Abstract
Objective
To test the hypothesis that foreign language and music instruction in early life are associated with lower incidence of mild cognitive impairment (MCI) and slower rate of cognitive decline in old age.
Method
At enrollment in a longitudinal cohort study, 964 older persons without cognitive impairment estimated years of foreign language and music instruction by age 18. Annually thereafter they completed clinical evaluations that included cognitive testing and clinical classification of MCI.
Results
There were 264 persons with no foreign language instruction, 576 with 1–4 years, and 124 with >4 years; 346 persons with no music instruction, 360 with 1–4 years, and 258 with >4 years. During a mean of 5.8 years of observation, 396 participants (41.1%) developed MCI. In a proportional hazards model adjusted for age, sex, and education, higher levels (>4 years) of foreign language (hazard ratio [HR] = 0.687, 95% confidence interval [ CI]: 0.482, 0.961) and music (HR = 0.708, 95% CI: 0.539, 0.930) instruction by the age of 18 were each associated with reduced risk of MCI. The association persisted after adjustment for other early life indicators of an enriched cognitive environment, and it was stronger for nonamnestic than amnestic MCI. Both foreign language and music instruction were associated with higher initial level of cognitive function, but neither instruction measure was associated with cognitive decline.
Conclusions
Higher levels of foreign language and music instruction during childhood and adolescence are associated in old age with lower risk of developing MCI but not with rate of cognitive decline.
Keywords: mild cognitive impairment, longitudinal studies, cognitive activity, foreign language, instruction, music instruction
INTRODUCTION
Indicators of a cognitively enriched childhood environment, including household and community socioeconomic level (Kaplan et al., 2001; Singh-Manoux, Richards, & Marmot, 2005; Wilson, Scherr, Bienias, et al., 2005; Wilson, Scherr, Hoganson, et al., 2005; Fors, Lennartsson, & Lundberg, 2009) and frequency of cognitively stimulating activities (Everson-Rose, Mendes de Leon, Bienias, Wilson, & Evans, 2003; Fritsch et al., 2007; Wilson, Barnes, et al., 2005; Wilson, Scherr, Schneider, Tang, & Bennett, 2007; Wilson et al., 2013), have been associated with late-life cognitive functioning. However, there is limited knowledge about how differential childhood exposure to specific forms of cognitive enrichment is related to individual differences in cognitive aging and whether these associations are primarily with late life level of cognitive function (Everson-Rose et al., 2003; Wilson, Barnes, et al., 2005; Wilson, Scherr, Bienias, et al,. 2005; Wilson, Scherr, Hoganson, et al., 2005)or also with rate of cognitive decline (Wilson et al., 2007; Wilson et al., 2013). The present analyses focus on 2 learning experiences, foreign language instruction and music instruction, that are common but not universal during childhood, making for wide individual differences in exposure. In adulthood, bilingualism (Salvatierral & Rosselli 2010; Gold, Kim, Johnson, Kryscig, & Smith, 2013)and musical expertise (Hanna-Pladdy & MacKay, 2011; Hanna-Pladdy & Gajewski, 2012)are associated with higher level of cognitive function, suggesting that early life experience in these domains might be related to late life cognitive health, but few prior studies have investigated these issues(White-Schwoch, Carr, Anderson, Strait, & Kraus, 2013).
We used data from the Rush Memory and Aging Project to test the hypotheses that higher levels of foreign language and music instruction during childhood are associated in old age with lower risk of developing mild cognitive impairment (MCI) and slower rate of cognitive decline. Participants are 964 older persons without cognitive impairment at the time of enrollment. At baseline, participants indicated years of foreign language instruction and music lessons by age 18. Thereafter, they completed annual evaluations that included clinical classification of MCI and administration of a battery of cognitive function tests. In analyses, we tested for the hypothesized association of each form of instruction with incident MCI and trajectories of change in cognitive function.
METHODS
Participants
All participants are from the Rush Memory and Aging Project (Bennett, Schneider, Buchman, Mendes de Leon, & Wilson, 2005; Bennett et al., 2012), an ongoing longitudinal clinical-pathologic cohort study that began in 1997 and is continuing. Eligibility requires agreement to detailed annual clinical evaluations and brain autopsy at death. Older persons were recruited from retirement communities, social service agencies, subsidized housing facilities, and churches in the Chicago area. Following a presentation about the project, persons who expressed interest met with study personnel and written informed consent was obtained. The project was approved by the institutional review board of Rush University Medical Center.
At the time of these analyses, 1,543 individuals had completed the baseline evaluation and assessment of early life instruction. We excluded 458 persons found to have cognitive impairment at the baseline evaluation (64 with dementia and 394 with MCI). Of the remaining 1,085 individuals, 23 persons died before the first annual follow-up and 39 persons had been enrolled less than 1 year. Follow-up data were available on 964 of the 1,023 persons who were eligible for follow-up (94.2%) and analyses are based on this group. They had a mean age of 78.7 years at baseline (SD=7.4) and a mean of 14.6 years of education (SD=3.2); 740 (76.8%) were women.
Assessment of Early Life Instruction
As part of a structured interview at the baseline evaluation, participants were asked “By the age of 18, had you received any instruction in a foreign language ?”Those responding yes were asked “How many years?” All participants were also asked, “By the age of 18, had you taken any music lesions?” followed by “How many years?” for those responding yes.
