–24 mo after treatment and 56.8% (46/81) obtained at 24 mo or more. Of the positive scans, 44.8% (126/281) were obtained 6–24 mo after treatment and 55.2% (155/281) were obtained at 24 mo or more.
Cox Regression Models and Patient Outcome
Age, sex, race, smoking status, histology (adenocarcinoma vs. non–small cell lung cancer vs. squamous cell carcinoma vs. other), stage (early stage, defined as stage I or II, vs. advanced stage, defined as stage III or IV), treatment type (surgery vs. chemotherapy vs. radiation), and PET/CT result (positive for tumor vs. negative for tumor) were included in the univariate and multivariate Cox regression models. Significant variables in the univariate analysis included age, smoking, sex, treatment, and PET result (Table 2). Only variables significant in the univariate analysis were included in the multivariate Cox model. After adjustment for these covariates, age (P < 0.0001) and PET/CT result (P < 0.0001) were the only variables significantly associated with OS (Table 3). We also performed a hierarchical regression analysis including the statistically significant clinical variables (age, sex, smoking, and treatment type) in the first step and then the PET/CT result. There was a statistically significant change in the model statistics (log likelihood 32.5, χ2 64.99, to log likelihood 41.9, χ2 82.39; P < 0.0001).
TABLE 2.
Univariate Cox Regression Analysis
| Characteristic | Estimate | 95% CI | P |
|---|---|---|---|
| Age | 0.040 | 0.02, 0.05 | <0.0001* |
| Smoking | −0.200 | −0.39, −0.03 | 0.0218* |
