Abstract
Background
Testosterone may be a biological factor that protects males against eating disorders. Elevated prenatal testosterone exposure is linked to lower levels of disordered eating symptoms, but effects emerge only after mid-puberty. Whether circulating levels of testosterone account for decreased risk for disordered eating in boys after mid-puberty is currently unknown; however, animal data support this possibility. In rodents, prenatal testosterone’s masculinizing effects on sex-differentiated behaviors emerge during puberty when circulating levels of testosterone increase and ‘activate’ the expression of masculinized phenotypes. This study investigated whether higher levels of circulating testosterone predict lower levels of disordered eating symptoms in adolescent boys, and in particular whether effects are associated with advancing pubertal maturation.
Method
Participants were 213 male twins from the Michigan State University Twin Registry. The Minnesota Eating Behavior Survey and Eating Disorder Examination Questionnaire assessed several disordered eating symptoms. The Pubertal Development Scale assessed pubertal status. Afternoon saliva samples were assayed for testosterone using enzyme immunoassays.
Results
Consistent with animal data, higher levels of circulating testosterone predicted lower levels of disordered eating symptoms in adolescent boys and effects emerged with advancing puberty. Results were not accounted for by several important covariates, including age, adiposity, or mood/anxiety symptoms.
Conclusions
Findings suggest that elevated circulating testosterone may be protective and underlie decreased risk for eating pathology in males during/after puberty, whereas lower levels of testosterone may increase risk and explain why some, albeit relatively few, males develop eating disorders.
Keywords: Adolescence, disordered eating, eating disorders, males, puberty, testosterone
Introduction
Males account for one-third to one-tenth of eating disorder cases (Hudson et al. 2007; Swanson et al. 2011), yet the factors that contribute to decreased risk for eating disorders in males are largely unknown. Testosterone may be a key biological mechanism that exhibits protective effects. With a few exceptions (Baker et al. 2009; Lydecker et al. 2012), higher prenatal testosterone exposure (assessed via proxy measures: 2nd to 4th digit ratios or twin type comparisons) has been shown to decrease risk for disordered eating (Klump et al. 2006; Culbert et al. 2008, 2013; Smith et al. 2010; Oinonen & Bird, 2012). However, prenatal testosterone’s protective effects on disordered eating appear to become evident only after the onset of mid-puberty (Culbert et al. 2013). Findings indicated no effect of prenatal testosterone on disordered eating in pre–early puberty, whereas elevated prenatal testosterone predicted lower disordered eating symptoms during/after mid-puberty (Culbert et al. 2013). Lower disordered eating risk in boys after mid-puberty may be due to increases in circulating testosterone and/or pubertal changes in the central nervous system’s (CNS) responsiveness to circulating testosterone.
Animal data support both possibilities. Prenatal testosterone’s masculinizing (i.e. to make male-like) effects on sex-differentiated eating behavior in rodents (e.g. food intake: males>females) emerge during puberty when circulating levels of testosterone increase and begin to exert activational effects (i.e. transient; only in the presence of the hormone) that facilitate male-typical expression of behavior (e.g. Chai et al. 1999; Asarian & Geary, 2006). Nonetheless, the degree of pubertal maturation appears to be important for CNS responsiveness to these activational processes, as the exogenous administration of testosterone fails to activate sex-differentiated behavior (e.g. mating behavior) in pre-pubertal male rodents (Romeo et al. 2002). Thus, in addition to the rise in testosterone secretion, neural and behavioral responsiveness to testosterone also increase with advancing pubertal development (Romeo et al. 2002; Schulz et al. 2004, 2009).
A critical next step is to examine the effects of circulating testosterone on disordered eating in boys and whether advancing pubertal maturation is important for the emergence of such effects. The current study examined two plausible, but competing, hypotheses: (1) whether higher levels of circulating testosterone predict lower levels of disordered eating symptoms regardless of relative pubertal development (i.e. main effect of testosterone on disordered eating); or (2) whether testosterone’s protective effects on disordered eating in boys become prominent with advancing pubertal development. If data indicate a main effect of testosterone on disordered eating, then mid-puberty could be linked to a lower risk for disordered eating in males merely because it is an indirect marker of increased testosterone levels. If data indicate pubertal moderation of testosterone’s effects on disordered eating, then advancing pubertal maturation may have etiological significance. That is, mid-puberty may correspond to the developmental period when neural and behavioral responsiveness to testosterone increases. Several developmental (i.e. age), physical (i.e. adiposity) and psychological (i.e. depression and anxiety symptoms) factors that are associated with disordered eating, puberty and testosterone were controlled for in analyses to ensure that testosterone–disordered eating effects were not accounted for by these potential confounds.
Method
Participants
Participants were 213 male twins [same-sex: monozygotic (n=86) and dizygotic (n=62); opposite-sex dizygotic (n=65)1,†] aged 10–15 years from the Michigan State University Twin Registry (MSUTR) Twin Study of Hormones and Disordered Eating across Puberty (Klump & Burt, 2006; Burt & Klump,2013). Inclusion criteria included no medication use or medical condition (e.g. diabetes) that could alter hormone functioning. Participants were recruited by the Michigan Department of Community Health through the use of birth records (Klump & Burt,2006; Burt & Klump, 2013). This recruitment method has resulted in MSUTR samples that are demographically representative of the Michigan population (Culbert et al. 2008). Consistent with the recruitment region (www.michigan.gov/mdch), the majority of participants were Caucasian (85%). Caucasian v. non-Caucasian participants did not significantly differ on any variable/covariate (p’s=0.27–0.97).
Measures
Disordered eating symptoms
The Minnesota Eating Behavior Survey2 (MEBS; Von Ranson et al. 2005) and Eating Disorder Examination Questionnaire (EDE-Q; Fairburn & Beglin, 1994) were used to assess a range of disordered eating symptoms. The inclusion of both questionnaires allowed us to investigate whether our findings replicate across commonly used measures. MEBS and EDE-Q total/global scores were, as expected, positively associated (r=0.75).
The MEBS assesses body dissatisfaction (dissatisfaction with body size/shape), weight preoccupation (preoccupation with dieting, weight and the pursuit of thinness), binge eating (thoughts/behaviors conducive to binge eating) and compensatory behavior (thoughts/behaviors about compensatory behaviors, such as self-induced vomiting). The EDE-Q assesses dietary restraint (restraint over eating), eating concerns (preoccupation with food/eating), shape concerns (preoccupation/dissatisfaction with body shape) and weight concerns (preoccupation with body weight and desire to lose weight). MEBS and EDE-Q total/global scores are computed by summing all items (MEBS) or subscale items (EDE-Q). Higher scores indicate more pathological eating attitudes and behavior.