Classification of Mild Cognitive Impairment
Each annual clinical evaluation included a structured medical history, neurological examination, and cognitive testing. On the basis of this evaluation, an experienced clinician diagnosed MCI in a 2-step process, as previously described in this (Boyle, Wilson, Aggarwal, Tang, & Bennett, 2006)and other (Bennett et al., 2002; Wilson, Aggarwal, et al., 2009) cohorts. First, an algorithm rated impairment in 5 cognitive domains (i.e., orientation, attention, memory, language, perception) based on educationally adjusted cutoff scores on 11 cognitive tests (Table 1; Bennett et al., 2002; Bennett et al., 2006). Second, after reviewing all cognitive data, education, occupation, and ratings of sensory problems, motor problems, and the participant’s level of effort, a neuropsychologist agreed or disagreed with the algorithmic rating of each cognitive domain. In the event of disagreement, the neuropsychologist supplied a new impairment rating. The diagnosis of MCI required impairment in at least 1 of the 5 cognitive domains in the absence of dementia, defined following the criteria of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association as a history of cognitive decline and impairment in at least 2 cognitive domains (McKhann et al., 1984). MCI was classified as amnestic if the memory domain was impaired and nonamnestic if memory was not impaired, as previously reported (Wilson, Schneider, et al., 2007; Wilson, Leurgans, Boyle, & Bennett, 2011). These MCI criteria have been related to intermediate levels of cognitive decline (Boyle et al., 2006; Bennett et al., 2002; Wilson et al., 2010)mortality (Bennett et al., 2002; Wilson, Aggarwal, et al., 2009), and Alzheimer’s disease pathology (Bennett, Schneider, Wilson, Bienias, & Arnold, 2005) compared to dementia and no cognitive impairment.
Table 1.
Role of tests in determining cognitive impairment for clinical classification and assessing cognitive change over time
| Impairment Cutoffs by Education | Hypothesized Cognitive Domains | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Test (maximum score) | <8 | 8–11 | 12–16 | >16 | Clinical Classification | Longitudinal Analyses |
| MMSE orientation (10) | <7 | <8 | <8 | <9 | Orientation | |
| Complex Ideational Material (8) | <7 | <7 | <7 | <7 | Language | |
| Boston Naming Test (15) | <9 | <10 | <11 | <13 | Language | Semantic memory |
| Verbal Fluency (75) | <9 | <10 | <10 | <12 | Language | Semantic memory |
| Digital Span Backward (12) | <3 | <4 | <5 | <5 | Attention | Working memory |
| Symbol Digit Modalities Test (110) | <8 | <16 | <21 | <25 | Attention | Perceptual speed |
| Logical Memory IIa (25) | <3 | <4 | <5 | <9 | Memory | Episodic memory |
| Word List Recall (10) | <3 | <5 | <5 | <5 | Memory | Episodic memory |
| Word List Recognition (10) | <8 | <9 | <9 | <9 | Memory | Episodic memory |
| Judgment of Line Orientation (15) | <3 | <5 | <7 | <8 | Perception | Visuospatial ability |
| Standard Progressive Matrices (16) | <5 | <6 | <7 | <8 | Perception | Visuospatial ability |
| Reading Test (15) | Semantic memory | |||||
| Digit Span Forward (12) | Working memory | |||||
| Digit Ordering (16) | Working memory | |||||
| Number Comparison (48) | Perceptual speed | |||||
| Stroop reading (80) | Perceptual speed | |||||
| Stroop naming (80) | Perceptual speed | |||||
| Logical Memory Ia (25) | Episodic memory | |||||
| Word List Memory (30) | Episodic memory | |||||
| Immediate story recall (12) | Episodic memory | |||||
| Delayed story recall (12) | Episodic memory | |||||
Note. MMSE, Mini-Mental State Examination.
Assessment of Cognitive Function
At each annual evaluation, a battery of 21 cognitive tests was administered in an approximately 45-minute session. The tests, listed in Table 1, included the Mini-Mental State Examination, a 30-item measure of global cognition (Folstein, Folstein, & McHugh, 1975); 8 items from Complex Ideational Material, a measure of auditory language comprehension (Goodglass & Kaplan, 1983); a 15-item version (Welsh et al., 1994) of the Boston Naming Test (Kaplan, Goodglass & Weintraub, 1983); verbal fluency which involved naming examples of 2 semantic categories (animals, vegetables) in separate 1-minute trials (Welsh et al., 1994; Wilson et al; 2002); Digit Span Forward and Backward from the Wechsler Memory Scale-Revised (Wechsler, 1987); a modified form (Wilson et al., 2002) of Digit Odering (Cooper, Sagar, Jordan, Harvey, & Sullivan, 1991) in which strings of 2 to 9 digits were presented and the participant tried to say them back in ascending order; the oral form of the Symbol Digit Modalities Test involved rapidly identifying symbol digit matches in a 90-second trial (Smith, 1982); a modified version (Wilson et al; 2002) of Number Comparision (Ekstrom, French, Harman, & Kermen, 1976) that involved rapidly classifying pairs of 3-to 10 -digit strings as same or different in a 90-second trial; immediate and delayed story recall (Albert et al., 1991; Wilson et al., 2002) and Logical Memory Ia and IIa (Weschsler, 1987)which both involved recalling story details immediately and after a delay; 3 measures that required learning a list of 10 words (Welsh et al., 1994), with 3 trials of immediate recall (Word List Memory) and tests of delayed recall (Word List Recall) and recognition (Word List Recognition); a 15-item form (Wilson et al., 2002) of Judgment of Line Orientation (Benton, Sivan, Hamsher, Varney, & Spreen, 1994) that involved matching the angles subtended by lines; a 16-item form (Wilson et al., 2002) of Standard Progressive Matrices (Raven, Court, &Raven, 1972) which required identifying the missing section of a visual stimulus from 6 to 8 alternatives; a reading test, with 15 words selected from the National Adult Reading Test (Nelson, 1982) and later modifications (Blair & Spreen, 1989; Grober & Sliwinski, 1991); and a modified version (Wilson, Barnes, et al., 2005) of the Stroop Neurospychological Screening Test (Trenerry et al., 1989) which involved separate 30-second trials rapidly reading color names and naming colors.
