SUMMARY
Neoadjuvant therapy has proven to be effective in the reduction of locoregional recurrence and mortality for esophageal cancer. However, induction treatment has been reported to be associated with increased risk of postoperative complications. We therefore compared outcomes after esophagectomy for esophageal cancer for patients who underwent neoadjuvant therapy and patients treated with surgery alone. Using the American College of Surgeons National Surgical Quality Improvement Program database (2005–2011), we identified 1939 patients who underwent esophagectomy for esophageal cancer. Seven hundred and eight (36.5%) received neoadjuvant therapy, while 1231 (63.5%) received no neoadjuvant therapy within 90 days prior to surgery. Primary outcome was 30-day mortality, and secondary outcomes included overall and serious morbidity, length of stay, and operative time. Patients who underwent neoadjuvant treatment were younger (62.3 vs. 64.7, P < 0.001), were more likely to have experienced recent weight loss (29.4% vs. 15.9%, P < 0.001), and had worse preoperative hematological cell counts (white blood cells <4.5 or >11 × 109/L: 29.3% vs. 15.0%, P < 0.001; hematocrit <36%: 49.7% vs. 30.0%, P < 0.001). On unadjusted analysis, 30-day mortality, overall, and serious morbidity were comparable between the two groups, with the exception of the individual complications of venous thromboembolic events and bleeding transfusion, which were significantly lower in the surgery-only patients (5.71% vs. 8.27%, P = 0.027; 6.89% vs. 10.57%, P = 0.004; respectively). Multivariable and matched analysis confirmed that 30-day mortality, overall, and serious morbidity, as well as prolonged length of stay, were comparable between the two groups of patients. An increasing trend of preoperative neoadjuvant therapy for esophageal cancer was observed through the study years (from 29.0% in 2005–2006 to 44.0% in 2011, P< 0.001). According to our analysis, preoperative neoadjuvant therapy for esophageal cancer does not increase 30-day mortality or the overall risk of postoperative complications after esophagectomy.
Keywords: chemotherapy, esophageal cancer, esophagectomy, outcome, radiation therapy
INTRODUCTION
Esophageal cancer is estimated to affect more than 450 000 individuals worldwide and its incidence shows a net rising trend.1 The most common esophageal cancer subtype in the United States is adenocarcinoma, affecting predominantly white overweight males, while squamous esophageal cancer remains the most prominent histologic type worldwide.2 Even though the ongoing advancements in medical and surgical treatment have improved the prognosis for this condition, the overall 5-year survival of patients with resectable esophageal cancer remains disappointing, ranging from 15% to 34%.3 Surgery has traditionally been considered the mainstay of treatment for localized esophageal cancer; however, over the last decade, new treatment protocols have been proposed in order to increase disease free and overall survival. Numerous trials have been designed to evaluate the benefits of neoadjuvant therapies for esophageal cancer, and it is current practice to treat locally advanced disease with trimodality therapy (concurrent chemotherapy and radiation followed by surgery) after the encouraging results of the CROSS trial were published.4 This study reported a remarkable increase in survival in patients undergoing esophagectomy after chemoradiotherapy (CRT), when compared with patients treated with surgery alone, with similar rates of postoperative complications. The influence of induction therapy on the surgical outcomes after esophagectomy remains a subject of controversy in the literature, since some authors advocate that neoadjuvant therapy negatively impacts several organ systems leading to worse surgical results. Improvements in oncological outcomes after CRT, in particular, have been reported to come at the cost of significantly increased postoperative mortality.5 The analysis of a surgical, research-oriented database has the potential to offer a meaningful insight on the topic, providing complementary information to previously reported studies. We therefore queried the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, in order to analyze the effects of neoadjuvant therapies on 30-day outcomes of esophagectomy for cancer in the United States.
MATERIALS AND METHODS
Data source
This was a retrospective analysis using the ASC-NSQIP database from 2005 to 2011. NSQIP is a large nationally validated, risk-adjusted, outcomes-based program and it is used to measure and improve the quality of surgical care. Any hospital that performs surgery may join NSQIP, with requirements that a dedicated surgical clinical reviewer be hired to capture and review the data, a surgeon champion or leader be identified to lead the program at the hospital, the hospital agree to the program protocols and meet minimum case standards, and an annual participation fee be paid to American College of Surgeons. Nearly 500 hospitals that vary in size and academic affiliation participate in NSQIP.6 This program employs a prospective systematic data collection on 150 preoperative and intraoperative variables, as well as 30-day postoperative morbidity and mortality. The development history and current details of ACS-NSQIP are described elsewhere.7 This study was deemed exempt by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Inclusion criteria
This study was restricted to adult patients (aged 18 years or older) who underwent esophagectomy (defined as Current Procedural Terminology [CPT] codes of 43107, 43108, 43112, 43113, 43116, 43117, 43118, 43121, 43122, 43123, or 43124) for esophageal cancer (defined by International Classification of Diseases, 9th revision codes of 150, 150.1, 150.2, 150.3, 150.4, 150.5, 150.8, 150.9, 151, or 151.0). The neoadjuvant treatment group consisted of patients who received either chemotherapy, radiation therapy, or both prior to undergoing a surgical procedure. The primary surgery group consisted of patients who did not receive a neoadjuvant therapy prior to the operation. NSQIP provides information on receiving chemotherapy for malignancy 30 days prior to the operation and receiving radiation therapy within 90 days before the operation. The chemotherapy variable, as measured by NSQIP, may include oral and parenteral treatment with chemotherapeutic agents.7 Patients who underwent an emergency surgery (n = 17) and who were diagnosed with a disseminated cancer (n = 61) were excluded from the study. In addition, patients for whom data regarding neoadjuvant treatment information were missing (n = 286) were also excluded.
