Abstract
Objective
To examine the cross-sectional and longitudinal relations between dietary intake of isoflavones and BMD of the lumbar spine (LS) and femoral neck (FN) in Black, White, Chinese and Japanese women during the menopause transition (MT).
Methods
We tested whether tertiles of isoflavone intake were associated with baseline BMD, when all women were pre- or early perimenopausal. To analyze whether isoflavone intake was associated with longitudinal BMD, we fit piece-wise linear models to repeated measurements of baseline-normalized LS or FN BMD, as functions of time before or after the final menstrual period (FMP) date.
Results
Multiply-adjusted mean FN BMD values of premenopausal, Japanese women were monotonically, positively related to isoflavone consumption (p for trend = 0.0003). Otherwise, no statistically significant baseline associations were observed. During the time period of 1 year prior to through 5 years after the FMP, all participants lost LS and FN BMD. Loss was unrelated to isoflavone intake, with the exception of Japanese women during 1 year prior to 2 years after the FMP: higher tertiles of isoflavone intake were associated with greater annual LS BMD loss rates (p for trend = 0.01) and FN loss rates (p for trend = 0.04).
Conclusions
In Japanese women, higher isoflavone intake was associated with higher peak FN BMD but also with greater rates of LS and FN BMD loss during the MT. Results in the other racial/ethnic groups did not support a relation between dietary intake of isoflavones and either peak BMD or BMD loss during the MT.
INTRODUCTION
Phytoestrogens, heterocyclic phenols found in plant foods, consist mainly of isoflavones, lignans and coumestans. These compounds, especially the isoflavone class, gained prominence due to their associations with lower rates of hormone-dependent malignancies and have since been associated with the prevention of other chronic diseases, including osteoporosis [1–4].
Isoflavones may benefit bone because they have positive effects on the osteoblast cell line (favoring proliferation, differentiation and mineralization) and hinder osteoclast and adipocyte generation [5]. Some bone effects of isoflavones depend on estrogen receptors for mediation, while others are estrogen receptor independent. Isoflavones bind to α and β estrogen receptors and are predominantly agonists, based on transcriptional activation assays [6, 7]. However, it is challenging to predict the effects of isoflavones on estrogen pathways because compounds vary in affinity for α and β estrogen receptors, conformational effects on estrogen receptors differ, agonist or antagonist properties may depend on circulating estradiol levels or on concentrations of the phytoestrogens and isoflavones modify the metabolism and bioavailability of endogenous sex steroids in ways that may either increase or decrease endogenous hormone levels or bioavailability [3, 4, 6–10]. Some isoflavones are potent antioxidants, a characteristic that could also promote favorable bone balance [4, 11, 12].
Although isoflavones could be advantageous to bone by way of many biological pathways, results of observational and interventional studies of isoflavones using bone mineral density (BMD) outcomes remain equivocal [13]. A meta-analysis of 11 randomized controlled trials of soy isoflavone supplements in postmenopausal women concluded that a statistically significant gain in lumbar spine, but not hip, BMD resulted from supplement use [14]. However, 4 subsequent, large, randomized controlled trials of soy isoflavone supplements in postmenopausal women discerned no whole-body or regional BMD effects of these compounds [15–18]. Substantial doses of isoflavones (equal to or greater than amounts in many Asian diets) were used in most interventions, making inadequate dose an unlikely explanation for the null findings [14–17]. Other postulated reasons for null effects of interventions include: isolated supplements may not have the same effect as diets high in isoflavones (due to differences in bioavailability or representation of specific compound, for example); long durations of exposure may be needed (as in life-long dietary intake); and timing of exposure during the life course might matter. While observational studies have their own set of limitations, complementary information may be obtained from cohort studies of diet and bone outcomes.
Longitudinal cohort studies of the bone density effects of isoflavones are few and yield results that vary by sex, menopause transition stage and bone site measured. [19–23]. Two large, longitudinal studies of soy foods and fractures among postmenopausal women in China reported a lower risk of any fracture [24] or hip fracture [25] with higher intake, suggesting that long-term consumption of dietary soy isoflavones has a bone-protective effect. To our knowledge, no prior study has investigated the relation between isoflavones and bone loss during the menopause transition (MT).