The aims of cognitive testing were to support diagnostic decisions, as described in the previous section, and to characterize change in cognitive function over time. To reduce measurement error in longitudinal analyses, particularly floor and ceiling artifacts, we used composite outcomes based on 2 or more cognitive tests. The Mini-Mental State Examination and Complex Ideational Material were not included in these analyses because of their skewed distributions. All of the remaining 19 tests were used to form a composite measure of global cognition. Supported by factor analyses in this (Wilson, Barnes, & Bennett, 2003; Wilson, Barnes, et al., 2005)and other (Wilson et al., 2002; Wilson, Aggarwal, et al., 2009; Krueger, Wilson, Bennett, & Aggarwal, 2009) groups, composite measures of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability were also formed ( Table 1). In each case, raw test scores were converted to z scores using the baseline mean and SD in the entire cohort, and z scores of component tests were averaged to yield the composite score. Further information on the individual tests and derivation of the composite measures is published elsewhere (Wilson et al., 2002; Wilson, et al., 2003; Wilson, Barnes, et al., 2005).
Assessment of Other Covariates
Educational attainment was based on years of formal schooling. There were 3 indicators of early life socioeconomic status : mother’s years of schooling, father’s years of schooling, and the number of children in the family. Each indicator was converted to a z score, using the mean and SD of the entire cohort, and the z scores were averaged (with values for number of children reversed to be consistent with the other indicators), as previously described (Wilson, Scherr, Hoganson, et al., 2005). At baseline, persons retrospectively rated cognitive resources and activities during childhood (Wilson, Barnes, et al., 2005). Specifically, a measure of the home cognitive environment was based on the number of 8 cognitive resources present in the home at age 12 (e.g., newspaper subscription, dictionary, encyclopedia, globe). They also rated frequency of participation in cognitively stimulating activities (e.g., reading a book, playing games like chess or checkers) from 1 (once a year or less) to 5 (every day or about every day). There were 11 items for ages 6 (3 items) and 12 (8 items) and 9 items for age 18. As in prior research (Wilson, Barnes, et al., 2005; Wilson et al, 2013), separate measures of childhood cognitive activity were formed by averaging the age 6–12 items and the age 18 items. Based on the medical history, we assessed 3 vascular risk factors: smoking (former or current), determined at baseline, and diabetes and hypertension, determined annually. The mean number present during the study period was used to quantify risk of vascular disease (Wilson, Arnold, Beck, Bienias, & Bennett, 2008; Wilson et al., 2012).
Statistical Analysis
All analyses were adjusted for age, sex, and education. We tested for the hypothesized association of early life instruction with incidence of MCI in a series of proportional hazards models. Because years of instruction was skewed (skewness of 2.8 for foreign language, 1.2 for music), we treated instruction as a categorical variable. To be consistent with prior research (White-Schwoch et al., 2013), we used 3 levels of instruction and contrasted a no instruction reference group with lower (1–4 years) and higher (>4 years) levels of instruction. We first modeled the 2 forms of instruction separately, then modeled them together, and then added covariates. We used a cause-specific relative hazard model (Lau, Cole, & Gange, 2009) to simultaneously estimate the association of instruction to amnestic and nonamnestic MCI. In a linear regression model, we regressed age at MCI diagnosis on the instruction measures. We used mixed-effects models to test for the association of the instruction measures with level of cognition at baseline and rate of cognitive change during follow-up. The initial outcome was the composite measure of global cognition. Subsequent models used composite measures of specific cognitive functions as outcomes.
RESULTS
There were 264 persons (27.4%) with no foreign language instruction by age 18, 576 (59.8%) with 1 to 4 years of instruction, and 124 (12.9%) with more than 4 years of instruction. There were 346 persons (35.9%) with no music instruction by age 18, 360 (37.3%) with 1 to 4 years, and 258 (26.8%) with more than 4 years. Higher level of foreign language instruction was associated with higher level of music instruction (χ2[4] =68.8, p<0.001). Higher level of foreign language (F[2,961]=8.6, p<0.001) but not music (F[2,961]=1.2, p=0.308) instruction was associated with older age at baseline. There were no gender differences in foreign language instruction (χ2[2]=1.7, p=0.420), but women reported more music instruction than men (χ2[2]=7.6, p=0.023). Higher levels of both instruction measures were associated with more years of education (foreign language F[2,961]=71.4, p<0.001; music F[2,961]=63.0, p<0.001), higher early life household socioeconomic level (foreign language F[2,955]=47.5, p<0.001; music F[2,955]=67.9, p<0.001), more cognitive resources in the early home environment (foreign language F[2,958]=45.2, p<0.001; music F[2,958]= 67.9, p<0.001), and more frequent participation in cognitively stimulating activities at ages 6–12 (foreign language F[2,960]=23.8, p<0.001; music F[2,960]=25.6, p<0.001) and 18 (foreign language F[2,961]=83.3, p<0.001; music F[2,961]=77.1, p<0.001).
Early Life Instruction and Mild Cognitive Impairment
During a mean of 5.8 years (SD=3.5) of annual follow-up evaluations, 396 individuals (41.1%) developed MCI. As in previous research (Luck et al., 2010; Plassman et al., 2011; Roberts et al., 2012), MCI incidence rates increased with age, with a rate of 0.004 for ages less than 75 (33 cases in 833.03 person-years), 0.093 for ages 75–84 (179 cases in 1916.13 person-years), and 0.143 for ages 85 and older ( 184 cases in 1285.84 person years). These rates are higher than early estimates of the incidence of MCI (Larrieu et al., 2002; Busse, Bischkopf, Riedel-Heller, & Angermeyer, 2003; Solfrizzi et al., 2004) but are comparable to more recent population-based estimates (Manly et al., 2008; Luck et al., 2010; Plassman et al., 2011; Roberts et al., 2012). Some methodological features of the present study may contribute to high incidence rates including the high follow-up participation, relatively brief interval between follow-up observations, and high proportion of observations proximate to death. Compared to the 568 who remained cognitively intact, those who developed MCI were older (mean age of 80.8 vs 77.2, t[942.6]=7.8, p<0.001) and had a lower baseline level of cognition (mean Mini-Mental State Examination score of 28.2 vs 28.7, t[700.3]=5.0, p<0.001; mean global cognitive score of 0.124 vs 0.383, t[962]=9.6, p<0.001), but they had a similar percent of women (74.0 vs 78.7, χ2[1]=2.9, p=0.089) and level of education (mean of 14.7 vs 14.5, t[962]=1.0, p=0.326).