Baseline characteristics of patients
Patient baseline characteristics were compared between the groups of patients. As a subgroup analysis, the surgery-only patients were compared with the chemotherapy only group, radiation therapy only, and both chemo and radiation therapy patients, respectively. Demographic characteristics included age, gender, and race (white, black, other, or unknown/not reported). Clinical characteristics consisted of American Society of Anesthesiology (ASA) classification of patient physical condition, body mass index (BMI), and preoperative comorbidities such as diabetes mellitus (with oral agents or insulin), current history of smoking (within 1 year before the operation), history of alcohol consumption (of more than two drinks per day in 2 weeks before admission), dyspnea, hypertension requiring medication, previous cardiac surgery, weight loss (<10% of body weight in last 6 months), steroid use for chronic condition, pre-operative hematological cell counts (white blood cell [WBC] and hematocrit), and lastly, history of chronic obstructive pulmonary disease (COPD), congestive heart failure, and myocardial infarction (MI) within 6 months. On average, the blood samples were collected 8 days prior to undergoing esophagectomy.
Outcomes
In NSQIP, patients are followed after surgery for a maximum of 30 days. Complications or death after that period are not included. Intraoperative and postoperative outcomes between the two groups of patients were compared. Thirty-day mortality was the primary outcome of interest. The secondary outcomes included overall and serious morbidity, length of stay (LOS) and total operative time. Overall morbidity was defined by presence of at least one of the following NSQIP complications: wound infection, pneumonia, urinary tract infection, return to operating room (OR), venous thromboembolic events (VTE), cardiac complication, shock/sepsis, unplanned intubation, bleeding requiring transfusion (at least one unit of packed or whole red blood cells given from the surgical start time up to and including 72 hours postoperatively), renal complication, ventilator dependency >48 hours, and organ/space surgical site infection (SSI). Of note, there is no specific code for anastomotic leak in the NSQIP database; however, the variable ‘postoperative organ space surgical site infection’ has been previously used as a logical proxy for anastomotic leaks, although it carries a relatively low sensitivity.8 Serious morbidity included occurrences of the following NSQIP complications: return to OR, cardiac complication, shock/sepsis, unplanned intubation, ventilator dependence for >48 hours, and organ/space SSI. From our data, prolonged length of stay (PLOS) was defined as a stay greater than or equal to 17 days, whereas prolonged operation time was defined as an operative time greater than or equal to 418.5 minutes. Similar NSQIP measured intraoperative and postoperative complications were combined into groups as follows: the wound infection variable was classified as the combination of superficial wound infection, deep incisional superficial SSI, and wound disruption; the cardiac complication variable included cardiac arrest requiring cardiopulmonary resuscitation and MI; the renal complication variable was defined as a postoperative acute failure or progressive renal insufficiency; and the VTE variable consisted of deep vein thrombosis/thrombophlebitis and pulmonary embolism. In addition, LOS and operation time were defined as prolonged if they were greater than or equal to the 75th percentile.