| Sex | 0.150 | 0.03, 0.26 | 0.0112* |
| Race | 0.1134 | ||
| White | 0.056 | −0.17, 0.32 | |
| Black | 0.273 | 0.01, 0.556 | |
| Histology | 0.1735 | ||
| Adenocarcinoma | −0.051 | −0.26, 0.15 | |
| NSCLC | 0.072 | −0.11, 0.26 | |
| SCC | 0.203 | −0.01, 0.41 | |
| Stage | −0.070 | −0.19, 0.05 | 0.263 |
| Treatment | 0.0154* | ||
| Surgery | −0.100 | −0.2, 0.05 | |
| Radiation | 0.250 | 0.08, 0.42 | |
| Time to scan | −0.001 | −0.01, 0.002 | 0.4647 |
| Clinical suspicion | −0.060 | −0.18, 0.05 | 0.2669 |
| PET result | −0.290 | −0.41, −0.18 | <0.0001* |
Significant variables.
NSCLC = non–small cell lung cancer; SCC = squamous cell carcinoma.
TABLE 3.
Multivariate Cox Regression Analysis
| Characteristic | Estimate | 95% CI | P |
|---|---|---|---|
| Age | 0.037 | 0.025, 0.048 | <0.0001* |
| Smoking | −0.118 | −0.310, 0.058 | 0.1934 |
| Sex | 0.1222 | 0.003, 0.241 | 0.0544 |
| Treatment | 0.0554 | ||
| Surgery | −0.135 | −0.306, 0.034 | |
| Radiation | 0.250 | 0.08, 0.42 | |
| PET result | −0.239 | −0.346, −0.010 | 0.0003* |
Significant variables.
PET/CT Result and Kaplan–Meier Survival Analysis
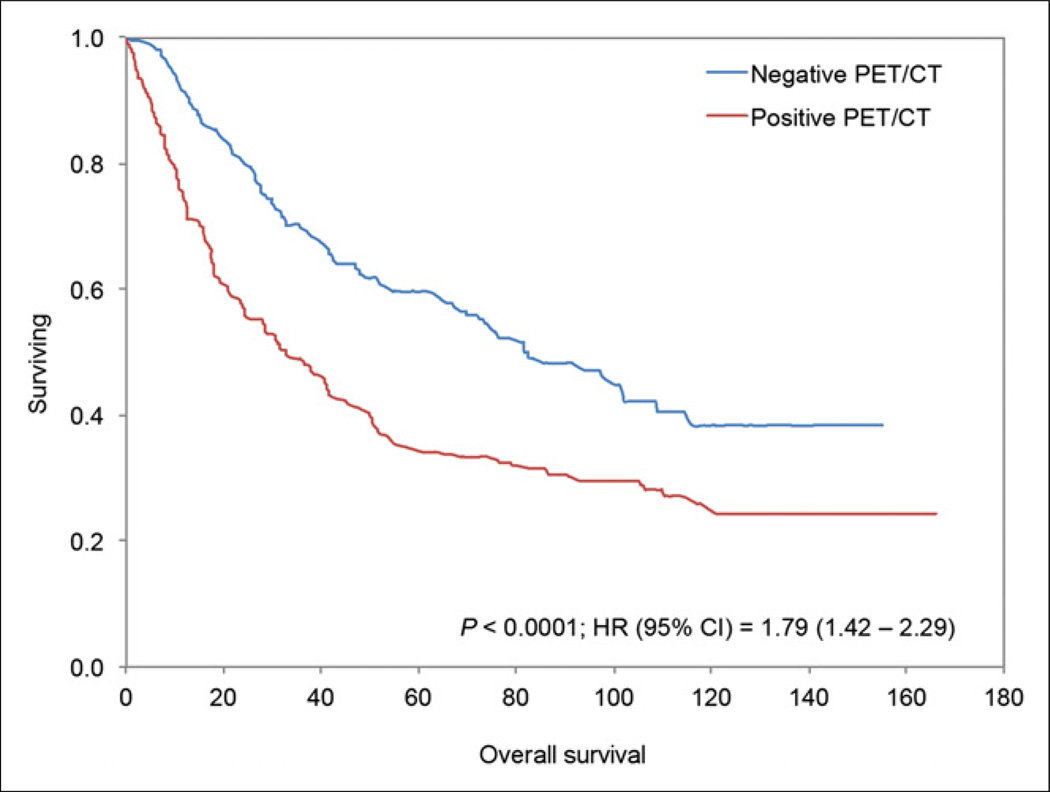
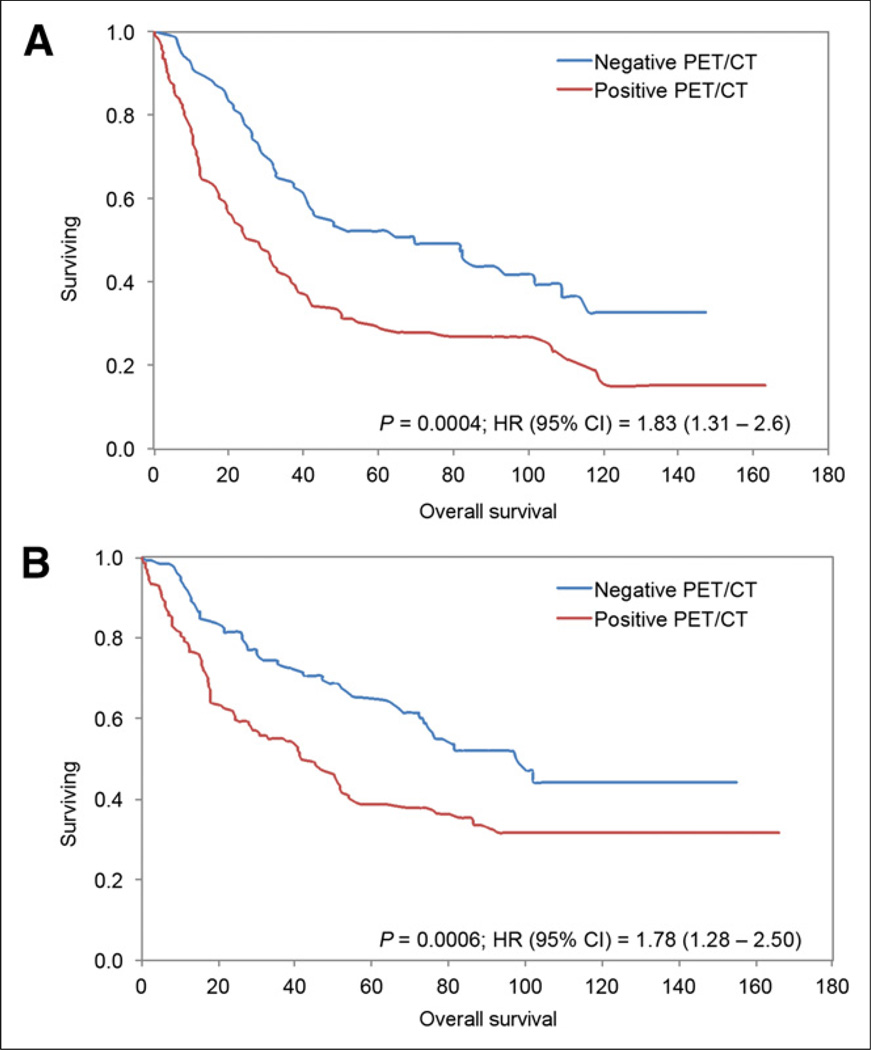
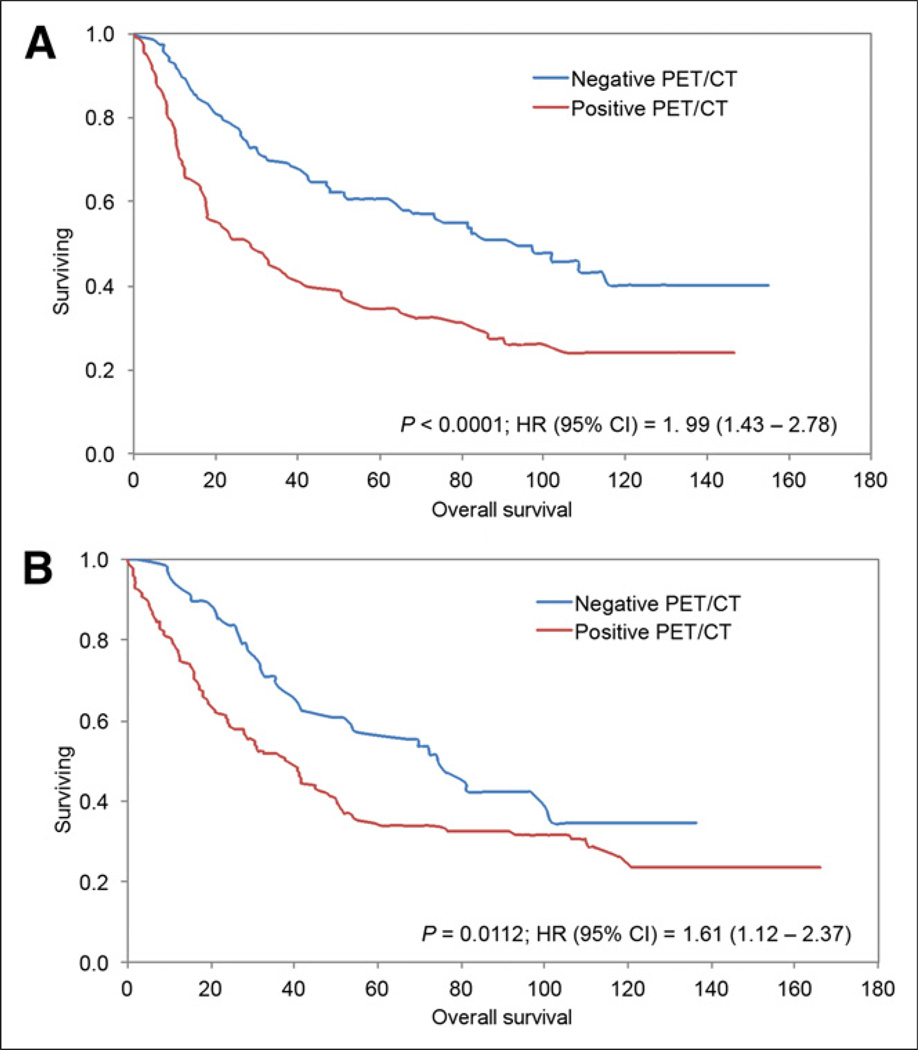
A total of 165 patients died during the study period: 135 (81.8%) had at least one positive PET/CT result and 30 (18.2%) had all negative PET/CT results (P < 0.0001). Of the 488 scans included in this study, overall median survival from the time of the PET/CT study was 48.5 mo (range, 0–166 mo). The median survival of PET-positive and PET-negative groups was 32.9 mo and 81.6 mo, respectively (P < 0.0001). The Kaplan–Meier analysis based on PET/CT results showed a significant difference in OS between those who had a positive result and those who had a negative result (log-rank P < 0.0001; hazard ratio [HR], 1.79; 95% confidence interval [CI], 1.42–2.29) (Fig. 1). A subgroup analysis demonstrated a similar difference in OS between PET-positive and PET-negative groups for 212 scans completed between 6 and 24 mo after primary treatment completion (log-rank P = 0.0004; HR, 1.83; 95% CI, 1.31–2.6) and 276 scans completed more than 24 mo after primary treatment completion (log-rank P = 0.0006; HR, 1.78; 95% CI, 1.28–2.5) (Fig. 2).
FIGURE 1.
Kaplan–Meier survival plot for all scans (n = 488) in our study. OS between PET/CT scans positive for lung tumor and scans negative for tumor differed significantly.
FIGURE 2.
Kaplan–Meier survival plots showing OS for patients with scans performed 6–24 mo after treatment (A) and scans performed more than 24 mo after treatment (B). OS differed significantly between patients with PET/CT scans positive for lung tumor and patients with PET/CT scans negative for lung tumor in both periods.
Influence of Age on the Impact of PET/CT Result and OS
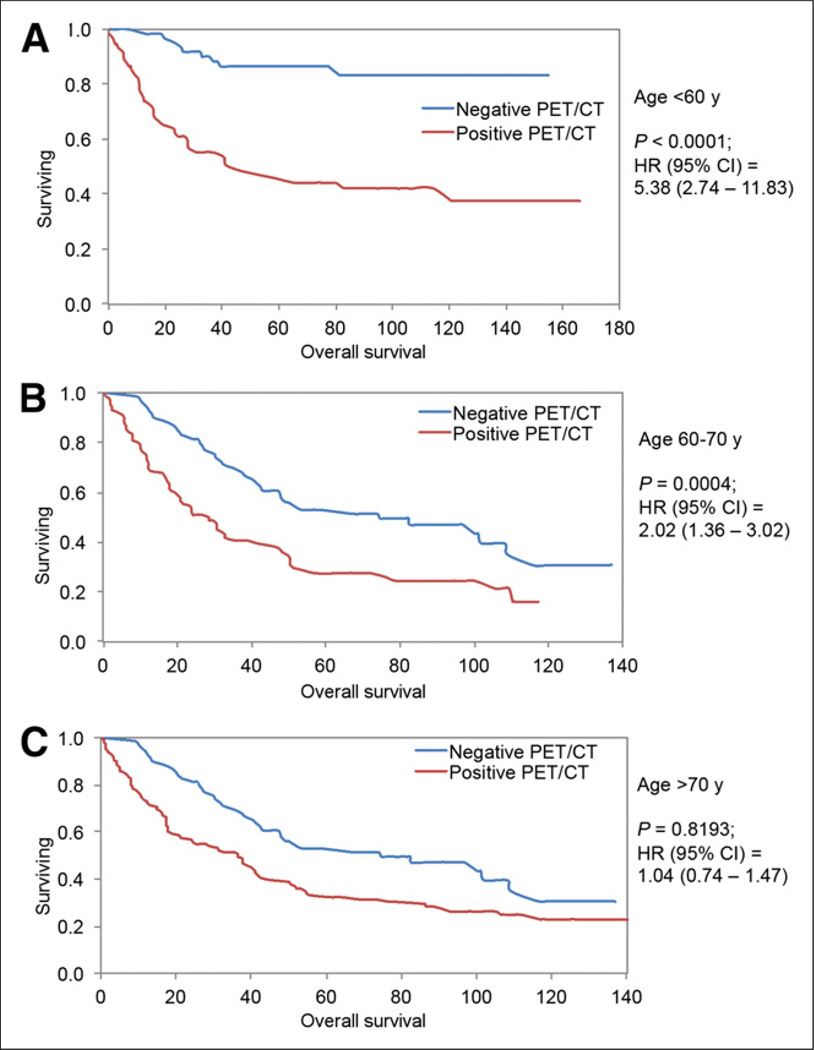