We constructed a series of proportional hazards models to test for the hypothesized association of early life instruction with late-life risk of MCI. These and all subsequent analyses included terms to control for the potentially confounding effects of age (at baseline), sex, and years of formal education. In the first analysis (table 2, model A), both lower (1–4 years) and higher (>4 years) levels of foreign language instruction were associated with reduced risk of developing MCI relative to individuals with no foreign language instruction. In a subsequent analysis (table 2, model B), higher level of music instruction was associated with lower risk of MCI relative to those with no music instruction, with a nearly significant association for lower level of music instruction. With both forms of instruction in the same analysis (table 2, model C), higher levels of both forms of instruction were associated with lower risk of MCI with similar but not quite significant effects for lower levels of both forms of instruction.
Table 2.
Relation of early life instruction to late-life risk of incident mild cognitive impairment
| Model A | Model B | Model C | Model D | |||||
|---|---|---|---|---|---|---|---|---|
| Instruction Years by Age 18 | HR | 95% CI | HR | 95% CI | HR | 95%, CI | HR | 95% CI |
| Foreign language | ||||||||
| None (reference group) | ||||||||
| 1–4 years | 0.740 | 0.570, 0.960 | 0.796 | 0.624, 1.017 | 0.802 | 0.621, 1.035 | ||
| >4 years | 0.616 | 0.428, 0.888 | 0.681 | 0.482, 0.961 | 0.692 | 0.485, 0.987 | ||
| Music | ||||||||
| None (reference group) | ||||||||
| 1–4 years | 0.783 | 0.609, 1.005 | 0.815 | 0.644, 1.030 | 0.818 | 0.641, 1.044 | ||
| >4 years | 0.650 | 0.488, 0.866 | 0.708 | 0.539, 0.930 | 0.716 | 0.538, 0.953 | ||
Note. From 4 separate proportional hazards models adjusted for age, sex, and education. Model D also adjusted for early life indicators of cognitive activity frequency and household socioeconomic status. HR, hazard ration; CI, confidence interval.
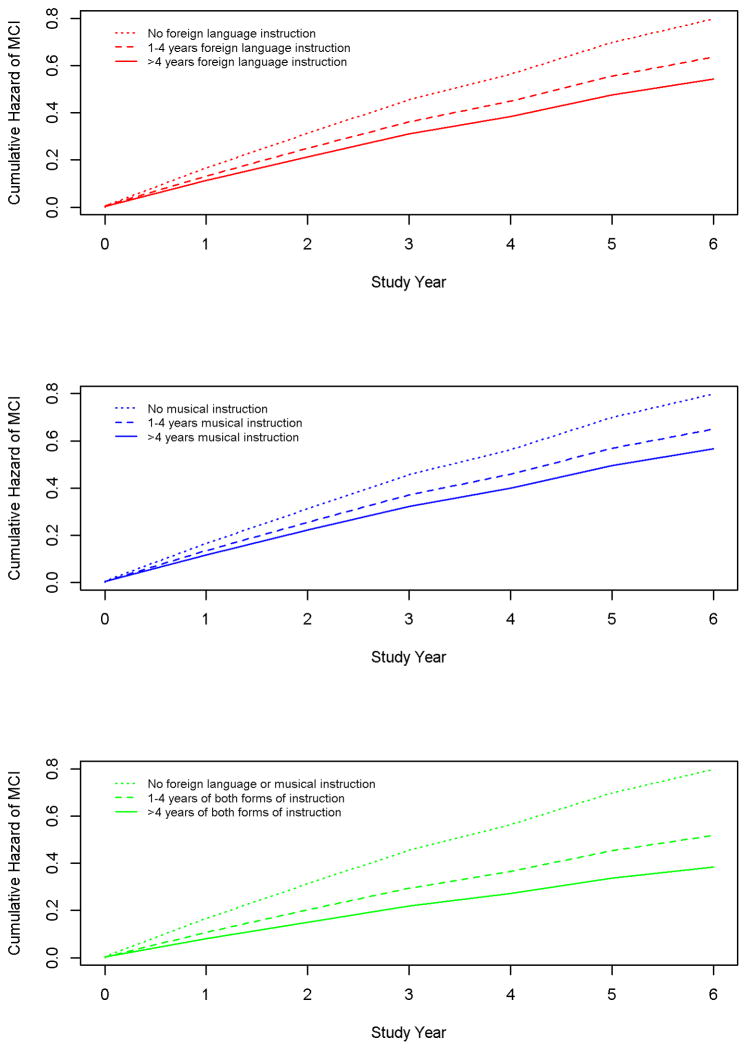
Figure 1, which is based on model C, shows that higher levels (solid lines) of foreign language (upper panel) and music (middle panel) instruction were each associated with an approximately 30% lower risk of MCI compared to no instruction (dotted lines). The lower panel shows that these effects are additive, with risk of MCI approximately 60% lower with high levels of both forms of instruction (solid line; 4.6% of participants) compared with no foreign language or music instruction (dotted line; 15.0% of participants).
Figure 1.
Relation of early life years of foreign language instruction (upper panel in red), music instruction ( middle panel in blue), and the 2 forms of instruction combined (lower panel in green) to cumulative hazard of developing mild cognitive impairment in late-life, from a proportional hazards model adjusted for age, sex, and education.
To determine whether cognitive activity or socioeconomic factors could account for the results, we repeated the previous analysis with 4 additional terms: frequency of cognitive activity at ages 6 and 12 (mean=3.02, SD=0.69), frequency of cognitive activity at age 18 (mean=3.07, SD=0.69), early life household socioeconomic status (mean=0.03, SD=0.74), and household cognitive resources at age 12 (mean=4.25, SD=2.03). As shown in table 1(model D), higher level of each form of early life instruction continued to be associated with lower risk of MCI.