Statistical analysis
Patients’ baseline characteristics and outcomes were compared between the two groups using Pearson’s chi-squared test for categorical variables and Student’s t-test for continuous variables. Fisher’s exact test was used when appropriate. As the two groups were noted to be significantly different at a baseline, a matching algorithm was employed to generate a control cohort of neoadjuvant patients that were well matched to the surgery-only patients with regards to the demographic and clinical characteristics. In particular, the one-to-one nearest neighbor matching algorithm without replacement was used. This strategy resulted in successful balancing of the treatment groups with regards to baseline variables. In addition to using the matching algorithm, multivariable logistic regression analysis was applied to confirm the finding from the matched analysis by predicting the odds of 30-day mortality, overall, and serious morbidity, as well as PLOS for the neoadjuvant patients in comparison to surgery-only patients. First, exploratory data analysis was performed using univariate logistic regression. Initially, the four models included all covariates with associations in exploratory analysis at the P < 0.25 level as recommended by Hosmer and Lemeshow.9 Additionally, all covariates of clinical importance were included, regardless of statistical significance. Models were then refined based on clinical importance of covariates and their impact on overall fit as assessed by likelihood ratio tests. As a result, the 30-day mortality model was adjusted for treatment received, age, BMI, ASA, alcohol consumption, and diabetes; the overall and serious morbidity models were adjusted for treatment received, age, gender, ASA, alcohol consumption, diabetes, dyspnea, history of COPD, hypertension, year of operation, WBC, hematocrit, and prolonged operative time; and lastly, the PLOS model was adjusted for age, ASA, smoking status, alcohol consumption, diabetes, dyspnea, history of COPD, hypertension, year of operation, WBC, hematocrit, and prolonged operative time. The fit of these four models was validated with the goodness of fit test. All data analyses and management were performed using Stata/MP version 12 (StataCorp LP, College Station, TX, USA). Statistical significance was indicated by P < 0.05.
RESULTS
Patient characteristics
One thousand nine hundred and thirty-six patients who underwent esophagectomy for esophageal cancer and whose preoperative neoadjuvant therapy information was available were identified. Ivor Lewis was the most common procedure in the study (41.8%) followed by transhiatal esophagectomy (34.3%), three-field (18.3%), intestinal conduit (4.0%), and other (1.7%). Of the 1936 patients, 708 (36.5%) received either chemotherapy, radiation therapy, or both prior to undergoing an operation. An increasing rate of preoperative neoadjuvant therapy for esophageal cancer was observed (from 29.0% in 2005–2006 to 44.0% in 2011, P < 0.001) (Fig. 1). The neoadjuvant patients were significantly younger (62.3 vs. 64.7 years, P < 0.001), had lower BMIs, and had significantly lower rates of diabetes, hypertension, and worse preoperative hematological cell counts (Table 1). As expected, weight loss was significantly greater in patients who underwent preoperative neoadjuvant therapy.
Fig. 1.
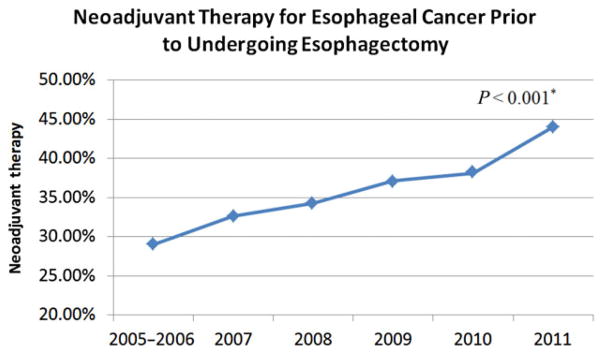
Rates of neoadjuvant therapy over the years. *P-value for the difference in proportion of patients who received neoadjuvant treatment prior to undergoing esophagectomy in 2005–2006 (29.0%) versus 2011 (44.0%).
Table 1.
Baseline demographic and clinical characteristics
| Characteristic | Total
|
Neoadjuvant patients
|
Surgery-only patients
|
P |
|---|---|---|---|---|
| n = 1939 | n = 708 (36.5%) | n = 1231 (63.5%) | ||
| Age, mean (median) | 63.9 ± 10.5 (65) | 62.3 ± 10.2 (63) | 64.7 ± 10.5 (65) | <0.001 |
| Male (%) | 1618 (83.6) | 601 (84.9) | 1017 (82.8) | 0.237 |
| Race (%) | 0.382 | |||
| White | 1634 (84.3) | 610 (86.2) | 1024 (83.2) | |
| Black | 57 (2.9) | 19 (2.7) | 38 (3.1) | |
| Other | 61 (3.2) | 19 (2.7) | 42 (3.4) | |
| Unknown | 187 (9.6) | 60 (8.5) | 127 (10.3) | |
| ASA classification (%) | 0.446 | |||
| No disturb/mild disturb | 414 (21.4) | 142 (20.1) | 272 (22.1) | |
| Serious disturb | 1386 (71.5) | 511 (72.2) | 875 (71.1) | |
| Life threat/moribund | 138 (7.1) | 55 (7.8) | 83 (6.8) | |