We examined the total/global scores as omnibus measures of disordered eating symptoms because, relative to subscales, these scores have shown stronger psychometric properties (e.g. 3-year stability, internal consistency) and are more robust indicators of disordered eating difficulties in males and children/adolescents (Carter et al. 2001; Von Ranson et al. 2005; Lavender et al. 2010; Culbert et al. 2013; A. Marderosian et al. unpublished observations).3 The MEBS total score has also been shown to successfully discriminate between girls with an eating disorder v. controls (Von Ranson et al. 2005). In this study, the MEBS and EDE-Q total/global scores demonstrated excellent internal consistency (α=0.85–0.91; see Table 1) and expected correlations with external correlates [i.e. depression, anxiety, body fat percentage, body mass index (BMI); see Table 2]. Further, the wide range of endorsed symptoms (see Table 1) and no variance differences in symptoms between boys in pre–early v. mid-late puberty (p=0.65–0.71) demonstrates that the MEBS and EDE-Q total/global scores capture the full spectrum of disordered eating attitudes and behaviors in boys throughout puberty.
Table 1. MEBS and EDE-Q item endorsement frequencies and item-total correlationsa.
| Item endorsement frequency, % of n participants |
Item/subscale and total score correlations: r |
|||
|---|---|---|---|---|
| Categorical pubertal status |
||||
| Pre–early (n=99–149) |
Mid–late (n=37–64) |
Overall sample (n= 136–213) |
Overall sample (n= 136–213) |
|
| MEBS item descriptor | ||||
| Binge eating (α= 0.70) | 0.73*** | |||
| Score range (maximum= 7): 0–6 | ||||
| Eats in response to emotions | 6.7 | 14.1 | 8.9 | 0.23*** |
| Stuffs self with food | 41.6 | 48.4 | 43.7 | 0.39*** |
| Eats a large amount of food with a lack of control | 17.4 | 21.9 | 18.8 | 0.64*** |
| Frequently thinks about binge eating | 12.8 | 11.1 | 12.3 | 0.57*** |
| Restricts food around others, binges when alone | 17.4 | 17.2 | 17.4 | 0.45*** |
| Avoids eating in front of others | 7.4 | 7.8 | 7.5 | 0.50*** |
| Afraid will eat when sad | 3.4 | 4.7 | 3.8 | 0.47*** |
| Body dissatisfaction (α =0.81) | 0.72*** | |||
| Score range (maximum= 6): 0–6 | ||||
| Stomach is too large | 14.1 | 17.2 | 15.0 | 0.52*** |
| Thighs are too large | 15.4 | 12.5 | 14.6 | 0.50*** |
| Dislikes body shape | 15.4 | 17.2 | 16.0 | 0.47*** |
| Buttocks are too large | 4.0 | 4.7 | 4.2 | 0.42*** |
| Frequently wishes to be thinner | 12.1 | 15.6 | 13.1 | 0.66*** |
| Dislikes hip size | 11.4 | 17.2 | 13.1 | 0.41*** |
| Compensatory behavior (α=0.46) | 0.40*** | |||
| Score range (maximum= 6): 0–4 | ||||
| Thinks about using self-induced vomiting for weight loss | 2.7 | 3.1 | 2.8 | 0.34*** |
| Stops eating for >1 day for weight loss | 4.0 | 0.0 | 2.8 | 0.21** |
| Uses laxatives to control weight | 2.7 | 1.6 | 2.3 | 0.23*** |
| Uses diet pills for weight loss | 0.7 | 3.1 | 1.4 | 0.26*** |
| Uses self-induced vomiting to control weight | 0.7 | 1.6 | 0.9 | 0.14* |
| Uses diuretics to control weight | 0.0 | 1.6 | 0.5 | 0.17* |
| Weight preoccupation (α=0.73) | 0.83*** | |||
| Score range (maximum=8): 0–8 | ||||
| Frequently diets to control weight | 12.1 | 7.8 | 10.8 | 0.36*** |
| Frequently thinks about dieting or weight loss | 15.4 | 17.2 | 16.0 | 0.67*** |
| Feels guilty about overeating | 18.8 | 10.9 | 16.4 | 0.49*** |
| Fears gaining weight | 22.8 | 20.3 | 22.1 | 0.58*** |
| Places undue importance on body weight | 40.9 | 43.8 | 41.8 | 0.41*** |
| Feels fat after eating a normal amount of food | 16.1 | 17.2 | 16.4 | 0.51*** |
| Worries about weight gain | 9.4 | 6.3 | 8.5 | 0.57*** |
| Frequently engages in weight checking | 26.2 | 18.8 | 23.9 | 0.41*** |
| Total score/other included items (α=0.85) | - | |||
| Score range (maximum=30): 0–19 | ||||
| Worries after eating sweets or starches | 13.4 | 10.9 | 12.7 | 0.39*** |
| Difficulty perceiving hunger | 36.9 | 32.8 | 35.7 | 0.51*** |
| Uses exercise for weight control more than peers | 30.9 | 23.4 | 28.6 | 0.44*** |
| Frequently weighs self to check for weight gain | 26.2 | 18.8 | 23.9 | 0.41*** |
| EDE-Q item descriptor | ||||
| Dietary restraint (α=0.69) | 0.69*** | |||
| Score range (maximum=6): 0–4.80 | ||||
| Attempts to limit food intake to influence shape/weight | 26.2 | 34.4 | 28.6 | 0.58*** |
| Avoids eating for long time period to alter shape/weight | 11.4 | 3.1 | 8.9 | 0.34*** |
| Tries to avoid eating certain foods to alter shape/weight | 29.5 | 26.6 | 28.6 | 0.58*** |
| Tries to follow dietary rules to influence shape/weight | 20.8 | 23.3 | 21.6 | 0.46*** |
| Wants stomach to be empty | 11.4 | 7.8 | 10.3 | 0.27** |
| Eating concerns (α=0.65) | 0.82*** | |||
| Score range (maximum=6): 0–4.20 | ||||
| Preoccupation with food/eating | 20.2 | 8.1 | 16.9 | 0.41*** |
| Afraid of losing control over eating | 21.2 | 8.1 | 17.6 | 0.72*** |
| Eats in secret | 27.3 | 10.8 | 22.8 | 0.13 |
| Feels guilty after eating | 29.3 | 27.0 | 28.7 | 0.73*** |
| Uncomfortable eating in front of others | 19.2 | 24.3 | 20.6 | 0.60*** |
| Shape concerns (α=0.85) | 0.94*** | |||
| Score range (maximum=6): 0–5.13 | ||||
| Wants flat stomach | 34.3 | 40.5 | 36.0 | 0.59*** |
| Cognitive preoccupation with shape/weight | 18.2 | 13.5 | 16.9 | 0.65*** |
| Fear of gaining weight or becoming fat | 33.3 | 27.0 | 31.6 | 0.71*** |
| Feelings of fatness | 28.3 | 29.7 | 28.7 | 0.68*** |
| Body shape influences judgment of self | 25.3 | 45.9 | 30.9 | 0.67*** |
| Dissatisfied with body shape | 26.3 | 45.9 | 31.6 | 0.76*** |
| Uncomfortable seeing one’s body | 26.3 | 27.0 | 26.5 | 0.73*** |
| Uncomfortable with others seeing one’s body | 38.4 | 54.1 | 42.6 | 0.65*** |
| Weight concerns (α=0.75) | 0.90*** | |||
| Score range (maximum=6): 0–5.20 | ||||
| Strong desire to lose weight | 26.3 | 32.4 | 27.9 | 0.69*** |
| Body weight influences judgment of self | 23.2 | 45.9 | 29.4 | 0.67*** |
| Cognitive preoccupation with shape/weight | 18.2 | 13.5 | 16.9 | 0.65*** |
| Would be upset if had to weigh self once per week | 35.4 | 21.6 | 31.6 | 0.48*** |
| Dissatisfied with body weight | 30.3 | 35.1 | 31.6 | 0.76*** |
| Global score (α=0.91) | – | |||
| Score range (maximum=6): 0–4.26 | ||||
MEBS, Minnesota Eating Behaviors Survey; EDE-Q, Eating Disorder Examination Questionnaire.