Because vascular disease contributes to cognitive impairment in old age (Srikanth et al., 2003), we repeated model C with a term for the mean number of vascular risk factors during the study. Higher vascular risk was associated with higher risk of developing MCI (HR=1.389; 95% CI: 1.028, 1.878), but both foreign language instruction (HR for lower level = 0.775; 95% CI: 0.597, 1.008; HR for higher level = 0.641; 95% CI: 0.443, 0.926) and music instruction (HR for lower level = 0.791; 95% CI: 0.615, 1.016; HR for higher level = 0.674;95% CI: 0.504, 0.900) continued to be associated with lower risk of MCI. In a subsequent analysis, there was no evidence that the vascular risk index modified the association of early life instruction with late-life risk of MCI.
In studies of prevalent MCI, bilingualism has been associated with later retrospective estimate of age of onset (Ossher, Bialystok, Craik, Murphy, & Troyer, 2013; Bialystok, Craik, Binus, Ossher, & Freedman, 2013). Therefore, we regressed prospectively estimated age of MCI onset (n=396: mean=84.0, SD=6.5) on the early life instruction indicators. Foreign language instruction was associated with later age of onset (estimate for lower level = 0.790, SE=0.309, p=0.011; estimate for higher level = 1.071, SE=0.434, p=0.014) but music instruction was not (estimate for lower level = 0.574, SE=0.297, p=0.054; estimate for higher level = 0.490, SE=0.340, p=0.150).
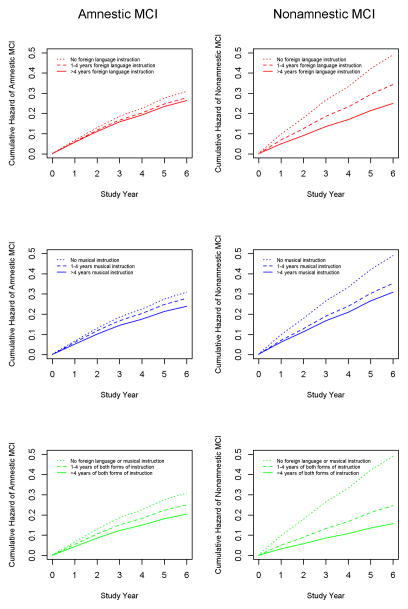
MCI was amnestic in 219 (55.3%) and nonamnestic in 177 (44.7%). Compared to those who developed amnestic MCI, people who developed nonamnestic MCI were younger at baseline (mean of 79.5 vs 81.8, t[344.4]=3.6, p<0.001) and more likely to be female (80.2 vs 69.0, χ2 [1]=6.5, p=0.011) but had similar levels of education (mean of 15 vs 14.5, t[394]=1.5, p=0.134) and global cognition at baseline (mean Mini-Mental State Examination score of 28.3 vs 28.1, t[394]=1.0, p=0.299; mean global cognitive score of 0.161 vs 0.094, t[394]=1.6, p=0.109). To see if early life instruction was differentially related to MCI subtypes, we constructed a cause-specific relative hazard model which made it possible to include both MCI subtypes in a single model (Table 3). Both forms of early life instruction were associated with lower incidence of nonamnestic MCI (right side of Figure 2), but neither was associated with incidence of amnestic MCI (left side of Figure 2).
Table 3.
Relation of early life instruction to subtypes of incident MCI
| Amnestic MCI (n=219) | Nonamnestic MCI (n=177) | |||
|---|---|---|---|---|
| Instruction Years by Age 18 | HR | 95% CI | HR | 95% CI |
| Foreign language | ||||
| None (reference group) | ||||
| 1–4 years | 0.888 | 0.626, 1.261 | 0.683 | 0.474, 0.986 |
| >4 years | 0.841 | 0.527, 1.341 | 0.491 | 0.281, 0.857 |
| Music | ||||
| None (reference group) | ||||
| 1–4 years | 0.893 | 0.643, 1.240 | 0.705 | 0.490, 1.014 |
| >4 years | 0.760 | 0.519, 1.114 | 0.617 | 0.406, 0.937 |
Note. From a cause-specific relative hazard model adjusted for age, sex, and education. MCI, mild cognitive impairment; HR, hazard ratio; CI, confidence interval.
Figure 2.
Relation of early life years of foreign language instruction (upper panels in red), music instruction (middle panels in blue), and the 2 forms of instruction combined (lower panels in green) to cumulative hazard of developing amnestic (left side) and nonamnestic (right side) mild cognitive impairment in late-life, from a cause-specific relative hazard model adjusted for age, sex, and education.