Because age was one of the independent covariates associated with OS in Cox models, we further investigated the influence of patients’ age on the impact of PET/CT results for OS. The study population was stratified into 3 groups: younger than 60 y, between 60 and 70 y old, and older than 70 y. There was a significant difference in survival between 18F-FDG PET–positive results (median survival, 45.2 mo) and 18F-FDG PET–negative results (median not reached; only 20% had died at 160 mo) in patients younger than 60 y (log-rank P < 0.0001; HR, 5.38; 95% CI, 2.74–11.83) and between 18F-FDG PET–positive results (median survival, 28.5 mo) and 18F-FDG PET–negative results (median survival, 74.61 mo) in patients 60–70 y old (log-rank P = 0.0004; HR, 2.02; 95% CI, 1.36–3.02). There was no statistically significant difference between 18F-FDG PET results and OS in patients older than 70 y (log-rank P = 0.8193; HR, 1.04; 95% CI, 0.74–1.4) (Fig. 3).
FIGURE 3.
Kaplan–Meier survival plots showing OS for patients less than 60 y old (A), 60–70 y old (B), and more than 70 y old (C). OS differed significantly between PET/CT scans positive for lung tumor and PET/CT scans negative for lung tumor in patients < 60 y old and those 60–70 y old. No significant difference was observed in patients > 70 y old.
PET/CT Results by Clinical Assessment
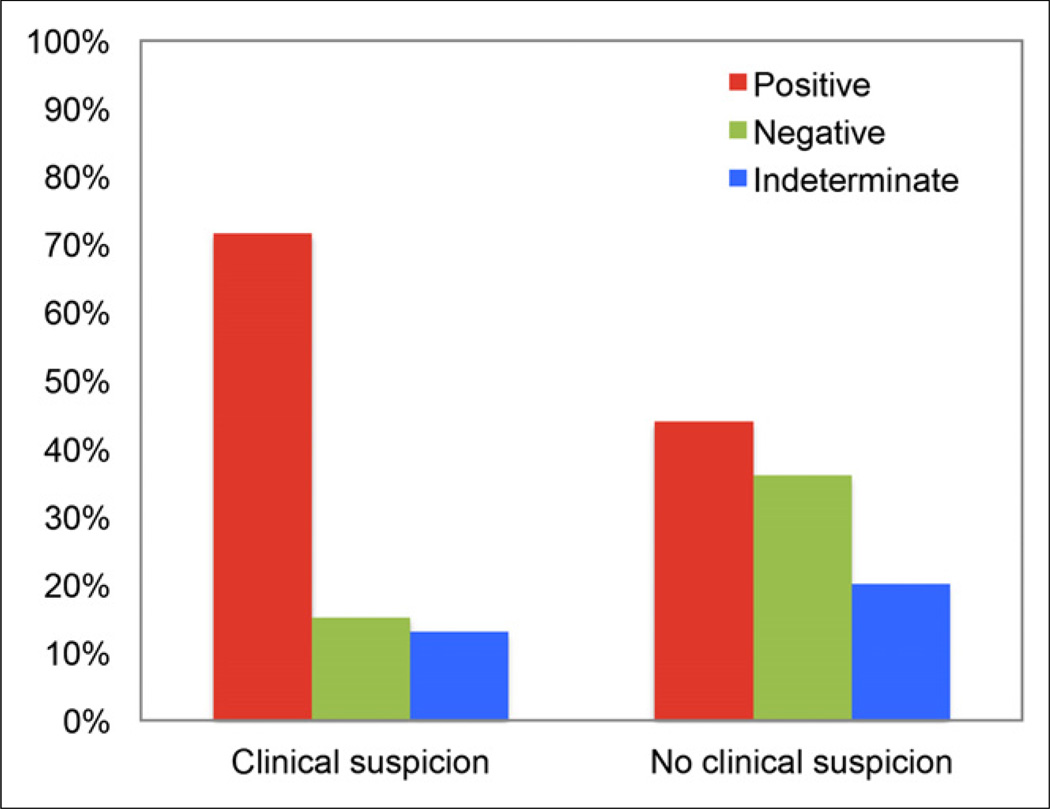
We evaluated the added value of 18F-FDG PET/CT to clinical assessment during follow-up. In this context, PET/CT identified recurrence or metastasis in 43.7% (107/245) of scans obtained without prior clinical suspicion and ruled out recurrence or metastasis in 15.2% (37/243) of scans obtained with prior clinical suspicion (Figs. 4–6).