Only EDE-Q and MEBS item descriptors (not the actual items) are presented above. MEBS items are rated using a true/false format, and thus, endorsement frequency reflects the percentage of ‘true’ ratings. EDE-Q items are rated based on the past 28 days and scored on a seven-point scale, and item endorsement frequencies refer to the percentage of ratings greater than 0 (i.e. endorsement of scores ≥1). Sample sizes vary across scales because a subset of participants did not complete the full EDE-Q scale.
p<0.05,
p<0.01,
p<0.001
Table 2. Descriptive statistics, correlations and mean differences between pubertal status groupsa.
| Pubertal status |
Pubertal status |
|||||
|---|---|---|---|---|---|---|
| Overall sample (n=136–213) |
Pre–early puberty (n=99–149) |
Mid–late puberty (n=37–64) |
t or z | p | Cohen’s d | |
| Raw mean (s.d.) | Mean difference | |||||
| MEBS total score | 4.15 (4.17) | 4.13 (4.06) | 4.20 (4.45) | 0.13 | 0.90 | 0.06 |
| % Above clinical cut-off (score ≥15.55) | 1.41 | 1.34 | 1.56 | |||
| EDE-Q global score | 0.61 (0.77) | 0.63 (0.83) | 0.55 (0.62) | 0.42 | 0.68 | 0.10 |
| Testosterone | 52.98 (34.24) | 39.49 (21.13) | 84.38 (38.27) | −9.78 | <0.001 | 10.45 |
| Covariates | ||||||
| Age | 12.74 (1.62) | 12.09 (1.40) | 14.20 (1.14) | −11.09 | <0.001 | 10.64 |
| CDI depression total score | 6.19 (5.39) | 5.86 (4.60) | 6.95 (6.88) | −0.59 | 0.56 | 0.09 |
| MASC anxiety total score | 46.85 (16.46) | 49.40 (15.63) | 40.82 (16.94) | 3.54 | <0.001 | 0.53 |
| Percentage body fat | 20.52 (7.84) | 20.41 (8.04) | 20.76 (7.41) | −0.29 | 0.78 | 0.04 |
| Body mass index | 19.82 (3.87) | 18.83 (3.38) | 21.92 (4.02) | −2.01 | <0.05 | 0.31 |
| Correlations with MEBS total score | Correlation comparison | |||||
| Pearson correlations | ||||||
| Testosterone | −0.07 | 0.10 | −0.40** | 3.44 | <0.01 | - |
| Age | −0.13† | −0.06 | −0.34** | 1.93 | <0.06 | - |
| CDI depression total score | 0.54** | 0.50** | 0.61** | −1.05 | 0.29 | - |
| MASC anxiety total score | 0.29** | 0.32** | 0.26* | 0.43 | 0.67 | - |
| Percentage body fat | 0.26** | 0.14 | 0.53** | −2.95 | <0.01 | - |
| Body mass index | 0.30** | 0.26** | 0.38** | −0.88 | 0.38 | - |
| Partial correlation, adjusted for covariates | ||||||
| Testosterone | −0.04 | 0.08 | −0.34** | 2.85 | <0.01 | - |
| Correlations with EDE-Q global score | ||||||
| Pearson correlations | ||||||
| Testosterone | −0.09 | 0.06 | −0.45** | 2.73 | <0.01 | - |
| Age | −0.22** | −0.17† | −0.45** | 1.57 | 0.11 | - |
| CDI depression total score | 0.38*** | 0.32** | 0.47** | −0.89 | 0.37 | - |
| MASC anxiety total score | 0.38*** | 0.36** | 0.47** | −0.67 | 0.50 | - |
| Percentage body fat | 0.22* | 0.16 | 0.36* | −1.08 | 0.28 | - |
| Body mass index | 0.35** | 0.34** | 0.41** | −0.41 | 0.68 | - |
| Partial correlation, adjusted for covariates | ||||||
| Testosterone | −0.01 | 0.08 | −0.39** | 2.47 | 0.01 | - |
Raw mean, Value not adjusted for any covariate; s.d., standard deviation; MEBS, Minnesota Eating Behavior Survey; EDE-Q, Eating Disorder Examination Questionnaire; CDI, Children’s Depression Inventory; MASC, Multidimensional Anxiety Scale for Children.
Salivary testosterone values are in pg/ml. Mean differences were tested with mixed linear models (t statistic) using Blom-transformed variables. Higher scores on the MEBS, CDI and MASC indicate higher levels of pathological symptoms. Partial correlations reflect associations between testosterone and disordered eating scores, controlling for all other variables: age, depression, anxiety, body fat percentage and body mass index. Pubertal status (i.e. pre–early v. mid–late) differences in the magnitude of correlation coefficients were tested using Fisher’s r-to-z transformation.
p<0.10,
p<0.05,
p<0.01.
Pubertal development
The Pubertal Development Scale (PDS; Petersen et al. 1988) assessed pubertal status across a range of secondary sex characteristics, including height spurts and body hair, skin, and voice changes. Participants rated items on a continuous four-point scale; development: (1) has not yet begun; (2) has barely started; (3) is definitely underway; and (4) seems complete. Higher scores indicate more advanced pubertal maturation. Data support the reliability and validity of the PDS, including high correlations between self and interviewer (r=0.70) ratings (Petersen et al. 1988) and excellent internal consistency in the current study (α=0.87).
Our analyses utilized the continuous pubertal status variable (i.e. average PDS score) in order to estimate testosterone–disordered eating effects across the full range of pubertal maturation. Descriptive statistics (see Tables 1 and 2) are, however, presented for our overall sample and separately for pre-to-early (PDS< 2.5) v. mid-to-late pubertal (PDS ≥2.5) categories since prior research demonstrates changes in etiological effects on disordered eating during mid–late puberty (e.g. Klump et al. 2007,2012; Culbert et al. 2009).
Testosterone
Circulating testosterone levels were measured via saliva samples. The saliva–serum correlation for testosterone is near unity in male samples (r=0.91–0.96; Granger et al. 1999; Shirtcliff et al. 2001); however, the use of saliva was considered advantageous over other biomarkers (e.g. serum, plasma) because it is less invasive and salivary concentrations of testosterone reflect only the unbound hormone, i.e. the biologically active fraction of this steroid (Vining et al. 1983; Shirtcliff et al. 2001).