Early Life Instruction and Cognitive Aging
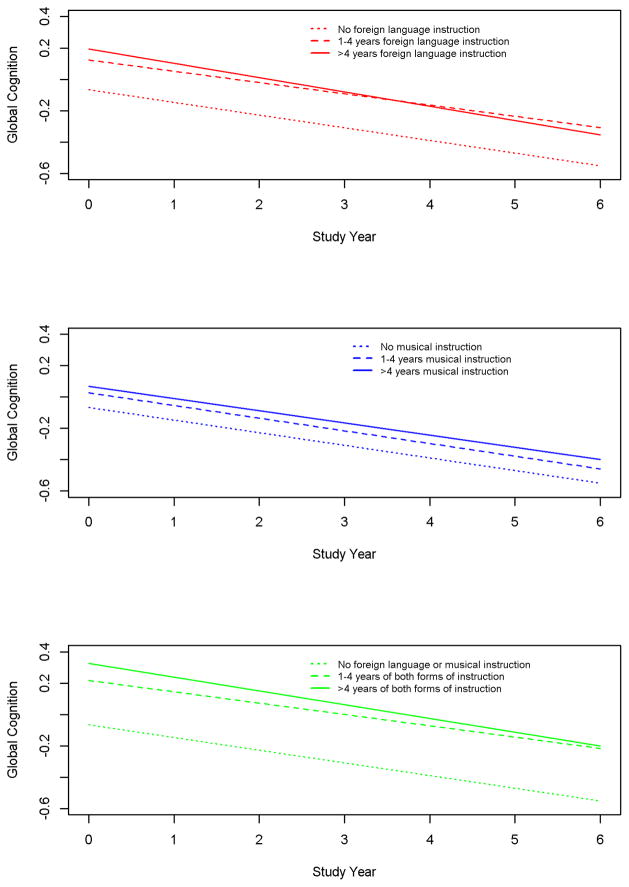
We analyzed change in cognitive function during the observation period in a mixed-effects model to determine whether early life instruction was related to risk of MCI because of an association with level of cognitive function, rate of cognitive decline, or both. In this analysis, lower (estimate=0.191, SE=0.031, p<0.001) and higher (estimate=0.260, SE=0.045, p<0.001) levels of foreign language instruction and lower (estimate=0.092, SE=0.031, p=0.003)and higher (estimate=0.133, SE=0.035, p<0.001)levels of music instruction were each associated with higher level of global cognitive function at baseline (mean=0.277, SD=0.434). However, neither foreign language instruction (estimate for lower level = 0.009, SE=0.008, p=0.291; estimate for higher level = −0.011, SE=0.012, p=0.354) nor music instruction (estimate for lower level =0.000, SE=0.008, p=0.992; estimate for higher level =0.003, SE=0.00 9, p=0.719) was related to rate of global cognitive decline. Figure 3, which is based on this analysis, shows a dose response type relationship between early life level of instruction and late life level of global cognition for foreign language instruction (upper panel), music instruction (middle panel), and the 2 forms of instruction combined (lower panel).
Figure 3.
Relation of early life years of foreign language instruction (upper panel in red), music instruction (middle panel in blue), and the 2 forms of instruction combined (lower panel in green)to level of and change in global cognition in late life, from a mixed-effects model adjusted for age, sex, and education.
To determine whether the association of early life instruction with late life cognition varied across cognitive domains, we repeated the previous analysis with composite measures of specific cognitive functions (Table 4). Both foreign language instruction and music instruction were associated with higher level of baseline function in all cognitive domains. However, with the exception of a marginal association of lower (but not higher) level of foreign language instruction with rate of decline in perceptual speed, the early life instruction measures were not associated with rate of cognitive decline.
Table 4.
Relation of early life instruction to late-life cognitive level and rate of change
| Episodic Memory | Semantic Memory | Working Memory | Perceptual Speed | Visuospatial Ability | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Term | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p |
| Time | −0.078 | 0.010 | <0.001 | −0.069 | 0.009 | <0.001 | −0.078 | 0.008 | <0.001 | −0.116 | 0.009 | <0.001 | −0.056 | 0.008 | <0.001 |
| Language 1–4 years | 0.187 | 0.041 | <0.001 | 0.203 | 0.038 | <0.001 | 0.161 | 0.044 | <0.001 | 0.217 | 0.047 | <0.001 | 0.112 | 0.043 | 0.009 |
| Language > 4 years | 0.271 | 0.059 | <0.001 | 0.219 | 0.055 | <0.001 | 0.185 | 0.064 | 0.004 | 0.259 | 0.068 | <0.001 | 0.165 | 0.063 | 0.009 |
| Music 1–4 years | 0.066 | 0.040 | 0.100 | 0.116 | 0.037 | 0.002 | 0.070 | 0.044 | 0.107 | 0.072 | 0.046 | 0.118 | 0.211 | 0.042 | <0.001 |
| Music > 4 years | 0.104 | 0.046 | 0.024 | 0.148 | 0.042 | <0.001 | 0.119 | 0.050 | 0.018 | 0.131 | 0.053 | 0.013 | 0.234 | 0.049 | <0.001 |
| Language 1–4 years × time | 0.015 | 0.010 | 0.112 | 0.004 | 0.009 | 0.656 | 0.010 | 0.008 | 0.204 | 0.004 | 0.009 | 0.627 | 0.023 | 0.009 | 0.008 |
| Language > 4 years × time | −0.003 | 0.014 | 0.828 | 0.003 | 0.013 | 0.794 | −0.002 | 0.012 | 0.898 | 0.005 | 0.013 | 0.675 | 0.014 | 0.012 | 0.247 |
| Music 1–4 years × time | 0.005 | 0.009 | 0.623 | −0.010 | 0.009 | 0.268 | 0.006 | 0.008 | 0.428 | 0.006 | 0.009 | 0.500 | −0.006 | 0.008 | 0.488 |
| Music > 4 years × time | 0.006 | 0.011 | 0.555 | −0.002 | 0.010 | 0.864 | 0.013 | 0.009 | 0.139 | 0.003 | 0.010 | 0.790 | −0.005 | 0.009 | 0.596 |
Note. From 5 separate mixed-effects models adjusted for age, sex, and education. SE, standard error.
DISCUSSION
In a group of nearly 1,000 older persons without evidence of cognitive impairment at study onset, more than 40% developed MCI during a mean of 5.8 years of follow-up observation. Foreign language and music instruction during youth (i.e., at least 5 years of instruction by age 18) were each associated with lower risk of developing MCI due to the association of instruction with higher initial level of cognitive function rather than with rate of cognitive decline. The results suggest that cognitively enriching experiences during childhood are related to cognitive health in old age.