FIGURE 4.
Added value of PET/CT to clinical assessment. PET/CT was helpful in excluding tumor in 15.2% (37/243) of patients with clinical suspicion of recurrence and in identifying recurrence in 43.7% (107/245) of patients with no prior clinical suspicion.
FIGURE 6.
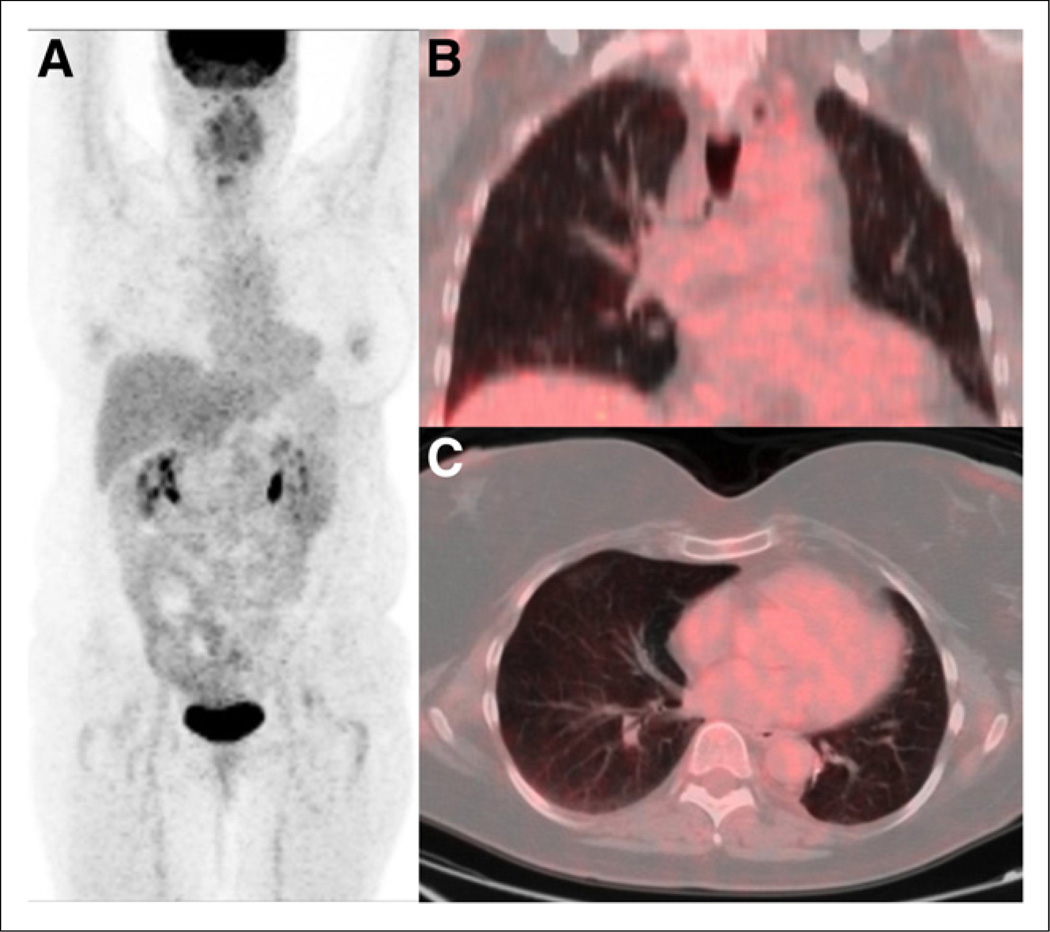
Clinical suspicion but negative PET results. Anterior maximum-intensity-projection (A), coronal PET/CT (B), and axial PET/CT (C) images of 51-y-old woman with limited-stage small cell carcinoma of left lung after left lower lobectomy and chemoradiation. Three years after completion of treatment, she presented with neurologic deficits including weakness of upper and lower extremities. Paraneoplastic syndrome was clinically suspected. Restaging PET/CT study showed no abnormal foci of metabolic activity to suggest active disease.
Of the 488 scans, 245 (50.2%) were obtained for routine follow-up without clinical suspicion of recurrent disease and 243 (49.8%) were obtained to evaluate for suspected disease. Of the routine scans, 48.6% (119/245) were obtained 6–24 mo after treatment and 51.4% (126/245) were obtained more than 24 mo after treatment. Of the clinical suspicion scans, 38.2% (93/243) were obtained 6–24 mo after treatment and 61.7% (150/243) were obtained more than 24 mo after treatment. Additionally, the probability of a 18F-FDG PET–positive result was significantly more likely to be associated with clinical suspicion than a routine scan, whereas an 18F-FDG PET–negative result was significantly more likely to be associated with a routine scan (Table 4).
TABLE 4.
