Abstract
BACKGROUND & AIMS
Combination therapy with infliximab and azathioprine has demonstrated benefit over monotherapy for moderate-to-severe Crohn’s Disease. Clinical trials and models have not accounted for age-specific risks associated with these therapies, including the risk of immunosuppression-related cancer and infection. After accounting for these risks, the strategy yielding the greatest benefit may vary with age.
METHODS
We assessed age-specific risks and benefits of combination therapy compared to infliximab monotherapy using Markov modeling. The base case was a 35 year-old male patient with a 1-year time horizon. We assumed the incidence of lymphoma to be 5.28-fold higher with combination therapy. Secondary analyses accounted for life expectancy, therapy beyond 1 year, and age-specific surgical and infection risks. Quality-adjusted life years (QALYs) were calculated for 25–75-years old individuals.
RESULTS
Combination therapy was found to be of greater benefit in the base case (0.7522 QALYs for combination therapy vs 0.7426 QALYs for monotherapy). Accounting for life years lost, monotherapy was the best approach if the hazard ratio for lymphoma with combination therapy was >8.1 patients 75 years old. Monotherapy provided greater net benefit to patients 55, 65, or 75 years old if therapy was extended for 9, 7, or 5 years, respectively. For 25 year-old men, monotherapy resulted in fewer deaths but only yielded greater QALYs if the annual incidence of hepatosplenic T-cell lymphoma exceeded 36/100,000 persons.
CONCLUSION
After accounting for age-specific risks of lymphoma, infection, and surgical complications, benefits of combination therapy outweighed the risks as a short-term and intermediate-term strategy for most patients with moderate-to-severe Crohn’s Disease younger than 65 years. For young male patients, combination therapy yields greater QALYs, but at cost of an increased risk of death from lymphoma.
Keywords: Infliximab, Azathioprine, Lymphoma, Crohn’s Disease
Combination therapy with anti-tumor necrosis factor alpha medications (anti-TNFs) and thiopurines is recommended in moderate-to-severe Crohn’s disease (CD)1–4. Concerns remain about the safety of this combination. The two most feared complications are infection and malignancy. There are conflicting data on whether anti-TNFs, and combination therapy in particular, increase the risk of serious infections such as pneumonia5, 6. An increased risk of malignancy, particularly lymphoma and non-melanoma skin cancer, has been demonstrated in several observational cohorts7–9. The existing evidence implicates thiopurines as the principal cause of lymphoma, with a possible synergistic effect when combined with anti-TNFs8, 10. Thiopurines also appear to be the dominant risk factor for hepatosplenic T-cell lymphoma (HSTCL), a rare but fatal lymphoma affecting young males11. Therefore, discerning whether combination therapy offers an overall benefit relative to anti-TNF monotherapy is complex.
The incidence of non-Hodgkin’s lymphoma (NHL) and surgical and infectious complications with combination therapy increases with age12, 13. Furthermore, the expected benefit of azathioprine monotherapy decreases in older populations as a consequence of increasing lymphoma risk14. In this study we explored the relationship between age-specific risks and the expected net benefit of combination therapy compared to infliximab monotherapy. We hypothesized that for certain individuals, age-specific risks of lymphoma and infection with combination therapy outweigh the potential benefit, mandating personalized therapy incorporating this risk-benefit balance.
Methods
We constructed a Markov model to assess age-specific risks of combination therapy with an anti-TNF and a thiopurine compared to anti-TNF monotherapy. The base case was a 35-year old male with moderate-to-severe CD, comparable to participants in the Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) trial1, initiating either combination therapy or infliximab monotherapy. It was assumed that surgery was the least desired option. The time horizon for the primary analysis was 1 year, with a 1-month cycle length.