Although there has been little prior research on the long term consequences of early life instruction (White-Schwoch et al., 2013), the results are broadly consistent with prior research showing experience dependent neuroplastic changes in animals (Marham & Greenough, 2004; Barnes & Finnerty, 2010; Qui, Huang, Lu, et al., 2012) and humans (Draganski, Gaser, Kempermann, et al, 2006; Schmidt-Wilcke, Rosengarth, Luerding, Bogdahn, & Greenlee, 2010; Woollett & Maguire, 2011) following exposure to novel environmental contingencies. The observed association of early life instruction in a foreign language or music with higher level of cognition in old age is consistent with previous cross-sectional research showing that adults with expertise in a foreign language (Salvatierra & Rosselli, 2010; Gold et al., 2013) or music (Hanna-Pladdy & Mackay, 2011; Hanna-Pladdy & Gajewski, 2012)perform better on cognitive tests than adults without such expertise. We found no association between early life training and late-life rate of cognitive decline. This is consistent with a previous study that found no association between bilingualism and cognitive decline in old age(Zahodne, Schofield, Farrell, Stern, & Manly, 2013) and a much broader literature showing that another measure of early life instruction, years of formal education, is related to level of cognition in old age but not to rate of decline (Van Dijk, Van Gerven, Van Boxtel, Van der Elst, & Jolles, 2008; Karlamangla et al., 2009; Wilson, Hebert, et al., 2009).
In persons with prevalent MCI, bilingualism has been associated with later age of symptom onset (Ossher, Bialystok, Craik, Murphy, & Troyer, 2013; Bialystok, Craik, Binus, Ossher, & Freedman, 2013), but the insidious onset of MCI makes it difficult to estimate, particularly retrospectively. The present results add to current knowledge by linking early life instruction to risk of MCI in a prospective study, by showing the association for 2 different forms of early life instruction, and by showing that early life instruction predicts incident MCI by virtue of an association with level of cognition rather than with rate of cognitive decline. An unexpected observation was that early life instruction was related to risk of nonamnestic MCI but not amnestic MCI. The significance of this finding is uncertain, however, since early life instruction was related to level of function in all cognitive domains, including episodic memory (Table 4).
These findings are consistent with the cognitive reserve hypothesis (Barulli & Stern, 2013) and research in life course epidemiology (Whalley, Dick, & McNeill, 2006) linking early life exposures to brain and cognitive development leading to higher peak performance during adulthood and thereby providing additional cognitive reserve that can delay the onset of MCI despite a lack of association with cognitive decline. That is, compared to people with less exposure to early life instruction those with more exposure begin late life with a higher level of cognitive ability, and because they decline at the same rate as individuals with less instruction exposure, it takes longer for their cognitive ability to slip to a level low enough to trigger a diagnosis of MCI. Thus, the observed association between early life instruction and late-life level of cognitive function was probably also present in early life given the strong correlation between early life cognitive ability and late-life cognitive ability (Gow et al, 2011; Deary, Pattie, & Starr, 2013). In prior research, music lessons (Schellenberg, 2004; Moreno et al., 2011) and school attendance (Ceci & Williams, 1997) have been associated with enhanced cognitive skills in children, suggesting that the instruction-cognition correlation in the present study is partly due to additional structured learning experience stimulating cognitive growth and development during childhood. In the present study, however, at least 5 years of foreign language instruction was associated with an additional 0.260-unit on the baseline global cognitive score, which is nearly 60% of the standard deviation at baseline (0.434), and the baseline global cognitive score of an individual with high levels of both forms of instruction was 0.393-unit higher than the score of an individual with no instruction in either area, a difference which is more than 90% of the baseline standard deviation. The substantial size of these effects suggests that other factors are probably involved. In particular, it is likely that the instruction-cognition association is reciprocal so that children with higher levels of cognitive ability likely gravitate toward more challenging academic pursuits and these additional structured learning experiences likely stimulate subsequent cognitive growth and development (Corrigall, Schellenberg, & Misura, 2013).
The strengths and limitations of these findings should be noted. The availability of a mean of 5.8 years of annual follow-up with a high rate of follow-participation in a relatively large cohort enhanced our ability to estimate the association of the instruction measures with cognitive outcomes. That results were comparable with different types of instruction suggests that they are reliable. Classification of MCI was based on a comprehensive neuropsychological assessment, as currently recommended (Bondi & Smith, 2014). Because the diagnosis of MCI was made by persons blinded to previously collected data, incidence of MCI and change in cognitive function are independent outcomes, and the 2 outcomes allowed us to show that the association of early life instruction with late-life cognitive health is clinically significant but does not involve cognitive decline. The main limitation is that we did not assess foreign language or musical experiences during the approximately 60 year period from age 18 to enrollment in the current study (mean age at baseline = 78.7). It is likely that some portion of the association of early life instruction with late-life cognition is mediated by the extent to which these skills were exercised during the adulthood. In addition, participants were selected and so the generalizability of the results remains to be determined, and information about early life instruction was obtained by retrospective report which may have biased results.
Acknowledgments
The authors thank the many Illinois residents for participating in the Rush Memory and Aging project; Traci Colvin, MPH, for managing the study; Donna Esbjornson, MS, for statistical programming; and John Gibbons, MS, and Greg Klein, MS, for data management.