Routine and Clinical-Suspicion PET/CT Results
| PET/CT result | n | P | ||
|---|---|---|---|---|
| Routine | Clinical suspicion | Total | ||
| Positive | 107 (38.1%) | 174 (61.9%) | 281(100%) | <0.0001* |
| Negative | 89 (70.6%) | 37 (29.4%) | 126 (100%) | |
| Indeterminate | 49 (60.5%) | 32 (39.5%) | 81 (100%) | |
| Total | 245 (50.2%) | 243 (49.8%) | 488 (100%) | |
Pearson χ2 test.
The Kaplan–Meier analysis showed a significant difference in OS between patients in the clinical suspicion group who had a positive PET/CT result (median survival, 38.6 mo) and those who had a negative result (median survival, 74.6 mo) (log-rank P = 0.0112; HR, 1.61; 95% CI, 1.12–2.37). The Kaplan–Meier analysis showed a significant difference in OS between patients in the routine scan group who had a positive PET/CT result (median survival, 28.3 mo) and those who had a negative result (median survival, 93.7 mo) (log-rank P < 0.0001; HR, 1.99; 95% CI, 1.43–2.78) (Fig. 7).
FIGURE 7.
Kaplan–Meier survival plots for scans performed under clinical suspicion (A) and scans performed as routine surveillance (B). OS differed significantly between patients with PET/CT scans positive for lung tumor and patients with PET/CT scans negative for lung tumor under both routine and clinically suggestive settings.
DISCUSSION
The primary objective of this study was to evaluate the prognostic value of 18F-FDG PET/CT in OS of lung cancer patients when performed between 6 and 295 mo after completion of curative primary treatment, with or without clinical suspicion of recurrence. Our study demonstrated that PET/CT was a significant prognostic indicator of OS regardless of the timing of the scan, especially in patients younger than 70 y. Additionally, PET/CT identified recurrence in 43.7% of patients without prior clinical suspicion and ruled out recurrence in 15.2% of patients with prior clinical suspicion.
Follow-up or surveillance 18F-FDG PET/CT has excellent accuracy and can bring about a change in management. Hicks et al. prospectively investigated a population of patients who were referred because of suspected relapse; patients were excluded if therapy had been completed less than 6 mo before imaging. The results demonstrated a PET sensitivity of 98% and specificity of 82%, with a change in management for 63% of patients within a 2-y study period (19). Gregory et al. prospectively analyzed the effect of PET/CT surveillance over a 5-y period and found a 42.3% change in management based on the PET/CT findings, with a 50.6% stage discordance between PET/CT and CT (20). Others (21–23) have also established a significant change in management of lung cancer patients based on the results of a PET study. The National Oncologic PET Registry has demonstrated a roughly 35% change in management—and more specifically a 13%–15% image-adjusted impact on management—based on the results of a PET study (23).
Using survival analysis, we demonstrated that PET/CT results positive for recurrence or metastases were associated with decreased OS, and this finding was significant regardless of the timing of the scan during follow-up or surveillance. We chose an interval of 24 mo because most of the accepted recommendations for CT surveillance in lung cancer are well defined in the first 2 y and become variable after that cutoff (1,5). Others have demonstrated the utility of surveillance PET/CT performed more than 6 mo after the completion of treatment. In the surveillance of asymptomatic patients with non–small cell lung cancer 1 y after curative-intent surgery, Dane et al. found recurrence in 16% of patients whereas Cho and Lee found recurrence in 31.4% of patients (5,24).
In our study, PET/CT identified recurrence or metastasis in 43.7% of scans obtained without prior clinical suspicion and excluded malignancy in 15.2% of scans obtained with prior clinical suspicion. The number of studies requested for routine surveillance (50.2%) was similar to the number requested because of clinical suspicion of disease progression (49.8%). The number of routine studies requested between 6 and 24 mo after treatment (48.6%) was similar to the number requested after 24 mo (51.4%). Among the studies requested because of clinical suspicion, fewer than two thirds were done more than 24 mo after treatment. This finding demonstrates a nonrandom probability and, further, that a PET/CT result is more likely to be positive if there is clinical suspicion of recurrence. However, as we expected, we found that the association between the PET/CT result and OS continued to be significant regardless of whether clinical suspicion was present before the study.
To account for additional covariates in our patient population, we used a multivariate Cox regression model. This demonstrated a significant positive relationship between OS and age, as well as a significant negative relationship between OS and a positive PET/CT result. The majority (85.5%) of our patients were older than 55 y, and we expected increased mortality with increasing morbidity in this age group. Stratification analysis by age showed a significant association between the PET/CT result and OS in the group younger than 60 y and the group 60–70 y old. However, there was no statistically significant association in the group older than 70 y. This result shows an overall relative decrease in survival for those older than 70 y after curative-intent therapy, thus reducing the impact of follow-up or surveillance PET/CT. Decreased survival in those 65 y or older after 5 or more years after curative intent surgery (25) has been demonstrated previously. Hence, it appears, follow-up or surveillance PET/CT is valuable for OS in patients younger than 70 y. However, the cause of mortality was not investigated, as our endpoint was OS. Non–cancer-related deaths could be a contributing factor in this age group.