This study uses data from the Study of Women's Health Across the Nation (SWAN), a study of the menopause transition in a multi-racial/ethnic sample and from the SWAN Phytoestrogen Study, a project that has greatly expanded the SWAN dietary phytonutrient data [26, 27]. In these analyses, we examine the cross-sectional relations between usual dietary consumption of isoflavones and BMD at SWAN baseline and the longitudinal association between dietary isoflavones and change in BMD. We previously demonstrated that during the 10-year interval surrounding the final menstrual period (FMP) rates of change in lumbar spine (LS) and femoral neck (FN) BMD were divisible into 3 linear phases with distinctive slopes [28]. Specifically, during the period of time between 5 years and 1 year prior to the FMP (the “pre-transmenopausal phase”), there was no measurable change in BMD. BMD loss began 1 year in advance of the FMP (the “transmenopausal phase”) and slowed, but did not cease, 2 years after it (the “postmenopausal phase”). The present longitudinal investigation asks whether women with higher dietary intakes of isoflavones, compared to those with lower intakes, have different rates of BMD loss in these 3 intervals surrounding the FMP.
METHODS
Study sample
The study sample is from the SWAN Phytoestrogen Ancillary Study, conducted within the parent SWAN cohort [26, 27]. Women were enrolled in SWAN between January 1996 and February 1997. Entry criteria were: age between 42 and 52 years, intact uterus and at least one intact ovary, not using hormone therapy, at least one menstrual period (in the 3 months before screening) and self-identification as a member of one of 5 eligible racial/ethnic groups. Boston, Chicago, Detroit, Pittsburgh, Los Angeles, Newark, and Oakland were the 7 clinical sites, each of which enrolled Whites. Boston, Chicago, Detroit, and Pittsburgh enrolled Blacks women. Los Angeles, Newark, and Oakland enrolled Japanese, Hispanic and Chinese women, respectively. The SWAN baseline cohort sample size was 3,302. SWAN collected dietary data at baseline and at annual follow-up visits 5 and 9. The SWAN Phytoestrogen sub-study excluded the Newark SWAN site because of high attrition and lack of dietary data at visit 9; 2870 women were eligible for the Phytoestrogen Study at the remaining 6 sites. At baseline, participants were excluded from the Phytoestrogen Study for the following reasons: no diet assessment (N = 17); intake of less than 4 or greater than 17 solid foods per day (N = 130); skipped more than 10 food items on the FFQ (N = 1); calculated daily energy intake of <500 kcal or >5,000 kcal (N = 24). If these dietary exclusion criteria were met at later visits, participants were censored at that time. Thus, the maximum SWAN Phytoestrogen Study sample sizes are 2721 at baseline, 1905 at follow-up visit 5, and 1677 at follow-up visit 9. The current study of phytoestrogens and bone mineral density (BMD) also required that participants have BMD data and no bone-based exclusions. The Newark (already excluded from the Phytoestrogen Study) and Chicago sites did not measure BMD; a maximum of 2413 participants were included in the SWAN bone density sub-cohort. The current analysis includes BMD data (baseline through follow-up visit 10, completed in February 2008) from Phytoestrogen Study participants who had an observed, natural FMP during that time interval and who were not taking hormone therapy or other pharmacological agents that affect bone at any time during the observation period, resulting in an analytic sample size of 853. All sites obtained IRB approvals for study protocols, and all participants gave written, informed consent.
Outcomes
Lumbar spine (LS) and femoral neck (FN) BMD (g/cm2) were measured annually using Hologic instruments (Hologic, Inc., Waltham, Massachusetts). Three sites used Hologic 4500A models throughout. Two sites upgraded from 2000 to 4500A models at follow-up visit 8; they scanned 40 women on both old and new machines to develop cross-calibration regression equations [28]. A standard quality control program, conducted with Synarc, Inc., included daily phantom measurements, 6 monthly-cross-calibration with a circulating anthropomorphic spine standard, local site review of all scans, central review of scans that met problem-flagging criteria, and central review of a 5% random sample of scans. Short-term in vivo measurement variability was 0.014 g/cm2 (1.4%) for the LS and 0.016 g/cm2 (2.2%) for the FN.