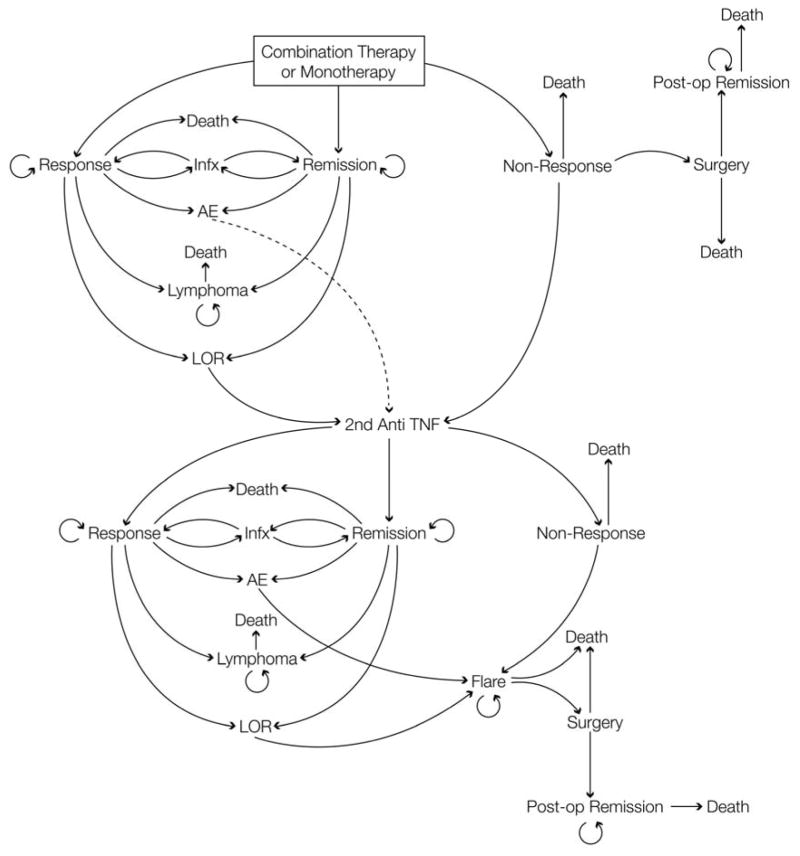
Combination therapy or monotherapy could initially result in remission, clinical response, or non-response (Figure 1). With response or remission, individuals could lose response, have a complication requiring cessation of the medication, experience a serious infectious complication requiring temporary withholding of medication for 1 cycle, develop lymphoma, or remain in their current state. Those without response and those that flared were transitioned to a second anti-TNF (adalimumab), with similar health states as with infliximab. All patients in the base model were continuously exposed to the age-specific probability of death of a male with CD, which was calculated using the baseline rate of death in US census data and a hazard ratio of 2.44 for those with CD on immunosuppressive therapy15, 16.
Figure 1. Model structure for combination therapy and monotherapy.
This is the structure of the model for the combination therapy arm. The monotherapy arm is identical, without inclusion of azathioprine.
Individuals entering a lymphoma state remained there, and were exposed to both age-specific and sex-specific all-cause and lymphoma-specific mortality. Lymphoma-specific mortality was derived from Surveillance, Epidemiology, and End Results (SEER) age- and sex-specific data22, 23. It was assumed that all patients received standard of care chemotherapy for lymphoma.
Patients undergoing surgery were exposed to an increased risk of peri-operative mortality for one cycle. They then entered a post-surgical remission state for the remainder of the study period, without exposure to medications and their risks.
Transition probabilities and outcome estimates
Transition probabilities were derived from relevant clinical trials (Table1, Supplemental Methods). The transition probabilities related to infliximab induction, maintenance, and complications were derived from the SONIC trial1. The Gauging Adalimumab Efficacy in Infliximab Non-responders (GAIN) study was used to inform initial remission and response rates for adalimumab17. Relapse, infection, and adverse event rates for adalimumab were derived from the Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance (CHARM) study. As there was no clear difference between combination therapy and monotherapy with adalimumab for relapse in CHARM, which has been confirmed in two recent meta-analysis of adalimumab combination and monotherapy, these transition probabilities were considered equivalent and were derived from those previously exposed to infliximab in CHARM18–20.
Table 1. Transition probabilities and QALY estimates.
Transition probabilities and QALY estimates for both combination therapy and monotherapy. All transition probabilities were assessed via one-way sensitivity analysis over a range of +/− 25%. For QALY inputs used for the model, sensitivity analyses were conducted varying the value by +/− 15%.