Prior to saliva collection, participants refrained from eating and drinking for 4 h and from brushing their teeth and/or chewing gum for at least 30 min. Collection occurred in the laboratory between 13.00 and 17.00 hours since diurnal variations in testosterone are more minimal during the late afternoon as compared with the morning or midday (Grumbach & Styne, 1998; Granger et al. 1999). Participants passively drooled 4 ml of saliva into a cryovial. Samples were immediately placed in the freezer and stored at −20 °C until they were shipped to Salimetrics, Inc. (Pennsylvania State University, College Park, USA) for testing.
Samples were centrifuged at 3000 revolutions per min for 15 min on the day of testing to remove mucins. Clear samples were transferred into testing wells to screen for potential problems with pH. Samples that fell out of the pH range of 4–9 were diluted in phosphate-buffered saline to correct pH prior to testing. Samples were assayed in duplicate using high-sensitive enzyme immunoassays developed by Salimetrics, Inc.
The testosterone assay had a minimum detection limit of 1 pg/ml and average intra- and inter-assay coefficients of variations that were less than 4.60% and 8.25%, respectively. Method accuracy, determined by spike recovery and linearity, was within acceptable ranges (104.4% and 99.9%). Testosterone concentrations in our sample were above the minimum detection limit (range: 9.59–183.05 pg/ml) and similar to levels attained in other studies of adolescent boys (Granger et al. 2004). The salivary testosterone–PDS correlation in our sample (r=0.69) is also on par with prior research (r’s=0.47–0.62; Granger et al. 1999).
Covariates
Age, adiposity, depression and anxiety have been associated with testosterone and/or disordered eating in prior research (Jacobi et al. 2004; Schulz et al. 2009; Ferreiro et al. 2011; Culbert et al. 2013), and, thus, were covaried in analyses to ensure that testosterone–disordered eating associations were not accounted for by their potential confounding effects.
Adiposity
Adiposity was examined using percentage body fat and BMI. Percentage body fat was assessed using hand-to-foot (i.e. whole body) bioelectrical impedance analysis (BIA) and calculated using the Houtkooper formula (Houtkooper et al. 1996): percentage body fat=0.61×height (cm)2/resistance (Ω)+0.25× weight (kg)+1.31. BIA is a painless procedure that passes an electrical signal through fat, lean mass and water, and has been shown to provide valid and reliable estimates of adiposity (Houtkooper et al. 1996). According to the National Institute of Health’s guidelines, participants fasted (no food or drink) for 4 h and voided within 30 min prior to their BIA assessment. BMI was calculated as weight (kg)/height (m)2 and adjusted for age. Height and weight measurements were obtained with a wall-mounted ruler and digital scale.
Anxiety and depression symptoms
Total scores from the self-reported Multidimensional Anxiety Scale for Children (MASC; March et al. 1997) and the Children’s Depression Inventory (CDI; Kovacs, 1985) assessed overall levels of anxiety (e.g. panic, social anxiety, physical symptoms) and depressive symptoms (e.g. sadness, inappropriate guilt/self-blame), respectively. Higher scores on both scales indicate higher levels of anxiety/depressive symptoms. The CDI and MASC have demonstrated excellent psychometric properties in boys (Kovacs, 1985; March et al. 1997). Internal consistencies were also good (CDI: α=0.82; MASC: α=0.91) in this study.
Statistical analyses
Data preparation
Subscale scores were pro-rated for participants missing ≤10% of items on the CDI. Data were coded as missing for one participant missing more than 10% of items on the MASC anxiety total score. There were no missing data for the PDS or MEBS scores. Due to protocol changes, the full EDE-Q was administered to only a subset of participants (n=136; 63.85% of the total sample). Data were also missing for a small subset of participants who did not complete the BIA procedure (n=17) due to home-based assessments. Project staff conducted home assessments when families were unable to travel to the laboratory (e.g. lack of transportation, childcare constraints, etc.). Participants with v. without complete EDE-Q or BIA body fat percentage data did not significantly differ on any study variable/covariate (p’s=0.20–0.88).
Testosterone, disordered eating, depression and BMI were rank-normalized and Blom-transformed prior to analyses to adjust for slight positive skew (skewness =1.16–2.03). Blom transformation was selected over square-root or log transformation since it produced skewness values closest to zero (skewness after transformation=0–0.24).
Statistical models
Mixed linear models (MLMs) were used to examine testosterone–disordered eating associations. The use of MLMs allowed us to control for the non-independence of twin data by nesting a level-1 variable (individual twin) within a level-2 unit (twin pair). Since MLMs provide unstandardized estimates of predictor effects, continuous variables were standardized prior to analysis to allow for the interpretation of unstandardized coefficients as standardized coefficients and to ease interpretation of effect sizes.
Model 1: Main Effect Models
The initial main effect model examined the main effect of testosterone on disordered eating symptoms, adjusting for pubertal status only. This model examined whether higher levels of circulating testosterone predict lower levels of disordered eating when pubertal status is controlled. After fitting this initial model, we tested a covariate model that controlled for age, adiposity, anxiety and depression, in addition to pubertal status, to ensure that any significant testosterone effects were not accounted for by these variables.
In contrast, moderation models aimed to investigate whether the protective effects of circulating testosterone on disordered eating symptoms are dependent upon advancing pubertal development.
Model 2: Moderation Models
The moderation models examined the effects of pubertal status, testosterone, and the pubertal status×testosterone interaction on disordered eating symptoms. The interaction effect between pubertal status and testosterone was of primary interest since a significant interaction would indicate that the influence of testosterone on disordered eating varies by pubertal maturation (e.g. the magnitude of the association increases with advancing pubertal development). After fitting initial models, covariate models were tested to ensure that observed associations were not due to age, adiposity, anxiety and/or depression.
Results
Descriptive statistics
A total of 1.41% boys scored above the mean clinical cut-off on the MEBS total score (score=15.55; Von Ranson et al. 2005; see Table 2), but, notably, a full range of disordered eating symptoms were endorsed on the MEBS and EDE-Q by participants falling below this cut-off (see Tables 1 and 2). Thus, the MEBS and EDE-Q scale distributions spanned a spectrum of severity and total/global scores, representative of a variety of disordered eating attitudes and behaviors. Consistent with the majority of prior research (for a review, see Klump, 2013), mean levels of disordered eating did not significantly differ between males in pre–early v. mid–late puberty (see Table 2) and pubertal status–disordered eating associations were small and non-significant (r’s=−0.03 to 0.004, p’s=0.75–0.95). Mean levels of testosterone were significantly higher for males in mid–late puberty compared with males in pre–early puberty as would be expected (Grumbach & Styne, 1998; Table 2). Importantly, disordered eating and testosterone showed adequate variability (see s.d.s in Table 2) in the full sample and within pre–early and mid–late pubertal groups, indicating that this study had ample variability to detect testosterone–disordered eating associations across varying levels of pubertal maturation. Pearson (and partial) correlations provided initial indication for puberty moderation of testosterone–disordered eating associations, as significant inverse associations were evident in mid–late, but not pre–early, puberty (see Table 2).