We acknowledge several limitations of the current study. This was a retrospective study, and such studies have inherent errors of confounding when the exposure is not controlled for as in prospective studies. Indeterminate imaging results were included, whose root cause we did not analyze. The median OS for indeterminate results was 48 mo, which is closer to the PET-positive than the PET-negative median OS. To control for these indeterminate results, we favored underestimating the effect of a PET-positive study rather than overestimating it by including the indeterminate results with the PET/CT-negative group. Clinical suspicion of recurrence was retrospectively determined from our electronic medical and imaging records. Lung cancer stage, especially for earlier medical records, was not reported, and the effect of stage on OS may be underestimated. Since many patients were referred from outside our institution, we could not directly establish the effect of diagnostic CT or the added value of 18F-FDG PET/CT over diagnostic CT.
CONCLUSION
18F-FDG PET/CT performed during follow-up more than 6 mo after the completion of primary treatment is a prognostic marker of OS in lung cancer patients, regardless of the timing of the scan, especially in patients younger than 70 y. PET/CT adds value to clinical judgment by excluding recurrence in 15% of patients in whom it is suspected and by identifying recurrence in 43% of patients in whom it is not clinically suspected.
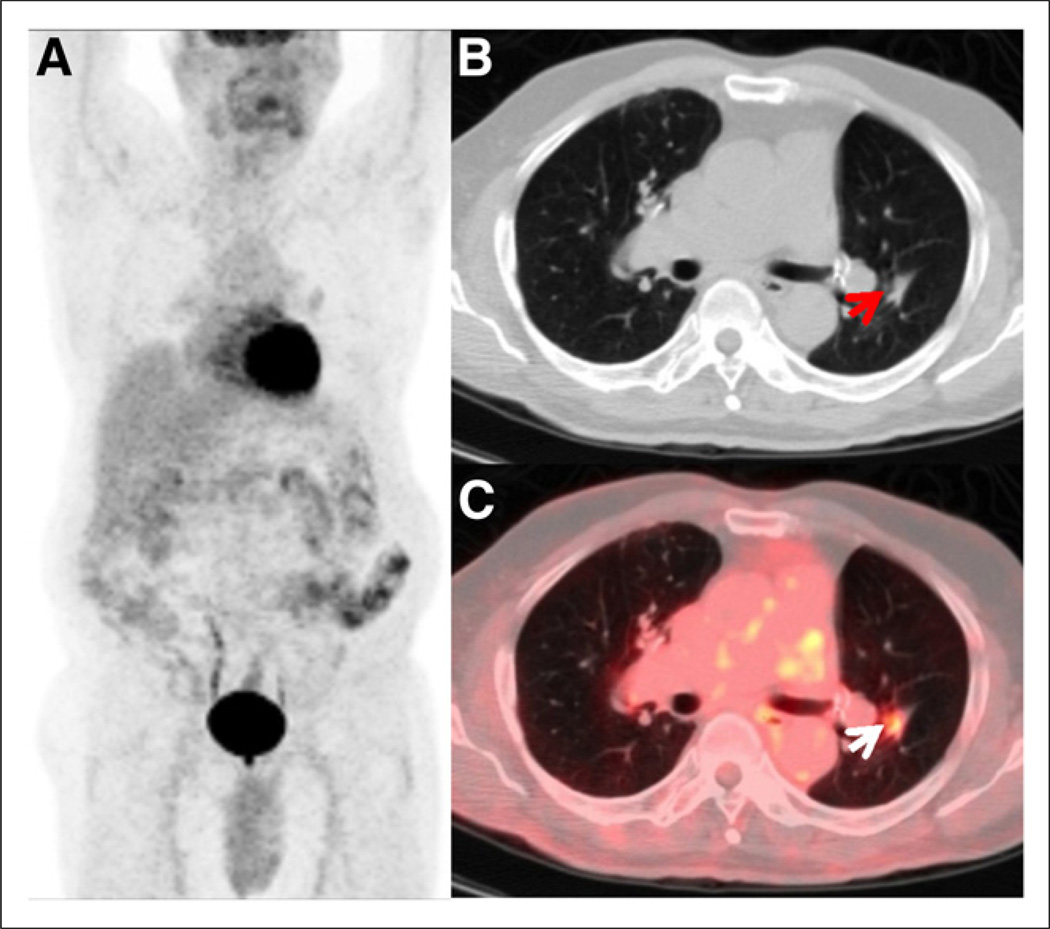
FIGURE 5.
No clinical suspicion but positive PET results. Anterior maximum-intensity-projection (A), axial CT (B), and axial PET/CT (C) images of 76-y-old man with T1N0 non–small cell lung carcinoma after right upper lobectomy and adjuvant chemotherapy. Clinically, patient was comfortable, with no complaints during follow-up at 3 y after completion of treatment. Restaging PET/CT study showed hypermetabolic focus (arrows) within left lower-lobe nodule, consistent with disease recurrence. Patient completed additional chemotherapy based on the results of this study.
Footnotes
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi89–vi98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975–2010. [Accessed April 14, 2014];National Cancer Institute. website. http://seer.cancer.gov/csr/1975_2010/. Based on November 2012 SEER data submission. Posted to the SEER website April 2013. Updated June 14, 2013.