| Transition probability for: | Value | Source |
|---|---|---|
| Remission with combination therapy with infliximab | 0.3254 | 1 |
| Clinical response with combination therapy with infliximab* | 0.3077 | 1 |
| Remission with combination therapy with adalimumab | 0.2192 | 17 |
| Flaring when in remission with combination therapy with infliximab, per year | 0.5385 | 1 |
| Flaring with clinical response with combination therapy with infliximab per year | 0.213 | 1 |
| Clinical response with combination therapy with adalimumab | 0.3836 | 17 |
| Adverse event requiring drug cessation with combination therapy with infliximab | 0.207 | 1 |
| Adverse event requiring drug cessation with combination therapy with adalimumab, per year | 0.058 | 18 |
| Infectious complication with combination therapy with infliximab per year | 0.0391 | 1 |
| Infectious complication with combination therapy with adalimumab per year | 0.0271 | 18 |
| Remission with monotherapy with infliximab | 0.2959 | 1 |
| Clinical response with monotherapy with infliximab* | 0.2485 | 1 |
| Remission with monotherapy with adalimumab | 0.2093 | 17 |
| Clinical response with monotherapy with adalimumab | 0.3736 | 17 |
| Flaring when in remission with monotherapy with infliximab, per year | 0.6509 | 1 |
| Flaring with clinical response with monotherapy with infliximab per year | 0.2784 | 1 |
| Adverse event requiring drug cessation with monotherapy with infliximab per year | 0.1779 | 1 |
| Adverse event requiring drug cessation with monotherapy with adalimumab per year | 0.058 | 18 |
| Flaring with clinical response with adalimumab per year | 0.5562 | 18 |
| Flaring in remission with adalimumab per year | 0.6795 | 18 |
| Infectious complication with monotherapy with infliximab per year | 0.0491 | 1 |
| Infectious complication with monotherapy with adalimumab per year | 0.0271 | 18 |
| Surgery during acute flare | 0.1 | 14 |
| Mortality rate with an infectious complication | 0.001 | 14 |
| QALY Estimates | ||
| Medical Remission | 0.89 | 14, 23 |
| Clinical Response | 0.76 | ** |
| Severe CD | 0.62 | 14, 23 |
| Surgical Remission | 0.8 | 14, 23 |
| Surgery | 0.25 | 14, 23 |
| Infectious Complication | 0.62 | 14, 23 |
| Adverse Event | 0.62 | 14, 23 |
| Lymphoma | 0.47 | 14, 23 |
Clinical response rates for combination therapy and monotherapy with infliximab were derived from residual response rates (CDAI decrease >100pts) after subtracting % with remission at 6 weeks in SONIC.
For clinical response, the average between medical remission and severe CD was used, per expert opinion.
It was assumed that the hazard ratios (HR) for azathioprine and infliximab were independent of each other. The baseline HR for azathioprine was determined to be 5.28 from the Cancers Et Surrisque Associé aux Maladies Inflammatoires Intestinales En France (CESAME) cohort, and was treated as a continuous risk8. The baseline HR for infliximab was 1.0, based on the non-significant standardized incidence ratios (SIR) in CESAME and the TREAT registry21. These hazards were applied to the age-specific rate of lymphoma as determined by SEER22.
Quality adjusted life year (QALY) estimates were derived from previously published estimates and expert opinion (Supplemental Methods)14, 23, 24. QALY estimates were assumed to be constant over all age ranges (Table 1).
Statistical Analysis
Analyses were conducted from age 25 to 75 using TreeAge Pro 2013 (TreeAge Software, Inc., Williamstown, MA). Means and standard deviations (SD) for QALY estimates were derived from first-order Monte Carlo simulations (FOMCS) using 50,000 subjects. Probabilistic analyses were performed using distributions derived from clinical trials for all transition probabilities and QALY estimates (Supplemental Methods)25. To simulate outcomes at the end of 1 year, Markov cohort analysis was performed using a cohort of 1,000,000 patients for all age ranges.
Sensitivity Analyses
One-way sensitivity analyses were performed for all transition probabilities, hazard ratios, and rewards. Alternative model designs were also examined assessing the impact of 1) using a second anti-TNF by conducting an analysis allowing only infliximab; 2) life-years lost due to death during the first year of therapy by modifying the final reward; 3) increased risk of perioperative mortality in those over 65 years of age; 4) increasing age-specific risks of serious infection and infection-related mortality for those over 65 years of age; 5) lymphoma-specific life years lost for duration of therapy up to 9 years; 6) a gradual increase rather than instantaneous risk of lymphoma with azathioprine; and 7) including an additional risk of HSTCL for 25-year-olds treated with combination therapy (Supplemental Methods).
Results
Combination therapy with infliximab and azathioprine was the preferred option in the base model (Expected QALYs: 0.7522 versus 0.7426, Incremental effectiveness (IE) 0.0096). This benefit was also appreciated in first order Monte Carlo analysis (IE 0.0097) and probabilistic analyses (mean Expected QALYs 0.7521 versus 0.7426, IE0.0095 (95% Cl −0.0076–0.0268)). Over 50,000 iterations of the probabilistic model, combination therapy was the preferred strategy 86.1% of the time. In Markov Cohort analysis, combination therapy resulted in a greater number of patients in remission (22.9% versus 20.7%) and with response (26.8% versus 22.5%), fewer in post-operative remission (25.5% versus 30.1%), and fewer with active disease (24.4% versus 26.3%) at one year (Supplemental Figure 1). Mortality rates were similar between groups, with 19 fewer deaths per million individuals at one year in combination therapy (Table 2).