MLM models
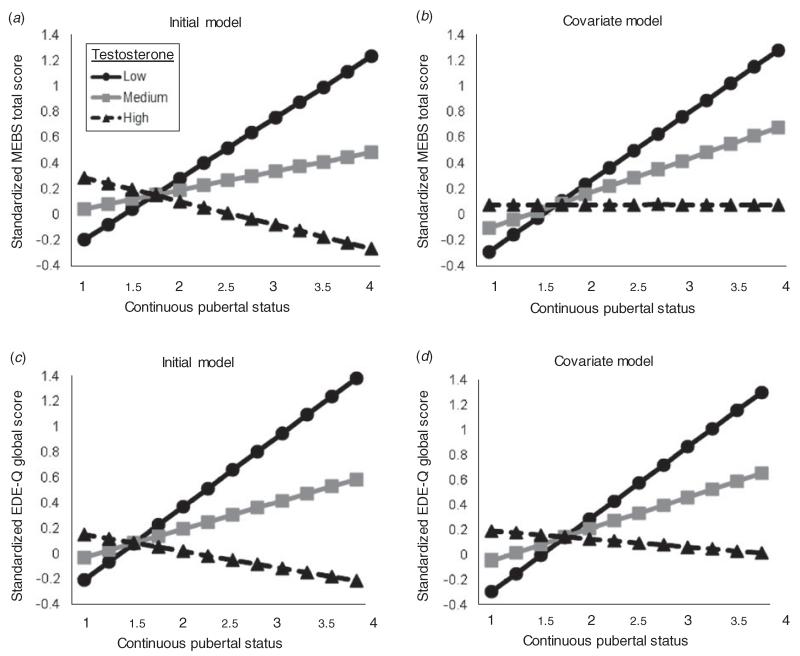
MLM main effect models indicated no significant main effect of testosterone on disordered eating symptoms, when controlling for puberty only or puberty and additional covariates (see Table 3). However, in MLM moderation models, a significant testosterone× pubertal status interaction was observed for disordered eating (see Table 4). Circulating levels of testosterone inversely predicted levels of disordered eating symptoms (i.e. boys with higher levels of testosterone showed lower levels of disordered eating and vice versa), but the magnitude of effects became prominent with advancing pubertal development (see Fig. 1). These effects remained significant even after adjusting for age, adiposity, depression and anxiety4 (see Table 4 and Fig. 1). Together, these results demonstrate that pubertal maturation plays an important predictive role in testosterone–disordered eating associations.
Table 3. MLMs examining testosterone main effects on disordered eating symptomsa.
| Initial model |
Covariate model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1: main effect of testosterone | Coefficient (s.e.) | t (df) | p | Pseudo R2 | Coefficient (s.e.) | t (df) | p | Pseudo R2 |
| Minnesota Eating Behavior Survey | ||||||||
| Total score | 0.00 | 0.35 | ||||||
| Intercept | 0.00 (0.08) | 0.01 (204) | 0.99 | −0.01 (0.08) | −0.18 (185) | 0.86 | ||
| Testosterone | −0.07 (0.08) | −0.90 (208) | 0.37 | −0.02 (0.08) | −0.19 (188) | 0.85 | ||
| Covariates | ||||||||
| Pubertal status | 0.03 (0.03) | 0.17 (207) | 0.87 | 0.05 (0.17) | 0.28 (186) | 0.78 | ||
| Anxiety | - | - | - | 0.11 (0.06) | 10.71 (187) | 0.09 | ||
| Age | - | - | - | −0.08 (00.08) | −0.97 (183) | 0.34 | ||
| BMI | - | - | - | 0.31 (00.09) | 30.64 (185) | <0.001 | ||
| Body fat percentage | - | - | - | −0.05 (00.09) | −0.63 (188) | 0.53 | ||
| Depressive symptoms | - | - | - | 0044 (00.06) | 60.85 (188) | <0.001 | ||
| Eating Disorder Examination Questionnaire | ||||||||
| Global score | 0.00 | 0.30 | ||||||
| Intercept | 0.00 (0.08) | −0.01 (119) | 0.99 | 0.02 (0.07) | 0.27 (114) | 0.79 | ||
| Testosterone | −0.16 (0.11) | −1.41 (117) | 0.16 | −0.05 (0.11) | −0.48 (109) | 0.63 | ||
| Covariates | ||||||||
| Pubertal status | 0.08 (0.11) | 0.73 (120) | 0.47 | 0.13 (0.11) | 10.18 (114) | 0.24 | ||
| Anxiety | - | - | - | 0.24 (0.09) | 20.81 (114) | 0.006 | ||
| Age | - | - | - | −0.16 (0.12) | −10.30 (110) | 0.20 | ||
| BMI | - | - | - | 0.31 (0.10) | 30.07 (114) | 0.003 | ||
| Body fat percentage | - | - | - | −0.08 (0.10) | −0.78 (109) | 0.44 | ||
| Depressive symptoms | - | - | - | 0.27 (0.09) | 30.06 (113) | 0.003 | ||
MLM, Mixed linear model; S.E., standard error; df, degrees of freedom; BMI, body mass index.
Initial models were not adjusted for any covariate. Covariate models were adjusted for pubertal status, anxiety, depression, body fat percentage, BMI and age. Variables were standardized prior to analysis to allow for the interpretation of unstandardized coefficients as standardized coefficients. Total variance explained (i.e. R2) by the MLMs was calculated using pseudo R2 (Kenny et al. 2006).
Table 4. MLMs examining testosterone and pubertal status interaction effects on disordered eating symptomsa.
| Initial model |
Covariate model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 2: pubertal status moderation effects | Coefficient (s.e.) | t (df) | p | Pseudo R2 | Coefficient (s.e.) | t (df) | p | Pseudo R2 |
| Minnesota Eating Behavior Survey | ||||||||
| Total score | 0.07 | 0.38 | ||||||
| Intercept | 0.18 (0.07) | 2.38 (206) | 0.02 | 0.13 (0.07) | 1.92 (186) | 0.06 | ||
| Testosterone | −0.07 (0.08) | −0.78 (209) | 0.44 | −0.06 (0.08) | −0.77 (186) | 0.45 | ||
| Pubertal status | 0.11 (0.09) | 1.33 (209) | 0.19 | 0.20 (0.09) | 2.28 (187) | 0.02 | ||
| Testosterone×pubertal status | −0.26 (0.06) | −4.22 (206) | <0.001 | −0.20 (0.06) | −3.32 (183) | 0.001 | ||
| Covariates | ||||||||
| Anxiety | - | - | - | 0.12 (0.06) | 1.86 (187) | 0.06 | ||
| Age | - | - | - | −0.14 (0.08) | −1.67 (182) | 0.10 | ||
| BMI | - | - | - | 0.33 (0.08) | 3.94 (185) | <0.001 | ||
| Body fat percentage | - | - | - | −0.07 (0.08) | −0.81 (187) | 0.42 | ||
| Depressive symptoms | - | - | - | 0.41 (0.06) | 6.43 (187) | <0.001 | ||
| Eating Disorder Examination Questionnaire | ||||||||
| Global score | 0.07 | 0.36 | ||||||
| Intercept | 0.17 (0.10) | 1.72 (117) | 0.09 | 0.17 (0.08) | 2.12 (112) | 0.04 | ||
| Testosterone | −0.14 (0.11) | −1.27 (118) | 0.21 | −0.04 (0.10) | −0.42 (111) | 0.68 | ||
| Pubertal status | 0.17 (0.11) | 1.52 (119) | 0.13 | 0.19 (0.11) | 1.75 (112) | 0.08 | ||
| Testosterone×pubertal status | −0.26 (0.08) | −3.29 (114) | 0.001 | −0.24 (0.07) | −3.46 (110) | 0.001 | ||
| Covariates | ||||||||
| Anxiety | - | - | - | 0.21 (0.08) | 2.53 (112) | 0.01 | ||
| Age | - | - | - | −0.15 (0.12) | −1.29 (106) | 0.20 | ||
| BMI | - | - | - | 0.34 (0.10) | 3.43 (112) | 0.001 | ||
| Body fat percentage | - | - | - | −0.09 (0.09) | −0.92 (111) | 0.36 | ||
| Depressive symptoms | - | - | - | 0.25 (0.08) | 3.01 (113) | 0.003 | ||
MLM, Mixed linear model; s.e., standard error; df, degrees of freedom; BMI, body mass index.