| Body mass index | 27.7 ± 6.1 | 26.8 ± 5.8 | 28.2 ± 6.2 | <0.001 |
| Diabetes (%) | 360 (18.6) | 113 (16.0) | 247 (20.1) | 0.025 |
| Current smoker (%) | 502 (25.9) | 206 (29.1) | 296 (24.1) | 0.015 |
| Alcohol consumption (%) | 93 (4.8) | 29 (4.1) | 64 (5.2) | 0.274 |
| Dyspnea (%) | 211 (10.9) | 76 (10.7) | 135 (11.0) | 0.874 |
| History of COPD (%) | 143 (7.4) | 51 (7.2) | 92 (7.5) | 0.827 |
| History of CHF (%) | 2 (0.1) | 2 (0.3) | 0 (0.0) | 0.133 |
| History of MI (%) | 13 (0.7) | 6 (0.9) | 7 (0.6) | 0.469 |
| Hypertension (%) | 1051 (54.2) | 335 (47.3) | 716 (58.2) | <0.001 |
| Previous cardiac surgery (%) | 133 (6.9) | 35 (4.9) | 98 (8.0) | 0.011 |
| Weight loss (%) | 404 (20.8) | 208 (29.4) | 196 (15.9) | <0.001 |
| Steroid use (%) | 29 (1.5) | 9 (1.3) | 20 (1.6) | 0.537 |
| Year of operation (%) | 0.003 | |||
| 2005–2007 | 473 (24.4) | 147 (20.8) | 326 (26.5) | |
| 2008–2009 | 720 (37.1) | 258 (36.4) | 462 (37.5) | |
| 2010–2011 | 746 (38.5) | 303 (42.80) | 443 (36.0) | |
| Preoperative WBC (%) | <0.001 | |||
| Normal (4.5–11 × 109/L) | 1507 (79.8) | 490 (70.7) | 1017 (85.0) | |
| Abnormal (<4.5 or >11 × 109/L) | 382 (20.2) | 203 (29.3) | 179 (15.0) | |
| Preoperative hematocrit (%) | <0.001 | |||
| Normal (≥36) | 1178 (62.8) | 347 (50.3) | 831 (70.0) | |
| Abnormal (<36) | 699 (37.2) | 343 (49.7) | 356 (30.0) |
Different denominators due to missing data: gender (n = 1936; n1 = 708; n2 = 1228); WBC (n = 1889; n1 = 693; n2 = 1196); Hematocrit (n = 1877; n1 = 690; n2 = 1187). Bold indicates statistically significant values. ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; WBC, white blood cell.
Unadjusted and adjusted outcomes
Thirty-day mortality, overall, and serious morbidity were comparable between the two groups with the exception of the individual complications of VTE and bleeding transfusion, which were significantly lower in the surgery-only patients (5.4% vs. 7.8%, P = 0.042; 6.7% vs. 10.7%, P = 0.002; respectively) (Table 2). Overall length of hospital stay and operative time were similar between the two groups, as were PLOS and prolonged operative time. In the subgroup analysis, the outcomes of interest were comparable in of all three separate analyses. However, patients with no preoperative neoadjuvant treatment had lower rates of VTE (5.7% vs. 9.1%, P = 0.013) when compared with radiation therapy patients, and rates for bleeding transfusion were lower for the non-treatment group when compared with the chemotherapy group and both treatments combined (6.89% vs. 13.92%, P = 0.020; 6.89% vs. 15.94%, P < 0.001; respectively).
Table 2.
Observed unadjusted rates of intraoperative and postoperative outcomes
| Outcome | Total
|
Neoadjuvant patients
|
Surgery-only patients
|
P |
|---|---|---|---|---|
| n = 1939 | n = 708 (36.5%) | n = 1231 (63.5%) | ||
| 30-day mortality (%) | 70 (3.6) | 21 (3.0) | 49 (4.0) | 0.249 |
| Overall morbidity† (%) | 956 (49.3) | 343 (48.5) | 613 (49.8) | 0.567 |
| Wound infection | 265 (13.7) | 100 (14.1) | 165 (13.4) | 0.657 |
| Pneumonia | 303 (15.6) | 90 (12.7) | 213 (17.3) | 0.007 |
| Urinary tract infection | 68 (3.5) | 18 (2.5) | 50 (4.1) | 0.080 |
| Return to OR | 240 (12.4) | 197 (13.7) | 143 (11.6) | 0.180 |
| Venous thromboembolism | 122 (6.3) | 55 (7.8) | 67 (5.4) | 0.042 |
| Cardiac complication | 57 (2.9) | 17 (2.4) | 40 (3.3) | 0.287 |
| Shock/sepsis | 361 (18.6) | 117 (16.5) | 244 (19.8) | 0.073 |
| Unplanned intubation | 266 (13.7) | 89 (12.6) | 177 (14.4) | 0.265 |
| Bleeding transfusion | 158 (8.2) | 76 (10.7) | 82 (6.7) | 0.002 |
| Renal complication | 39 (1.93) | 10 (1.36) | 29 (2.27) | 0.151 |
| On ventilator >48 hours | 293 (15.1) | 96 (13.6) | 197 (16.0) | 0.148 |
| Organ space SSI | 113 (5.8) | 40 (5.7) | 73 (5.9) | 0.800 |
| Serious morbidity‡ (%) | 651 (33.6) | 223 (31.5) | 428 (34.8) | 0.142 |
| Length of stay, days (median) | 15.8 ± 21.0 (11) | 15.2 ± 14.4 (11) | 16.2 ± 24.0 (12) | 0.279 |
| Prolonged length of stay (%) | 512 (26.4) | 173 (24.4) | 339 (27.5) | 0.136 |
| Operative time, minute (median) | 340.4 ± 124.7 (325) | 344.3 ± 122.3 (325) | 338.1 ± 126.0 (324) | 0.290 |
| Prolonged operative time (%) | 485 (25.0) | 175 (24.7) | 310 (25.2) | 0.820 |
Overall morbidity: wound infection, pneumonia, urinary tract infection, venous thromboembolism, bleeding transfusion, renal complication, return to OR, cardiac complication, shock/sepsis, unplanned intubation, on ventilator >48 hours, and organ space SSI.