Table 2. Markov Cohort Analysis: Age-specific results.
Age-specific outcomes for a cohort of 1,000,000 individuals demonstrating those in remission, clinical response, post-operative remission, death, active CD, and lymphoma states at 1 year
| Combination therapy | Monotherapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Medically induced remission |
Clinical Response |
Post- Operative Remissions |
Death (All causes) |
Moderate -to- severely active disease |
Lymphoma | Medically induced remission |
Clinical Response |
Post- Operative Remissions |
Death (All causes) |
Moderate -to- severely active disease |
Lymphoma |
| 25 | 224056 | 261653 | 248714 | 3607 | 237847 | 132 | 202108 | 219355 | 292800 | 3637 | 255823 | 24 |
| 35 | 223855 | 261421 | 248557 | 4245 | 237665 | 293 | 201967 | 219207 | 292623 | 4264 | 255652 | 51 |
| 45 | 222640 | 260018 | 247405 | 9056 | 236433 | 623 | 200954 | 218122 | 291283 | 9058 | 254366 | 106 |
| 55 | 219846 | 256792 | 244720 | 20288 | 233577 | 1262 | 198594 | 215597 | 288148 | 20259 | 251367 | 216 |
| 65 | 214381 | 250484 | 239482 | 42234 | 228004 | 2601 | 193995 | 210673 | 282037 | 42087 | 245526 | 446 |
| 75 | 201129 | 235178 | 226224 | 98134 | 214139 | 4196 | 182402 | 198259 | 266465 | 97679 | 230731 | 722 |
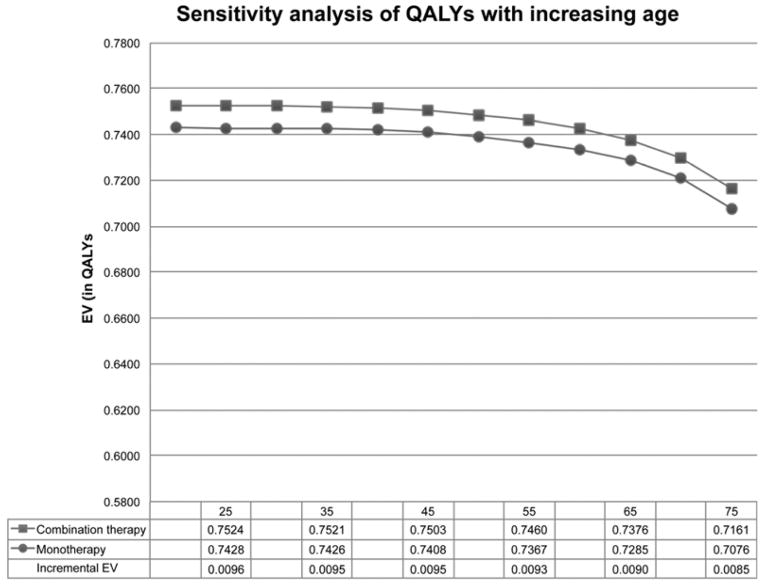
Combination therapy remained the preferred therapy throughout the lifespan (Figure 2). The increase in rates of lymphoma with age, particularly in the combination therapy arm (Supplemental Figure 5), resulted in increased mortality in the combination therapy arm compared to monotherapy for those older than 55. Per 1,000,000 patients treated, there were 29 more deaths with combination therapy at age 55, 147 at age 65, and 455 at age 75 (Table 2).
Figure 2. Impact of age on overall QALY estimates.
The margin of benefit of short-term combination therapy declined with increasing age.
Alternative model structures
Combination therapy yielded greater QALYs in alternative models that: 1) did not allow for crossover to a second anti-TNF (Expected Value (EV) 0.7341 versus 0.7232, IE 0.0109); 2) utilized final rewards to account for remaining life-years in the base case (EV 43.0403 versus 43.00299, IE 0.0104) and throughout the lifespan (data not shown); and 3) increased the risk for perioperative mortality in those over 65 by 2-fold (65 year old: 0.7375 versus 0.7285, IE 0.0090, 75 year old: 0.7160 versus 0.7075, IE 0.0085) or 5-fold (data not shown).