This research was supported by NIH grants R01AG17917, R01AG15819, R01AG33678, and R01AG34374, and by the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- Albert M, Scherr P, Taylor J, Evans D, Funkensten H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010;16:186–198. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Science. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer’s disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, de S, Varney NR, Spreen O. Contributions to neuropsychological assessment. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Bialystok E, Craik FIM, Binns MA, Ossher L, Freedman M. Effects of bilingualism on the age of onset and progression of MCI and AD: evidence from executive function tests. Neuropsychology. 2014;28:290–304. doi: 10.1037/neu0000023. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting permorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bondi MW, Smith GE. Mild cognitive impairment: a concept and diagnostic entity in need of input from neuropsychology. Journal of the International Neuropsychological Society. 2014;20:129–134. doi: 10.1017/S1355617714000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer’s disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria. British Journal of Psychiatry. 2003;182:449–454. [PubMed] [Google Scholar]
- Ceci SJ, Williams WM. Schooling, intelligence and income. American Psychologist. 1997;52:1051–1058. [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114:2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Corrigall KA, Schellenberg EG, Misura NM. Music training, cognition, and personality. Frontiers in Psychology. 2013;4:222. doi: 10.3389/fpsyg.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Pattie A, Starr JM. The stability of intelligence from age 11 to age 90 years: the Lothian cohort of 1921. Psychological Science. 2013;24:2361–2368. doi: 10.1177/0956797613486487. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Kermen D. Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. American Journal of Epidemiology. 2003;158:1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-Mental State: a practical method for grading the mental state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. Journal of Neuroscience. 2013;33:387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan D. The assessment of aphasia and related disorders. 2 . Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gow AJ, Johnson W, Pattie A, Brett CE, Roberts B, Starr JM, Deary IJ. Stability and change in intelligence from age 11 to ages 70, 79, and 87: the Lothian Birth Cohorts of 1921 and 1936. Psychology and Aging. 2011;26:232–240. doi: 10.1037/a0021072. [DOI] [PubMed] [Google Scholar]
- Grober E, Silwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Gajewski B. Recent and past musical activity predicts cognitive aging variability: direct comparison with general lifestyle activities. Frontiers in Human Neuroscience. 2012;6:198. doi: 10.3389/fnhum.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B, MacKay A. The relation between instrumental musical activity and cognitive aging. Neuropsychology. 2011;25:378–386. doi: 10.1037/a0021895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia, Pa: Lea & Febiger; 1983. [Google Scholar]
- Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. International Journal of Epidemiology. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Bennett DA, Aggarwal NT. A battery of tests for assessing cognitive function in older Latino persons. Alzheimer Disease and Associated Disorders. 2009;23:384–388. doi: 10.1097/WAD.0b013e31819e0bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. American Journal of Epidemiology. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck T, Luppa M, Briel S, Matschinger H, Hans-Helmut K, Bleich S, Riedel-Heller S. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. Journal of the American Geriatric Society. 2010;58:1903–1910. doi: 10.1111/j.1532-5415.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Moreno S, Bialystok E, Barac R, Schellenberg EG, Cepeda NJ, Chau T. Short-term music training enhances verbal intelligence and executive function. Psychological Science. 2011;22:1425–1433. doi: 10.1177/0956797611416999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. National Adult Reading Test (NART): Test manual. Windsor, England: NFER Nelson; 1982. [Google Scholar]
- Ossher L, Bialystok E, Craik FIM, Murphy KJ, Troyer AK. The effect of bilingualism on amnestic mild cognitive impairment. Journals of Gerontology B Series Psychological Sciences and Social Sciences. 2013;68:8–12. doi: 10.1093/geronb/gbs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, McCammon RJ, Gwenith F, Potter GG, Burke JR, Wallace RB. Incidence of Dementia and Cognitive Impairment, Not Dementia in the United States. Annuals of Neurology. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui X, Huang CX, Lu W, Yang S, Li C, Shi XY, Tang Y. Effects of a 4 month enriched environment on the hippocampus and the myelinated fibers in the hippocampus of middle-aged rats. Brain Research. 2012;1465:26–33. doi: 10.1016/j.brainres.2012.05.025. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven’s progressive matrices and vocabulary: Standard Progressive Matrices. Oxford, England: Oxford Psychologists Press; 1992. [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankartz VS, Boeve BF, Petersen RC. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatierra JL, Rosselli M. The effect of bilingualism and age on inhibitory control. International Journal of Bilingualism. 2010;15:26–37. [Google Scholar]
- Schellenberg EG. Music lessons enhance IQ. Psychological Science. 2004;15:511–514. doi: 10.1111/j.0956-7976.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage. 2010;51:1234–1241. doi: 10.1016/j.neuroimage.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test manual-revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Solfrizzi V, Panza F, Colacicco AM, D’Introno A, Capurso C, Torres F, Capurso A. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- Srikanth VK, Thrift AG, Saling MM, Anderson JFI, Dewey HM, Macdonnell RAL, Donnan GA. Increased risk of cognitive impairment 3 months after mild to moderate first-ever stroke: a community-based prospective study of nonaphasic english-speaking survivors. Stroke. 2003;34:1136–1143. doi: 10.1161/01.STR.0000069161.35736.39. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. The Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Van Dijk KRA, Van Gerven PWM, Van Boxtel MPJ, Van der Elst W, Jolles J. No protective effect of education during normal cognitive aging: results from the 6 - year follow-up of the Maastricht Aging Study. Psychology and Aging. 2008;23:119–130. doi: 10.1037/0882-7974.23.1.119. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part V: a normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurology. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- White-Schwoch T, Carr KW, Anderson S, Strait DL, Kraus N. Older adults benefit from music training early in life: biological evidence for long-term training-driven plasticity. The Journal of Neuroscience. 2013;33:17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Bienias JL, Mendes de Leon CF, Evans DA. Biracial population study of mortality in mild cognitive impairment and Alzheimer’s disease. Archives of Neurology. 2009;66:767–772. doi: 10.1001/archneurol.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Aggarwal NT, Barnes LL, Mendes de Leon CF, Herbert LE, Evans DA. Cognitive decline in incident Alzheimer’s disease in a community population. Neurology. 2010;74:951–955. doi: 10.1212/WNL.0b013e3181d64786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Archives of General Psychiatry. 2008;65:439–446. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsychology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychology and Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Archives of Neurology. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Bienias JL, Mendes de Leon CF, Everson-Rose SA, Bennett DA, Evans DA. Socioeconomic characteristics of the community in childhood and cognition in old age. Experimental Aging Research. 2005;31:393–407. doi: 10.1080/03610730500206683. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25:8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in old age. Archives of General Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Buchman AS, Boyle PA, Hizel LP, Bennett DA. Terminal decline in motor function. Psychology and Aging. 2012;27:998–1007. doi: 10.1037/a0028182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett K, Maguire EA. Acquiring “the knowledge” of London’s layout drives structural brain changes. Current Biology. 2011;21:2109–2114. doi: 10.1016/j.cub.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Schofield PW, Farrell MT, Stern Y, Manly JJ. Bilingualism does not alter cognitive decline or dementia risk among Spanish-speaking immigrants. Neuropsychology. 2014;28:238–246. doi: 10.1037/neu0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]