Serious morbidity: return to OR, cardiac complication, shock/sepsis, unplanned intubation, on ventilator >48 hours, and organ space SSI. Bold indicates statistically significant values. OR, operative room; SSI, surgical site infection.
After performing matching analysis, there were 495 neoadjuvant patients that were identified and well matched in terms of baseline characteristics to the surgery-only patients (Table 3). Overall, there were 39 mortalities with no differences between the groups. There was also no significant difference between the two groups in terms of overall and serious morbidity, LOS, and operative time. Of note, the rate of venous thromboembolism was significantly higher in neoadjuvant patients when compared with the surgery-only patients (9.3% vs. 5.3%, P = 0.014) (Table 4).
Table 3.
Baseline demographic and clinical characteristics in the matched cohort
| Characteristic | Total
|
Neoadjuvant patients
|
Surgery-only patients
|
P |
|---|---|---|---|---|
| n = 990 | n = 495 | n = 495 | ||
| Age, mean (median) | 62.8 ± 10.3 (63) | 62.8 ± 9.8 (63) | 62.8 ± 10.7 (63) | 0.958 |
| Male (%) | 847 (85.6) | 429 (86.7) | 418 (84.4) | 0.320 |
| Race (%) | 0.091 | |||
| White | 850 (85.9) | 429 (86.7) | 421 (85.1) | |
| Black | 32 (3.2) | 10 (2.0) | 22 (4.4) | |
| Other | 29 (2.9) | 12 (2.4) | 17 (3.4) | |
| Unknown | 79 (8.0) | 44 (8.9) | 35 (7.1) | |
| ASA classification (%) | 0.927 | |||
| No disturb/mild disturb | 219 (22.1) | 109 (22.0) | 110 (22.2) | |
| Serious disturb | 698 (70.5) | 351 (70.9) | 347 (70.1) | |
| Life threat/moribund | 73 (7.4) | 35 (7.1) | 38 (7.7) | |
| Body mass index | 27.5 ± 5.9 | 27.6 ± 5.8 | 27.5 ± 6.1 | 0.773 |
| Diabetes (%) | 178 (18.0) | 87 (17.6) | 91 (18.4) | 0.741 |
| Current smoker (%) | 280 (28.3) | 141 (28.5) | 139 (28.1) | 0.888 |
| Alcohol consumption (%) | 45 (4.6) | 21 (4.2) | 24 (4.9) | 0.647 |
| Dyspnea (%) | 117 (11.8) | 52 (10.5) | 65 (13.1) | 0.201 |
| History of COPD (%) | 79 (8.0) | 36 (7.3) | 43 (8.7) | 0.412 |
| History of CHF (%) | 2 (0.2) | 2 (0.4) | 0 (0.0) | 0.499 |
| History of MI (%) | 9 (0.9) | 5 (1.0) | 4 (0.8) | 0.999 |
| Hypertension (%) | 515 (52.0) | 256 (51.7) | 259 (52.3) | 0.849 |
| Previous cardiac surgery (%) | 53 (5.4) | 28 (5.7) | 25 (5.1) | 0.672 |
| Weight loss (%) | 210 (21.2) | 103 (20.8) | 107 (21.6) | 0.756 |
| Steroid use (%) | 16 (1.6) | 6 (1.2) | 10 (2.0) | 0.313 |
| Year of operation (%) | 0.991 | |||
| 2005–2007 | 211 (21.3) | 106 (21.4) | 105 (21.2) | |
| 2008–2009 | 372 (37.6) | 185 (37.4) | 187 (37.8) | |
| 2010–2011 | 407 (41.1) | 204 (41.2) | 203 (41.0) | |
| Preoperative WBC (×109/L) (%) | 0.491 | |||
| Normal (4.5–11 × 109/L) | 771 (77.9) | 381 (77.0) | 390 (78.8) | |
| Abnormal (<4.5 or >11 × 109/L) | 219 (22.1) | 114 (23.0) | 105 (21.2) | |
| Preoperative hematocrit (%) | 0.438 | |||
| Normal (≥36) | 586 (59.2) | 299 (60.4) | 287 (58.0) | |
| Abnormal (<36) | 404 (40.8) | 196 (39.6) | 208 (42.0) |
ASA, American Society of Anesthesiology; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; WBC, white blood cell.