Assessing the impact of HSTCL in younger males, combination therapy remained the preferred strategy (EV 0.7524 versus 0.7428, IE 0.0096). However, in our Markov cohort analysis, there were 37 excess deaths with combination therapy due to 67 additional HSTCL-related deaths. When accounting for life-years lost due to HSTCL, the margin of benefit was reduced compared to the base model (IE 0.0075). Monotherapy became the preferred strategy if the incidence of HSTCL was greater than 36.0 per 100,000, or 3.2-fold greater than the baseline estimate.
In one-way sensitivity analysis of the HR for azathioprine-related lymphoma accounting for life years lost due to death, monotherapy became the preferred strategy in 65 year olds if the HR for combination therapy was >13.6, and in 75 year olds if the HR was >8.1 (Figure 3AB).
Figure 3. One-way sensitivity analysis of the hazard ratio of lymphoma with azathioprine.
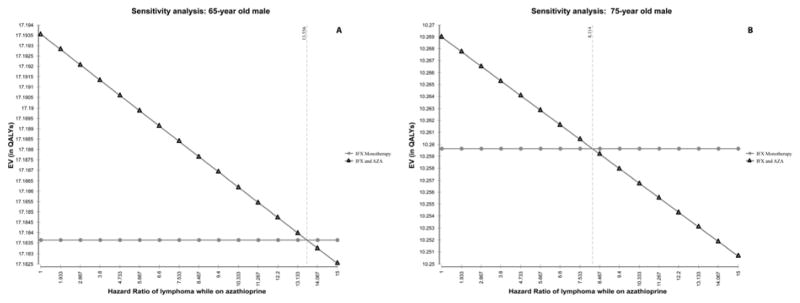
In models accounting for life-years lost due to death, monotherapy is preferred if the HR of lymphoma exceeded 13.6 in those 65 years of age (A), and if it exceeded 8.1 in those 75 or older (B).
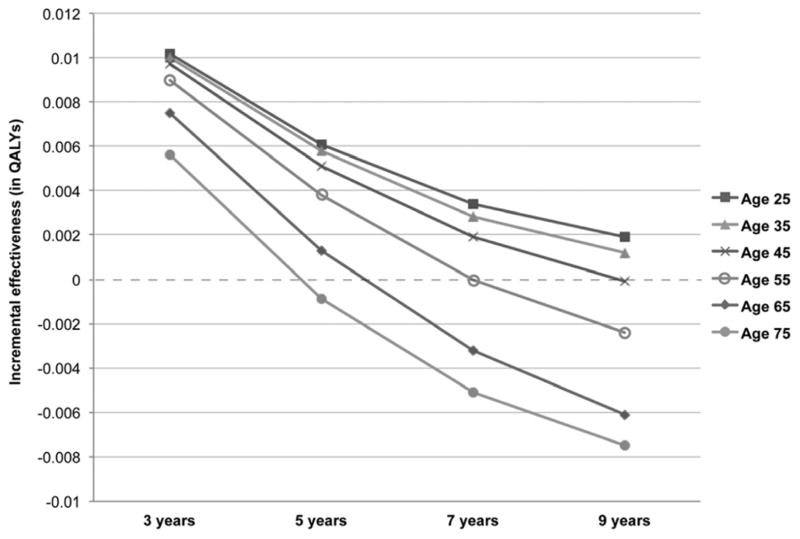
When extending the time horizon, combination therapy remained the preferred strategy for all ages for up to 3 years of therapy (Figure 4, Supplemental Table 1). Monotherapy was preferred in patients age 75 with more than 5 years of therapy and in those age 55 or older with over 9 years of therapy.
Figure 4. Impact of age with increasing time horizon.
Impact of age on preferred strategy in models that account for 3, 5, 7, and 9 years of exposure, from 25 years old to 75 years old.
Sensitivity analyses
The model was not sensitive to changes in transition probabilities across a range from 50% lower than to 50% greater than the base value for adverse event, response, relapse, mortality, or infection rates for combination or monotherapy. However, if the probability of remission with combination therapy with infliximab and azathioprine decreased to 20.8%, or 8.8% below the monotherapy remission probability, monotherapy became the preferred therapeutic option. If the remission rate with infliximab monotherapy exceeded 42.1%, monotherapy became the preferred strategy. The model was not sensitive to increases in the risk of lymphoma with infliximab over a range of HRs from 1 to 10 (Supplemental Methods, Supplemental Figure 4). The model was not sensitive to QALY estimates ranging 15% above or below baseline value, and was not sensitive to QALYs assigned to lymphoma over a wide range of values (0.20–0.80).
The model was robust to estimates of infection risk. The model was not sensitive to infection rates related to anti-TNFs or combination therapy when increased to up to 5 times of the base estimate, regardless of age (Supplemental Methods, Supplement Figure 3). In a 2-way sensitivity analysis, monotherapy became the preferred strategy when the odds of infection with combination therapy were >10x monotherapy and the infection-specific mortality exceeded 10% (Supplemental Figure 2).