- 3.Colice GL, Rubins J, Unger M, et al. Follow-up and surveillance of the lung cancer patient following curative-intent therapy. Chest. 2003;123(suppl):272S–283S. doi: 10.1378/chest.123.1_suppl.272s. [DOI] [PubMed] [Google Scholar]
- 4.Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–81. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Dane B, Grechushkin V, Plank A, et al. PET/CT vs. non-contrast CT alone for surveillance 1-year post lobectomy for stage I non-small-cell lung cancer. Am J Nucl Med Mol Imaging. 2013;3:408–416. [PMC free article] [PubMed] [Google Scholar]
- 6.Bury T, Corhay JL, Duysinx B, et al. Value of FDG-PET in detecting residual or recurrent nonsmall cell lung cancer. Eur Respir J. 1999;14:1376–1380. doi: 10.1183/09031936.99.14613769. [DOI] [PubMed] [Google Scholar]
- 7.Choi SH, Kim YT, Kim SK, et al. Positron emission tomography-computed tomography for postoperative surveillance in non-small cell lung cancer. Ann Thorac Surg. 2011;92:1826–1832. doi: 10.1016/j.athoracsur.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 8.He YQ, Gong HL, Deng YF, et al. Diagnostic efficacy of PET and PET/CT for recurrent lung cancer: a meta-analysis. Acta Radiol. 2014;55:309–317. doi: 10.1177/0284185113498536. [DOI] [PubMed] [Google Scholar]
- 9.Kanzaki R, Higashiyama M, Maeda J, et al. Clinical value of F18-fluorodeoxyglucose positron emission tomography-computed tomography in patients with non-small cell lung cancer after potentially curative surgery: experience with 241 patients. Interact Cardiovasc Thorac Surg. 2010;10:1009–1014. doi: 10.1510/icvts.2009.227538. [DOI] [PubMed] [Google Scholar]
- 10.Opoka L, Szołkowska M, Podgajny Z, et al. Assessment of recurrence of non-small cell lung cancer after therapy using CT and integrated PET/CT. Pneumonol Alergol Pol. 2013;81:214–220. [PubMed] [Google Scholar]
- 11.Saunders CA, Dussek JE, O’Doherty MJ, et al. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999;67:790–797. doi: 10.1016/s0003-4975(98)01257-0. [DOI] [PubMed] [Google Scholar]
- 12.Lardinois D, Weder W, Hany TF, et al. Staging of non–small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348:2500–2507. doi: 10.1056/NEJMoa022136. [DOI] [PubMed] [Google Scholar]
- 13.Hellwig D, Gröschel A, Graeter TP, et al. Diagnostic performance and prognostic impact of FDG-PET in suspected recurrence of surgically treated non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2006;33:13–21. doi: 10.1007/s00259-005-1919-4. [DOI] [PubMed] [Google Scholar]
- 14.Fischer BM, Mortensen J. The future in diagnosis and staging of lung cancer: positron emission tomography. Respiration. 2006;73:267–276. doi: 10.1159/000092080. [DOI] [PubMed] [Google Scholar]
- 15.Aberle DR, Adams AM, Berg CD, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paidpally V, Tahari AK, Lam S, et al. Addition of 18F-FDG PET/CT to clinical assessment predicts overall survival in HNSCC: a retrospective analysis with follow-up for 12 years. J Nucl Med. 2013;54:2039–2045. doi: 10.2967/jnumed.113.121285. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal A, Chirindel A, Shah BA, Subramaniam RM. Evolving role of FDG PET/CT in multiple myeloma imaging and management. AJR. 2013;200:884–890. doi: 10.2214/AJR.12.9653. [DOI] [PubMed] [Google Scholar]
- 18.Birth, marriage, and death search page. [Accessed April 14, 2014]; Ancestry.com website. http://search.ancestry.com/search/category.aspx?cat=34. [Google Scholar]
- 19.Hicks RJ, Kalff V, MacManus MP, et al. The utility of 18F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–1613. [PubMed] [Google Scholar]
- 20.Gregory DL, Hicks RJ, Hogg A, et al. Effect of PET/CT on management of patients with non–small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med. 2012;53:1007–1015. doi: 10.2967/jnumed.111.099713. [DOI] [PubMed] [Google Scholar]
- 21.Keidar Z, Haim N, Guralnik L, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med. 2004;45:1640–1646. [PubMed] [Google Scholar]
- 22.Seltzer MA, Yap CS, Silverman DH, et al. The impact of PET on the management of lung cancer: the referring physician’s perspective. J Nucl Med. 2002;43:752–756. [PubMed] [Google Scholar]
- 23.Hillner BE, Siegel BA, Hanna L, et al. Impact of 18F-FDG PET used after initial treatment of cancer: comparison of the National Oncologic PET Registry 2006 and 2009 cohorts. J Nucl Med. 2012;53:831–837. doi: 10.2967/jnumed.112.103911. [DOI] [PubMed] [Google Scholar]
- 24.Cho S, Lee EB. A follow-up of integrated positron emission tomography/computed tomography after curative resection of non–small-cell lung cancer in asymptomatic patients. J Thorac Cardiovasc Surg. 2010;139:1447–1451. doi: 10.1016/j.jtcvs.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 25.van Rens MT, de la Rivière AB, Elbers HR, van Den Bosch JM. Prognostic assessment of 2,361 patients who underwent pulmonary resection for non-small cell lung cancer, stage I, II, and IIIA. Chest. 2000;117:374–379. doi: 10.1378/chest.117.2.374. [DOI] [PubMed] [Google Scholar]