Table 4.
Observed unadjusted rates of intraoperative and postoperative outcomes in the matched cohort
| Outcome | Total
|
Neoadjuvant patients
|
Surgery-only patients
|
P |
|---|---|---|---|---|
| n = 990 | n = 495 | n = 495 | ||
| 30-day mortality (%) | 39 (3.9) | 18 (3.6) | 21 (4.2) | 0.624 |
| Overall morbidity† (%) | 487 (49.2) | 242 (48.9) | 245 (49.5) | 0.849 |
| Wound infection | 134 (13.5) | 72 (14.6) | 62 (12.5) | 0.353 |
| Pneumonia | 146 (14.8) | 64 (12.9) | 82 (16.6) | 0.107 |
| Urinary tract infection | 33 (3.3) | 12 (2.4) | 21 (4.2) | 0.111 |
| Return to OR | 123 (12.4) | 66 (13.3) | 57 (11.5) | 0.386 |
| Venous thromboembolism | 72 (7.3) | 46 (9.3) | 26 (5.3) | 0.014 |
| Cardiac complication | 24 (2.4) | 10 (2.0) | 14 (2.8) | 0.408 |
| Shock/sepsis | 176 (17.8) | 84 (17.0) | 92 (18.6) | 0.506 |
| Unplanned intubation | 130 (13.1) | 64 (12.9) | 66 (13.3) | 0.851 |
| Bleeding transfusion | 85 (8.6) | 43 (8.7) | 42 (8.5) | 0.910 |
| Renal complication | 14 (1.4) | 5 (1.0) | 9 (1.8) | 0.282 |
| On ventilator >48 hours | 148 (15.0) | 72 (14.5) | 76 (15.4) | 0.721 |
| Organ space SSI | 53 (5.4) | 28 (5.7) | 25 (5.1) | 0.672 |
| Serious morbidity‡ (%) | 324 (32.7) | 156 (31.5) | 168 (33.9) | 0.416 |
| Length of stay, days (median) | 16.0 ± 26.8 (11) | 14.8 ± 14.6 (10) | 17.1 ± 34.8 (12) | 0.190 |
| Prolonged length of stay (%) | 250 (25.3) | 112 (22.6) | 138 (27.9) | 0.057 |
| Operative time, minute (median) | 341.3 ± 126.1 (326) | 342.2 ± 122.2 (324) | 340.5 ± 130.1 (328) | 0.832 |
| Prolonged operative time (%) | 246 (24.9) | 120 (24.2) | 126 (25.5) | 0.659 |
Overall morbidity: wound infection, pneumonia, urinary tract infection, venous thromboembolism, bleeding transfusion, renal complication, return to OR, cardiac complication, shock/sepsis, unplanned intubation, on ventilator >48 hours, and organ space SSI.
Serious morbidity: return to OR, cardiac complication, shock/sepsis, unplanned intubation, on ventilator >48 hours, and organ space SSI. Bold indicates statistically significant values. OR, operative room; SSI, surgical site infection.
Results using multivariable logistic regression analysis were consistent with the unadjusted and matched analyses. Multivariable analysis confirmed that 30-day mortality, overall, and serious morbidity, as well as PLOS were comparable between the neoadjuvant and surgery-only patients while adjusting for other factors (Table 5).
Table 5.
Multivariable logistic regression analyses
| Outcome | OR (95% CI) | P |
|---|---|---|
| 30-day mortality | 0.79 (0.46–1.35) | 0.392 |
| Overall morbidity† | 1.01 (0.82–1.24) | 0.930 |
| Serious morbidity‡ | 0.97 (0.78–1.20) | 0.768 |
| Prolonged length of stay | 0.84 (0.67–1.05) | 0.130 |
Reference group is the surgery-only patients.
Overall morbidity: wound infection, pneumonia, urinary tract infection, venous thromboembolism, bleeding transfusion, renal complication, return to OR, cardiac complication, shock/sepsis, unplanned intubation, on ventilator >48 hours, and organ space SSI.
Serious morbidity: return to OR, cardiac, shock/sepsis, intubation, on ventilator > 48 hours, and organ space SSI. CI, confidence interval; OR, odds ratio.