Discussion
Concerns about age-related risks with immunomodulators and biologic therapies in IBD have markedly impacted willingness to use our most effective therapies in the youngest and oldest patients. In this study, we modeled the age-specific risks and benefits associated with combination therapy for moderate-to-severe CD, demonstrating that combination therapy may yield the greatest clinical benefit for short-term therapy in patients aged 35 to 65. However, the risks associated with combination therapy may outweigh the benefits for those over 65, particularly with longer treatment periods. This appears to be driven by lymphoma-related complications as opposed to increased risks of surgical complications or infection, even though infectious complications are far more common than lymphoma. Our sensitivity analyses highlight this finding, demonstrating no change in the optimal therapy with increasing age-specific risks of infection or surgery.
We employed several novel techniques in estimating the age-specific benefits and risks of combination therapy. We utilized age-dependent rates of NHL, infection, and surgery to better capture these risks for all individuals, and assessed the impact of these rates on long-term therapy. Using these approaches, we demonstrated that monotherapy yielded greater clinical benefit in patients older than 75 when therapy exceeded 5 years, and was preferred in those over 55 years of age if therapy exceeded 9 years. This highlights the complicated risk benefit analyses required to ascertain the preferred strategy for a given individual.
We determined thresholds for lymphoma risk that would indicate change in preferred strategy. For only 1 year of therapy, the HR of lymphoma with combination therapy would need to be greater than 8.1 for monotherapy to become the preferred strategy in those over 75; this threshold is well within the 95% CI reported in CESAME (HR 5.28, 95% CI 2.01 – 13.9)8.
This model is the first to assess the impact of HSTCL in younger males. We demonstrated that there was a trade-off between the small increased risk of death from HSTCL and increased therapeutic efficacy with combination therapy. We estimated that the risk of HSTCL must exceed a threshold of 36 per 100,000 person-years of exposure to thiopurines for monotherapy to yield greater QALYs. It seems unlikely that the true incidence is this high. The annual incidence of NHL among 25-year old American males is approximately 4 per 100,000. Therefore, the estimated annual incidence in thiopurine-exposed is only 21 per 100,000 based on the relative risk estimates from CESAME, well below the threshold of 36 per 100,000.
We can also utilize this sensitivity analysis to consider the impact of other thiopurine-related complications in young males. Hemophagocytic lymphohistiocytosis (HLH) is a potentially fatal complication associated with primary EBV infection when taking azathioprine. CESAME reported two fatalities from complications related to primary EBV infection in young males using thiopurines, for an estimated incidence rate of 10 per 100,000 person-years8. When considering only young males who are EBV seronegative, the incidence of this event may be as high as 290 per 100,000 person-years26. Combining the more conservative estimate of 10 per 100,000 with our estimated rate of HSTCL, the overall incidence rate of these two fatal complications of thiopurine therapy is 21.2 per 100,000 person-years, which is much closer to the threshold we estimated. If the true incidence of fatal primary EBV infection among EBV seronegative patients is closer to 290 per 100,000 person-years, our model would strongly favor monotherapy in this sub-population.
There are several important caveats to interpreting our results. The overall incremental effectiveness is small. However, in Markov analysis, there were clear differences in favor of combination therapy, with 64,102 more individuals with clinical improvement and 62,072 fewer individuals requiring surgery, suffering with active disease, or dying.
The key transition probabilities were derived from large clinical trials. Notably, in a recent meta-analysis comparing combination therapy and monotherapy, the pooled odds of remission at 24 weeks was 1.64, favoring combination therapy, similar to the OR of 1.62 in SONIC 27. Pooled estimates of infection and adverse events were also comparable to those in SONIC27. Recent meta-analyses also support our assumption of equivalent relapse rates for adalimumab combination and monotherapy 19, 20
We did not model dose escalation or antibody measurement with loss of response. There are limited and conflicting data regarding the efficacy of this treatment strategy28, 29. As our model was insensitive to relapse transition probabilities, utilization of these tests would not markedly impact our results.
We assumed that the risk of lymphoma begins immediately with azathioprine initiation. This risk may increase over time with therapy30. We therefore assessed models with extended time horizons and performed a sensitivity analysis increasing azathioprine-related risk of lymphoma over time. These models yielded similar results, demonstrating that for those over 65, the risks of combination therapy beyond 6 years may outweigh the potential benefits.