Initial models were not adjusted for any covariate. Covariate models were adjusted for anxiety, depression, body fat percentage, BMI and age. Variables were standardized prior to analysis to allow for the interpretation of unstandardized coefficients as standardized coefficients. Total variance explained (i.e. R2) by the MLMs was calculated using pseudo R2 (Kenny et al. 2006).
Fig. 1.
Testosterone levels predicting overall levels of disordered eating symptoms during puberty as measured by the Minnesota Eating Behavior Survey (MEBS) total score (a, b) and Eating Disorder Examination Questionnaire (EDE-Q) global score (c, d). Initial models were not adjusted for any covariate. Covariate models were adjusted for depression, anxiety, body fat percentage, BMI and age. Predicted values on the y-axis represent Blom-transformed standardized disordered eating scores. Blom-transformed standardized testosterone levels were plotted according to low (−1=1 s.d. below the mean; corresponding untransformed raw value about 23 pg/ml), medium (0=the mean; corresponding untransformed raw value about 41 pg/ml) and high (1=1 s.d. above the mean; corresponding untransformed raw value about 88 pg/ml). Pubertal status scores <2.5 are considered ‘pre-to-early’ puberty, whereas pubertal status scores ≥2.5 are considered ‘mid-to-late’ puberty.
Post-hoc analyses were conducted to determine whether observed effects persist across a spectrum of disordered eating severity. We examined if circulating levels of testosterone inversely predict levels of disordered eating in boys at highest risk for an eating disorder, in particular boys scoring above the MEBS clinical cut-off (score ≥15.55, n=3; testosterone range=17.0–29.07 pg/ml), or more broadly, those scoring within the top 5% of the sample (score ≥14, n=12; testosterone range=17.0–98.76 pg/ml). Inverse testosterone-disordered eating associations were detected (MEBS ≥15.55, r=−0.17; MEBS ≥14, r=−0.49). Binary logistic regression analyses (MEBS score >14=‘high risk’, n=12; MEBS score of 0=‘low risk’, n=22) also replicated effects observed in our full sample, such that low levels of testosterone predicted eating disorder risk with advancing pubertal maturation (e.g. testosterone×puberty: coefficient=−1.55, t=−2.40, p=0.02) even after adjusting for covariates (p<0.05). Nonetheless, the active engagement in eating disorder symptoms (e.g. dieting, excessive exercise, weight loss) could result in decreased testosterone production, and, consequently, drive the inverse association between testosterone and disordered eating. Additional post-hoc analyses were therefore conducted to ensure that our results were not merely accounted for by the boys at highest eating disorder risk, i.e. scoring above the MEBS clinical cut-off (n=3) or within the top 5% of the sample (n=12). Indeed, even after eliminating boys scoring above these cut-offs, results indicated a significant testosterone×pubertal status interaction for disordered eating, independent of covariates (p<0.01). Together, the findings indicated that puberty moderates testosterone–disordered eating associations across a spectrum of severity and that any potential confounding effects of active engagement in disordered eating on levels of testosterone (e.g. reduced testosterone production) are unlikely to account for the results of this study.
Discussion
This study was the first to investigate testosterone as a biological factor underlying individual differences in risk for eating pathology in boys during puberty. Circulating testosterone did not exhibit a significant main effect on disordered eating symptoms, but, instead, interacted with pubertal maturation to predict disordered eating symptoms. Levels of testosterone inversely predicted disordered eating symptoms with advancing pubertal development, where boys with higher levels of testosterone exhibited significantly lower levels of disordered eating symptoms and vice versa. Our findings indicate that patterns observed in animals for general food intake and other sex-differentiated behaviors translate to disordered eating in boys; puberty appears to be a key developmental period for the emergence of testosterone’s activational effects on disordered eating symptoms. Consequently, average to elevated levels of circulating testosterone may therefore exert protective effects and decrease risk for the onset of disordered eating symptoms in males during and after puberty, whereas lower levels of testosterone may increase risk and serve to explain why some, albeit relatively few, males may develop eating disorders.
The fact that adiposity, anxiety and depression did not account for testosterone–disordered eating associations deserves note. It is clear from past research that these variables probably contribute to disordered eating risk (e.g. Olivardia et al. 1995; Kostanski & Gullone, 1998; McCabe & Vincent, 2003). Indeed, they are well-established correlates of, and predictors for, disordered eating and eating disorders across development (Jacobi et al. 2004). However, despite their predictive effects on eating pathology, our findings suggest that these factors do not account for testosterone’s activational effects on disordered eating in boys during puberty. The emergence of inverse associations between testosterone and disordered eating with advancing puberty even after excluding males with elevated disordered eating scores (i.e. above clinical cut-offs) also provides evidence that our observed effects were not driven or accounted for by eating disorder sequelae (e.g. low testosterone due to eating pathology). Further, effects were consistent across disordered eating scales even despite the smaller sample size for EDE-Q data. Thus, the predictive effects of testosterone on disordered eating symptoms during puberty appear to be quite robust across the spectrum of eating pathology.
Our findings add to a growing literature that implicates gonadal hormones and puberty as key developmental factors in the etiology of disordered eating symptoms (Baker et al. 2012; Klump, 2013). Importantly, testosterone’s protective effects on disordered eating in boys may be due to both its organizational (i.e. permanent changes to neural structure/function that persist after hormone exposure) and activational (i.e. transient – only in the presence of the hormone) effects on the CNS and behavior. While the perinatal period has long been identified as a critical period for testosterone-dependent organization and masculinization of the CNS (Breedlove, 1994), puberty is now recognized as a second developmental window for hormone-driven neural organization/masculinization (Schulz et al. 2009). Exogenous administration of testosterone does not activate adult male-like phenotypes (e.g. sexual behavior) in male rodents before puberty and fails to activate masculinized phenotypes after puberty if rodents are not exposed to testicular hormones during puberty (Romeo et al. 2002; Schulz et al. 2004, 2009). Thus, testosterone-dependent organization of male-typical phenotypes begins early in life and is completed during puberty, when testosterone refines neural circuits (Schulz et al. 2009). Circulating testosterone subsequently acts upon this organized neural template to ‘activate’ the expression of maletypical behavior (Schulz et al. 2009), such as facilitating low disordered eating symptoms.