DISCUSSION
In our study, results from both unadjusted and adjusted analysis showed substantially no differences in postoperative outcomes between patients who underwent neoadjuvant therapy prior to esophagectomy and those treated with surgery alone. Thirty-day mortality and morbidity and overall LOS were comparable between the two groups, with a few exceptions among individual complications. Despite the fact that neoadjuvant treatment has been described to increase the difficulty of a surgical procedure, we found similar operative times in our two groups.10
The differences noted at baseline between the two groups were to be expected, likely as a consequence of induction therapy; treated patients, in fact, demonstrated signs of neoadjuvant-induced anorexia and marrow suppression, such as lower BMI, preoperative weight loss, and lower blood counts. In parallel, it is justified to hypothesize that several preoperative characteristics of patients in the surgery-only group, such as more advanced age and comorbidities (diabetes, hypertension, previous cardiac surgery), might have contributed to the decision of avoiding neoadjuvant therapy. Even though we found no differences in mortality and global morbidity between the two groups, we observed that treated patients experienced more bleeding requiring transfusion and venous thromboembolism. Of note, after performing matching analysis, only the difference in the rate of venous thromboembolism remained significant. The occurrence of the aforementioned adverse events can easily be related to neoadjuvant therapy, which is known to impact several organ systems in a multifactorial fashion. A component of chemotherapy-induced myelotoxicity, e.g., is thrombocytopenia, which potentially increases the risk of bleeding in solid tumor patients, regardless of the specific chemotherapy regimen administered.11 In addition, radiation-induced fibrosis makes dissection between the anatomical planes during esophagectomy more difficult, likely predisposing to injury to the adjacent structures and related bleeding.10 Moreover, treated patients are more likely to present to surgery with a lower red cell count than non-treated patients, leading to a more pronounced postoperative anemia and hence increasing the likelihood of blood transfusion.
Both chemotherapy and radiotherapy have been associated with increased risk of developing venous thrombosis.12 Some authors reported that the addition of radiation therapy to the neoadjuvant regimen remarkably increases postoperative mortality, when compared with preoperative chemotherapy alone.13 Conversely, we only found isolated differences between CRT, chemotherapy alone, and radiation therapy alone in subgroup analysis, with comparable mortality and overall and serious morbidity.
Our findings correlate well with the results of a recent randomized trial by van Hagen et al., which showed no significant differences in the occurrence of surgical complications between patients who underwent preoperative CRT and patients treated with surgery only.4 Benefits of neoadjuvant CRT include clearance of micrometastatic disease and tumor down staging, which allows for a more radical surgical resection; these effects were reflected in their results in terms of increased survival and R0 resection rate in the neoadjuvant group. Of note, a remarkable percentage of patients in their CRT group experienced the same induction-related events that we described, such as anorexia and poor blood cell counts. Other investigations led to similar conclusions about safety of preoperative CRT for esophageal cancer: results from a recent multicenter study showed a comparable 90-day mortality rate between patients treated with neoadjuvant therapy and surgery alone.14 A meta-analysis by Lv et al. showed no difference in adverse events rate between preoperative CRT and surgery alone; however, the authors observed a trend in favor of surgery alone for operative mortality.15
The NSQIP database has already been queried for the purpose of studying the influence of neoadjuvant therapy on early outcomes of surgical procedures for other types of cancer, with results similar to ours. Fahy et al. compared patients who either received or not chemotherapy within 30 days prior to liver resections, finding no differences in 30-day survival and major complication rates between the two groups.16 Similarly, Cho et al. demonstrated comparable mortality and morbidity after pancreaticoduodenectomy for pancreatic cancer in patients treated with or without neoadjuvant radiation therapy.17 More recently, the NSQIP database has been used to study the impact of preoperative chemotherapy or radiotherapy on perioperative outcomes of surgery for retroperitoneal sarcoma and bladder cancer.18,19
Even though the characteristics of the NSQIP dataset don’t allow us to analyze oncologic outcomes, we observed an increasing trend of preoperative neoadjuvant therapy for esophageal cancer, from 29.0% in 2005–2006 to 44.0% in 2011, P < 0.001. Since numerous reports (especially the CROSS trial) have documented, over the last decade, an increased survival for esophageal cancer patients treated with multimodality therapy, it is reasonable to attribute our finding to a progressively augmented confidence toward the beneficial role of induction treatment.4
The limitations of our study are mostly due to the characteristics of the queried database. Specifically, NSQIP only provides information on receiving chemotherapy for malignancy 30 days prior to the operation and receiving radiation therapy within 90 days before the operation. This could potentially lead to misclassification of patients in the different treatment subgroups, in case a patient had undergone chemotherapy, radiotherapy, or both earlier than the treatment window recorded by NSQIP. In addition, we had to exclude 286 patients from our analysis, due to missing data on neoadjuvant treatment. Of note, NSQIP only collects information on patients who underwent surgery; as a consequence, individuals experiencing severe adverse complications of neoadjuvant treatment precluding surgery could not be included in our study. Moreover, we had no information on specific treatment protocols, which would have allowed for a study of correlation between different radiation doses or drugs and specific complications. Similarly, one could hypothesize that patients treated with neoadjuvant chemotherapy may be different at baseline from those receiving neoadjuvant CRT, in regard to patient’s or tumor’s characteristics. Unfortunately, NSQIP lacks sufficient details to explore the indications for neoadjuvant treatment. In the same vein, the dataset does not allow for studying the specific results of minimally invasive surgical techniques in this group of patients. NSQIP, in fact, relies on CPT codes for classifying surgical procedures and unfortunately, to date, there is no dedicated code for minimally invasive or hybrid esophagectomy. Of note, in addition, NSQIP dataset records the variable postoperative bleeding basing on the number of transfusions received. Since treated patients are more likely to present to surgery with a lower red cell count than non-treated patients, they have higher odds of developing a more pronounced postoperative anemia; this, rather than higher surgical bleeding, might justify the differences in the NSQIP variable ‘bleeding transfusion’. Finally, as mentioned previously, NSQIP only gives us information on the first 30 postoperative days, therefore precluding the opportunity of analyzing oncological outcomes.