Our estimated rate of HSTCL was based on limited data; if true rates are much lower, combination therapy would be the preferred strategy in younger males. Some reports suggest that HSTCL rarely occurs prior to several years of therapy with thiopurines, and we therefore may have over-estimated the impact of HSTCL. We did not model the impact of discontinuing azathioprine after the first few years of therapy in models with longer time horizons. As more data become available, future models should evaluate this potential strategy. We did not model the impact of combination therapy with methotrexate in young males, though a recent trial failed to demonstrate a therapeutic advantage with this regimen compared to anti-TNF monotherapy31.
Lastly, we focused on the risk of lymphoma as opposed to other neoplasms. Our model does not take into account increased rates of certain skin cancers with these medications or the possible increased risk of other tumors recently appreciated with combination therapy 9, 32, 33. We did, however, account for increasing age-related risks of infection and surgery and demonstrated that they did not impact the optimal strategy.
In summary, this study is the first to assess the impact of age-specific risks on the decision to use combination therapy versus monotherapy for patients with moderate-to-severe CD. In our model, increased lymphoma, infection, and surgery risks do not outweigh the greater efficacy of combination therapy for those aged 35 to 65 when considering therapy for up to 3 years. However, the risk of lymphoma may outweigh the benefits of combination therapy for those older than 65, particularly with long-term therapy. These data highlight the need to further examine de-escalation strategies with long-term remission. Our model also suggests that combination therapy in young adult males may be the preferred strategy, providing greater QALYs, albeit at the cost of an increased risk of HSTCL-related deaths. Our data also support a potential strategy of screening for EBV in those younger than 25 before embarking on combination therapy to prevent primary EBV infection-related complications. This may represent a greater risk than HSTCL in this population26. These data help to better inform conversations with individual patients of all ages.
Supplementary Material
Acknowledgments
Grant Support via NIH:
K08-DK095951-02, K24-DK078228, K08-DK084347-01, T32- DK007066-36, K08- DK098272, K12-CA-076931
Abbreviations used
- CD
Crohn’s Disease
- CESAME
Cancers Et Surrisque Associé aux Maladies Inflammatoires Intestinales En France
- CHARM
Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance
- EBV
Epstein Barr Virus
- FOMCS
First Order Monte Carlo Simulation
- GAIN
Gauging Adalimumab Efficacy in Infliximab Non-responders
- HLH
Hemophagocytic Lymphohistiocytosis
- HSTCL
Hepatosplenic T Cell Lymphoma
- IE
Incremental effectiveness
- NHL
Non-Hodgkin’s Lymphoma
- QALY
Quality Adjusted Life Year
- SD
Standard Deviation
- SEER
Surveillance, Epidemiology, and End Results
- SIR
Standardized Incidence Ratio
- SOMCS
Second Order Monte Carlo Simulation
- SONIC
Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease
- TNF
Tumor Necrosis Factor
Footnotes
Author Contributions:
Frank I. Scott, MD MSCE: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding
Ravy Vajravelu, MD: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Meenakshi Bewtra MD, PhD: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding
Ronac Mamtani MD MSCE: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Dale Lee MD: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
David Goldberg MD MSCE: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding
James D Lewis MD MSCE: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Disclosures:
Frank I. Scott, MD MSCE: nothing to disclose
Ravy Vajravelu, MD: nothing to disclose
Meenakshi Bewtra MD, PhD: Boehringer Ingelheim(research grant) Janssen (research grant), Imedex (CME presentation)
Ronac Mamtani MD MSCE: Takeda Pharmaceuticals
Dale Lee MD: nothing to disclose
David Goldberg MD MSCE: Bayer Pharmaceuticals (Research Grant)
James D Lewis MD MSCE: Dr. Lewis has served as a consultant for Merck, Shire, AstraZeneca, Pfizer, Lilly, Janssen, Amgen, AbbVie, Rebiotix, MedImmune, and Immune Pharmaceuticals. Dr. Lewis has had research funding from Takeda, Shire, Bayer, and Nestle.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 2.Lemann M, Mary JY, Duclos B, et al. Infliximab plus azathioprine for steroid-dependent Crohn’s disease patients: a randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–61. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 3.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–7. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 4.Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Use of Thiopurines, Methotrexate, and Anti-TNF-alpha Biologic Drugs for the Induction and Maintenance of Remission in Inflammatory Crohn’s Disease. Gastroenterology. 2013;145:1459–63. doi: 10.1053/j.gastro.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 5.Grijalva CG, Chen L, Delzell E, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240–8. doi: 10.1038/ajg.2012.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrinton LJ, Liu L, Weng X, et al. Role of Thiopurine and Anti-TNF Therapy in Lymphoma in Inflammatory Bowel Disease. Am J Gastroenterol. 2011;106:2146–2153. doi: 10.1038/ajg.2011.283. [DOI] [PubMed] [Google Scholar]