Our data cannot directly speak to whether testosterone’s effects on disordered eating during puberty are organizational or purely activational. However, it is clear that an activational, main effect of circulating testosterone alone is not enough to facilitate reductions in risk for disordered eating in boys, as testosterone’s effects only emerged in the presence of advancing pubertal development. Such findings point to the possibility that pubertal maturation of neural substrates, in conjunction with elevated testosterone, are necessary for low expression of disordered eating. Whether such changes in neural substrates are driven by organizational effects of testosterone and/or occur independent of testosterone (e.g. testosterone-independent cortical changes; Peper et al. 2011) remains to be elucidated, but our pattern of results is consistent with possible organizational effects since testosterone would first be expected to organize brain circuits and then facilitate reduced disordered eating risk via activation of those circuits.
Further, differences in the magnitude of male-typical behavior as a function of pubertal timing can also point to organizational effects, as sensitivity to testosterone’s organizational effects decreases with advancing adolescent age (Schulz et al. 2009). Although we controlled for possible pubertal timing effects (due to sample size constraints) by adjusting for age, exploratory post-hoc analyses indicate that testosterone’s protective effects on disordered eating are stronger for boys with relatively earlier pubertal timing. For example, the magnitude of testosterone–disordered eating associations was larger for males who reached mid-puberty earlier (≤ age 12 years, n=11; partial r mean=−0.63) than their peers (>age 12 years, n=53; partial r mean =−0.28), even after adjusting for adiposity, depression and anxiety. These findings corroborate experimental animal data (see Schulz et al. 2009) and indirectly suggest that the brain may be more sensitive to testosterone’s pubertal organizational and activational effects on disordered eating at a younger age. Future studies should directly explore whether organizational effects of testosterone during puberty are important for the etiology of disordered eating using translational animal (i.e. hormonal manipulations) and human (e.g. longitudinal examination of puberty hormones, brain development) models.
While findings from this study provide a piece of the puzzle in explaining disordered eating behavior in boys, and are thus quite novel, additional research is needed to replicate our results in larger samples and to improve upon study limitations. First, we aimed to control for the confounding effects of diurnal fluctuations in testosterone by collecting saliva in the afternoon. However, future studies may benefit from collecting samples at other time points (e.g. in the morning when levels are highest) and to capture diurnal variation, as these measurements would allow for the exploration of whether individual differences in testosterone levels and diurnal variations are predictive of disordered eating, as implicated for other phenotypes (e.g. anxiety–depression; Granger et al. 2003). Second, although salivary samples were tested in duplicate, samples were collected on a single day and were only assayed for the primary androgen, testosterone. Future research should replicate and extend our findings by exploring other potent androgens (e.g. androstenedione, dihydrotestosterone) and using hormone samples collected across multiple days to ensure the stability of effects. Third, testosterone concentrations were measured via saliva only. Despite high correlations between salivary and serum testosterone (r’s=0.91–0.96; Granger et al. 1999; Shirtcliff et al. 2001), the use of saliva has been shown to underestimate hormone–behavior associations (Granger et al. 2004). Examining both salivary and serum samples is an important future direction to ensure effects replicate across methods and assays.
Fourth, disordered eating symptoms were assessed in a community rather than a clinical sample, and the extent to which testosterone is predictive of clinical eating disorder risk is therefore unknown. However, it should be noted that examining our hypotheses in a clinical sample would be costly due to the low prevalence of eating disorders in males and a floor effect in testosterone levels in males with eating disorders, particularly those ill with anorexia nervosa (Beumont et al. 1972; Crisp et al. 1982; Lemaire et al. 1983). Nonetheless, now that we have shown this effect in a population sample and demonstrated that our effects are present across a spectrum of severity, the substantial extra cost of assembling a clinical sample of boys may be justified. In the meantime, because a full range of disordered eating symptoms was endorsed in our sample (see Table 1), and extant data indicate strong prospective associations between symptoms assessed in this study (e.g. weight preoccupation, body dissatisfaction) and eating disorder risk (Jacobi et al. 2004), our findings likely have etiological relevance and may speak to a variety of disordered eating symptoms.
Fifth, PDS self-report ratings were used as an indicator of pubertal maturation. Although participants’ self-report PDS ratings are highly correlated with parent reports in our data (r=0.82, p<0.001) and with interviewer and physician ratings in prior studies (r’s=0.61–0.86; Petersen et al. 1984; Carskadon & Acebo, 1993; Shirtcliff et al. 2009), future research may benefit from including health professional ratings (Dorn & Biro, 2011). Finally, our study was crosssectional, and, thus, we were unable to determine if within-person changes in testosterone predict withinperson changes in disordered eating across puberty. Longitudinal studies, which could elucidate when and how testosterone alters individual differences in risk for eating pathology in males, may be warranted based on these data.
In conclusion, our findings contribute to the literature on risk for disordered eating symptoms in adolescent boys and continue to highlight puberty as a key developmental period for the emergence of hormone-disordered eating associations. Moreover, while elevated levels of circulating testosterone may be protective and reduce risk for eating disorders in males during/after puberty, low levels of circulating testosterone may be a biological mechanism that increases eating disorder risk. These data provide a foundation upon which future studies can build. Identifying how testosterone and possibly other androgens contribute to pathophysiology and disordered eating risk will be an important next step. Androgens influence CNS structure and function and regulate several neurotransmitters and neuropeptides via their genomic and non-genomic effects (Rubinow & Schmidt, 1996), and delineating the role of these biological processes for testosterone’s effects on disordered eating risk may eventually point to key neural targets for pharmacological intervention and/or augmentation (Rubinow & Schmidt, 1996; Miller et al. 2004).
Acknowledgements
The research was supported by the National Institute of Mental Health: no. 1R21-MH070542-01 (to K.L.K., C.L.S. and J.T.N.), no. F31-MH084470 (to K.M.C.), no. T32-MH070343 (to K.M.C.), and no. T32-MH082761 (to K.M.C.); the Michigan State University College of Social Science Faculty Initiatives Fund (to K.L.K.), Graduate Student Research Enhancement Award (to K.M.C.), and John Hurley Endowed Fellowship (to K.M.C.); the Academy for Eating Disorders Student Research Grant (to K.M.C.); Blue Cross Blue Shield of Michigan Dissertation Research Award (1412.SAP; to K.M.C.); American Psychological Association Dissertation Research Award (to K.M.C.); American Psychological Foundation Clarence J. Rosecrans Scholarship (to K.M.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Males from same-sex and opposite-sex twin pairs were combined in analyses to maximize sample size since, as shown in prior research (Culbert et al. 2013), they exhibited similar levels of disordered eating (p’s=0.22–0.99). There also were no significant differences in the magnitude of testosterone–disordered eating correlation coefficients between males from same-sex v. opposite-sex twin pairs or between same-sex monozygotic v. dizygotic male twins in pre–early puberty (z scores=0.10–0.87, p’s=0.38–0.92) or mid-late puberty (z scores=0.04–1.33, p’s=0.18–0.97), further suggesting that combining these twin groups would not unduly influence results.