Despite the abovementioned limitations, we believe that the use of an outcomes specific oriented database, as well as the large number of patients analyzed in this study, represent a valuable tool for evaluating the comparative safety of esophagectomy performed alone or after induction therapy.
In conclusion, the results of our analysis show that preoperative neoadjuvant therapy for esophageal cancer does not increase 30-day mortality or the overall risk of postoperative complications after esophagectomy. In parallel, there is increasing evidence, in the literature, that induction therapy leads to longer survival than surgery alone. With these assumptions, we strongly believe that surgeons should not be hindered by the misleading perception that neoadjuvant treatment negatively affects postoperative outcomes to such an extent to outweigh its oncological benefits.
Acknowledgments
Mr. Edwin Lewis provided generous support of Dr Lidor’s Department of Surgery Research Fund.
References
- 1.International Agency for Research on Cancer. Oesophageal cancer: estimated incidence, mortality and prevalence worldwide in 2012. [Cited 9 Jan 2014.] Available from URL: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925–30. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Surgeons National Surgical Quality Improvement Program. Participants. Chicago, IL, USA: [Cited 9 Jan 2014.] Available from URL: http://site.acsnsqip.org/participants. [Google Scholar]
- 7.American College of Surgeons National Surgical Quality Improvement Program. ACS-NSQIP user guide for the 2011 Participant Data Use File. 2012 [Cited 9 Jan 2014.] Available from URL: http://site.acsnsqip.org/wp-content/uploads/2012/03/2011-User-Guide_Final.pdf.
- 8.Reinke CE, Showalter S, Mahmoud NN, Kelz RR. Comparison of anastomotic leak rate after colorectal surgery using different databases. Dis Colon Rectum. 2013;56:638–44. doi: 10.1097/DCR.0b013e31827886db. [DOI] [PubMed] [Google Scholar]
- 9.Hosmer DW, Lemeshow S. Applied Logistic Regression, 2nd edn. New York: Wiley. 2000;95:143–164. [Google Scholar]
- 10.Javed A, Pal S, Chaubal GN, Sahni P, Chattopadhyay TK. Management and outcome of intrathoracic bleeding due to vascular injury during transhiatal esophagectomy. J Gastrointest Surg. 2011;15:262–6. doi: 10.1007/s11605-010-1375-8. [DOI] [PubMed] [Google Scholar]
- 11.Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–46. doi: 10.1200/JCO.2001.19.4.1137. [DOI] [PubMed] [Google Scholar]
- 12.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 13.Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–6. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 14.Vallbohmer D, Holscher AH, DeMeester S, et al. A multicenter study of survival after neoadjuvant radiotherapy/chemotherapy and esophagectomy for ypT0N0M0R0 esophageal cancer. Ann Surg. 2010;252:744–9. doi: 10.1097/SLA.0b013e3181fb8dde. [DOI] [PubMed] [Google Scholar]
- 15.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15:4962–8. doi: 10.3748/wjg.15.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahy BN, Aloia TA, Jones SL, Bass BL, Fischer CP. Chemotherapy within 30 days prior to liver resection does not increase postoperative morbidity or mortality. HPB (Oxford) 2009;11:645–55. doi: 10.1111/j.1477-2574.2009.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SW, Tzeng CW, Johnston WC, et al. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) HPB (Oxford) 2013;16:350–6. doi: 10.1111/hpb.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett EK, Roses RE, Meise C, Fraker DL, Kelz RR, Karakousis GC. Preoperative radiation for retroperitoneal sarcoma is not associated with increased early postoperative morbidity. J Surg Oncol. 2014;109:606–11. doi: 10.1002/jso.23534. [DOI] [PubMed] [Google Scholar]
- 19.Johnson DC, Nielsen ME, Matthews J, et al. Neoadjuvant chemotherapy for bladder cancer does not increase risk of perioperative morbidity. BJU Int. 2013 doi: 10.1111/bju.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]