- 8.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–25. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 9.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–74. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrinton LJ, Liu L, Abramson O, et al. The incidence of hepatosplenic T-cell lymphoma in a large managed care organization, with reference to anti-tumor necrosis factor therapy, Northern California, 2000–2006. Pharmacoepidemiol Drug Saf. 2012;21:49–52. doi: 10.1002/pds.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261–9. doi: 10.1182/blood-2003-05-1675. [DOI] [PubMed] [Google Scholar]
- 12.Masoomi H, Kang CY, Chaudhry O, et al. Predictive factors of early bowel obstruction in colon and rectal surgery: data from the Nationwide Inpatient Sample, 2006–2008. J Am Coll Surg. 2012;214:831–7. doi: 10.1016/j.jamcollsurg.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 13.Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30–5. doi: 10.1016/j.cgh.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–24. doi: 10.1016/s0016-5085(00)70353-2. [DOI] [PubMed] [Google Scholar]
- 15.Lewis JD, Gelfand JM, Troxel AB, et al. Immunosuppressant Medications and Mortality in Inflammatory Bowel Disease. Am J Gastroenterol. 2008;103:1428–1435. doi: 10.1111/j.1572-0241.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- 16.Arias E. National Vital Statistics Reports. United States Life Tables, 2007. 2011;59:1–61. [PubMed] [Google Scholar]
- 17.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 18.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with adalimumab and immunomodulators for Crohn’s disease: A meta-analysis. Journal of Crohn’s and colitis. 2014;8:S50–S51. doi: 10.1016/j.crohns.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Jones J, Kaplan GG, Peyrin-Biroulet L, et al. Impact of Concomitant Immunomodulator Treatment on Efficacy and Safety of Anti-TNF Therapy in Crohn’s Disease: A Meta-Analysis of Placebo Controlled Trials With Individual Patient-Level Data. Gastroenterology. 2013;144:S179. [Google Scholar]
- 21.Lichtenstein GR, Cohen RD, Feagan BG, et al. Safety of Infliximab and Other Crohn’s Disease Therapies: Treat™ Registry Data With a Mean of 5 Years of Follow-up. Gastroenterology. 2011;140:S773. [Google Scholar]
- 22.Howlader N, Noone A, Krapcho M, et al. Institute. NC, editor. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: 2013. http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 23.Gregor J, McDonald J, Klar N, et al. An Evaluation of Utility Measurement in Crohn’s Disease. Inflammatory Bowel Diseases. 1997;3:265–276. [PubMed] [Google Scholar]
- 24.Olin RL, Kanetsky PA, Ten Have TR, et al. Determinants of the optimal firstline therapy for follicular lymphoma: a decision analysis. Am J Hematol. 2010;85:255–60. doi: 10.1002/ajh.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briggs AH, Claxton K, Sculpher MJ. Oxford handbooks in health economic evaluation. Oxford: Oxford University Press; 2006. Decision modelling for health economic evaluation; pp. 77–120. [Google Scholar]
- 26.Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut. 2012;61:476–83. doi: 10.1136/gutjnl-2011-301133. [DOI] [PubMed] [Google Scholar]
- 27.Lin Z, Bai Y, Zheng P. Meta-analysis: efficacy and safety of combination therapy of infliximab and immunosuppressives for Crohn’s disease. Eur J Gastroenterol Hepatol. 2011;23:1100–10. doi: 10.1097/MEG.0b013e32834b9544. [DOI] [PubMed] [Google Scholar]
- 28.Afif W, Loftus EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pariente B, Pineton de Chambrun G, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:1199–206. doi: 10.1002/ibd.21839. [DOI] [PubMed] [Google Scholar]
- 30.Khan N, Abbas AM, Lichtenstein GR, et al. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology. 2013;145:1007–1015. e3. doi: 10.1053/j.gastro.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 31.Feagan BG, McDonald JWD, Panaccione R, et al. Methotrexate in Combination with Infliximab is no More Effective than Infliximab Alone in Patients with Crohn’s Disease. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399. e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osterman MT, Sandborn WJ, Colombel J-F, et al. Increased Risk of Malignancy With Adalimumab Combination Therapy, Compared With Monotherapy, for Crohn’s Disease. Gastroenterology. 146:941–949.e2. doi: 10.1053/j.gastro.2013.12.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.