The MEBS (previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, FL 33549 from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, Polivy, Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Notably, secondary MLM analyses were conducted using MEBS and EDE-Q subscale scores to examine whether puberty × testosterone effects hold for a range of disordered eating symptoms, and to ensure that our focus on total/global scores did not unduly affect study interpretations or conclusions. The pattern of results across all subscales was identical to those reported herein for total/global scores (p<0.05; data available upon request).
We ensured results were not unduly affected by potential multicollinearity given substantial correlations amongst some variables (e.g. age and testosterone, r=0.64; body fat percentage and BMI, r=0.72). Tolerance and variance inflation factors (VIFs) were within the acceptable range for the covariate model (tolerance=0.34–0.81, threshold ≤0.10; VIF=1.24–2.54, threshold ≥10). Thus, all covariates were retained. We also ensured that our use of a continuous pubertal status variable did not unduly influence our pattern of findings. MLM models were re-run using two-group (pre–early v. mid–late puberty) and three-group (pre–puberty v. early puberty v. mid–late puberty) pubertal status categorizations. Findings were nearly identical to continuous model effects, in that inverse testosterone–disordered eating associations were detected, but only in the mid–late puberty group (p<0.001; data available upon request).
The notes appear after the main text.
Declaration of Interest
None.
Parts of this work were presented at the International Conference on Eating Disorders, Austin, Texas, 3–5 May 2012.
References
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London, SeriesB: Biological Sciences. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Girdler SS, Bulik CM. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Review of Obstetrics and Gynecology. 2012;7:573–583. doi: 10.1586/eog.12.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Lichtenstein P, Kendler KS. Intrauterine testosterone exposure and risk for disordered eating. British Journal of Psychiatry. 2009;194:375–376. doi: 10.1192/bjp.bp.108.054692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumont PJV, Beardwood CJ, Russell GFM. The occurrence of the syndrome of anorexia nervosa in male subjects. Psychological Medicine. 1972;2:216–231. doi: 10.1017/s0033291700042513. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Sexual differentiation of the human nervous system. Annual Review of Psychology. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): an update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. Journal of Adolescent Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Carter JC, Stewart DA, Fairburn CG. Eating Disorder Examination Questionnaire: norms for young adolescent girls. Behaviour Research and Therapy. 2001;39:625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1999;276:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Crisp AH, Hsu LKG, Chen CN, Wheeler M. Reproductive hormone profiles in male anorexia nervosa before, during and after restoration of body weight to normal. A study of twelve patients. International Journal of Eating Disorders. 1982;1:3–9. [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Archives of General Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. Journal of Abnormal Psychology. 2013;122:420–432. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL. Puberty and the genetic diathesis of disordered eating attitudes and behaviors. Journal of Abnormal Psychology. 2009;118:788–796. doi: 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: a decade in review. Journal of Research on Adolescence. 2011;21:180–195. [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Ferreiro F, Seoane G, Senra C. Gender-related risk and protective factors for depressive symptoms and disordered eating in adolescence: a 4-year longitudinal study. Journal of Youth and Adolescence. 2011;41:607–622. doi: 10.1007/s10964-011-9718-7. [DOI] [PubMed] [Google Scholar]
- Granger DA, Schwartz EB, Booth A, Arentz M. Salivary testosterone determination in studies of child health and development. Hormones and Behavior. 1999;35:18–27. doi: 10.1006/hbeh.1998.1492. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Booth A, Kivlighan KT, Schwartz EB. The ‘trouble’ with salivary testosterone. Psychoneuroendocrinology. 2004;29:1229–1240. doi: 10.1016/j.psyneuen.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Development and Psychopathology. 2003;15:431–449. [PubMed] [Google Scholar]
- Grumbach MM, Styne DM. Puberty: Ontogeny, Neuroendocrinology, Physiology, and Disorders. W. B. Saunders Company; Philadelphia, PA: 1998. [Google Scholar]
- Houtkooper LB, Lohman TG, Going SB, Howell WH. Why bioelectrical impedance analysis should be used for estimating adiposity. American Journal of Clinical Nutrition. 1996;64:436S–448S. doi: 10.1093/ajcn/64.3.436S. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Stewart A. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic Data Analysis. Guilford Press; New York: 2006. [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: a review of human and animal studies. Hormones and Behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychological Medicine. 2012;42:627–637. doi: 10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins P, Thorne D, Sisk CL, Breedlove SM. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychological Medicine. 2006;12:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Kostanski M, Gullone E. Adolescent body image dissatisfaction: relationships with self-esteem, anxiety, and depression controlling for body mass. Journal of Child Psychology and Psychiatry. 1998;39:255–262. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Lavender JM, De Young KP, Anderson DA. Eating Disorder Examination Questionnaire (EDE-Q): norms for undergraduate men. Eating Behaviors. 2010;11:119–121. doi: 10.1016/j.eatbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Lemaire A, Ardaens K, Lepretre J, Racadot A, Buvat-Herbaut M, Buvat J. Gonadal hormonesin male anorexia nervosa. International Journal of Eating Disorders. 1983;2:135–144. [Google Scholar]
- Lydecker JA, Pisetsky EM, Mitchell KS, Thornton LM, Kendler KS, Reichborn-Kjennerud T, Lichtenstein P, Bulik CM, Mazzeo SE. Association between co-twin sex and eating disorders in opposite sex twin pairs: evaluations in North American, Norwegian, and Swedish samples. Journal of Psychosomatic Research. 2012;72:73–77. doi: 10.1016/j.jpsychores.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker J, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- McCabe MP, Vincent MA. The role of biodevelopmental and psychological factors in disordered eating among adolescent males and females. European Eating Disorders Review. 2003;11:315–328. [Google Scholar]
- Miller KK, Deckersbach T, Rauch SL, Fischman AJ, Grieco KA, Herzog DB, Klibanski A. Testosterone administration attenuates regional brain hypometabolism in women with anorexia nervosa. Psychiatry Research: Neuroimaging. 2004;132:197–207. doi: 10.1016/j.pscychresns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Oinonen KA, Bird JL. Age at menarche and digit ratio (2D:4D): relationships with body dissatisfaction, drive for thinness, and bulimia symptoms in women. Body Image. 2012;9:302–306. doi: 10.1016/j.bodyim.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope HG, Mangweth B, Hudson JI. Eating disorders in college men. American Journal of Psychiatry. 1995;152:1279–1285. doi: 10.1176/ajp.152.9.1279. [DOI] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, Van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neuroscience and Biobehavioral Reviews. 2002;26:381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. American Journal of Psychiatry. 1996;153:974–984. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: the organizational–activational hypothesis adapted to puberty and adolescence. Hormones and Behavior. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Ostek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and Behavior. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz E, Curran MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: associations between digit ratio and disordered eating in males. International Journal of Eating Disorders. 2010;43:543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry. 2011;68:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clinical Chemistry. 1983;29:1752–1756. [PubMed] [Google Scholar]
- Von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: a brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;4:373–392. doi: 10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]