Primary exposure variables: dietary phytoestrogens
SWAN originally estimated dietary isoflavone intake based on the limited nutrient databases available through1994 [29, 30]. By adding nutrient information available through 2008, the SWAN Phytoestrogen Study greatly expanded phytoestrogen ascertainment [27]. In brief, the SWAN dietary assessment is a 3-component interviewer-administered instrument that gauges usual food consumption during the past year. It consists of: 1) a full food frequency questionnaire (FFQ); 2) an “Ethnic Foods Page”, and 3) open-ended questions. The English-language FFQ contains a 103-item core food list, based on Second National Health and Nutrition Examination Survey (NHANES II) [31]. The Chinese and Japanese versions include the same 103-item core food list plus 12 to 16 foods appropriate for each group (Ethnic Foods Pages). Finally, all participants were asked about other foods eaten at least weekly. The SWAN Phytoestrogen Study constructed a phytonutrient data base using all available phytonutrient data through 2008. The exposure used in the current analysis is the total daily intake of isoflavones (the sum of daidzein, genistein, formononetin and glycetin). Total dietary kilocalories were also computed.
Other predictors
FMP date, assessed by annual interview, was defined as the menstrual bleeding date during the visit immediately prior to the first visit when the participant was classified as postmenopausal (had 12 months of amenorrhea). The number of months before or after the FMP that the BMD was taken was computed using the month and year of the FMP and the month and year of each annual BMD assessment. Age [years], self-defined race/ethnicity [Black, White, Chinese, Japanese], hormone therapy use [yes/no, time-varying], use of bone-active medication [yes/no, time-varying]) were obtained using annual, standardized interviews. Menopause transition (MT) stages [cross-sectional analysis only, based on reported bleeding patterns] were defined as: premenopausal (regular menses, no change from individual's pattern) and early perimenopausal (cycles more irregular than they had been but no gaps in cycles of 3 or more months). Weight (kilograms, time-varying) and height (meters) were assessed annually, using calibrated scales and stadiometers. Body mass index (BMI, [weight in kilograms/(height in meters) 2]) was calculated annually. Because diet was not measured annually, total kilocalorie intake (continuous) was interpolated for visits at which diet was not measured (described below). For all covariates, values corresponding to the beginning of each segment of the piece-wise model were used in analyses (see data analysis).
Interpolation of dietary intake variables
Diet was assessed at SWAN baseline and follow up visits 5 and 9, while BMD outcomes were measured annually. To handle this difference in measurement schedules, dietary variables (isoflavones and total kilocalories) were interpolated (one-at-a-time) using random effects modeling; dietary variables were log transformed due to right-skewness and then modeled as a function of time on study [32]. Models were stratified by race/ethnicity, due to racial/ethnic differences in isoflavone consumption. Loess curves indicated linear time trends; thus, each model included a random (woman-specific) intercept and slope for time on study. The woman-specific regression coefficients are weighted averages of the coefficients from the full sample and the coefficients from each participant's data only [33]. Predictors other than time-on-study were not used in the final interpolation, because they did little to improve prediction, but missing data reduced the sample size. To assess the performance of interpolation models, we compared fitted values with observed values for visits when diet was actually assessed (baseline and follow-ups 5 and 9). Pearson correlations between fitted and observed, accounting for within-woman correlation ranged from 0.978 to 0.996, indicating excellent agreement [34]. Linear regressions of observed values on fitted values indicated no systematic bias: intercepts were close to 0 and slopes were close to 1. Finally, loess curves for observed dietary variable values in relation to time-on-study overlapped considerably with corresponding curves for fitted values. We interpolated (i.e., imputed) logged dietary variables for visits at which diet was not assessed, using the participant-specific intercept and slope coefficients and the relevant value of time on study for each study visit at which diet was not assessed. Due to the high agreement between observed and fitted values, we did not employ multiple imputation.
Data analysis
Coding of dietary exposure variables
All analyses were conducted using SAS version 9.2. Intakes of daidzein, genistein, formononetin and glycetin were summed to obtain total isoflavone exposure. Isoflavone distribution of was strongly bimodal, with almost no overlap between Chinese and Japanese intakes (higher) and White and Black intakes (lower). Therefore, we constructed Asian and non-Asian isoflavone tertiles. In longitudinal analyses, we used time-varying membership in the highest tertile of intake as the primary exposure, but maintained baseline tertile cut-points to identify the high-intake women at follow-up visits.
Cross-sectional analyses
We examined whether isoflavone intake (categorized into tertiles) was associated with baseline BMD. For comparability with the longitudinal results, only data from the women in the longitudinal analysis were included. We used race/ethnicity-stratified linear regression to adjust for age (continuous), BMI (continuous), total kilocalories (continuous), supplemental calcium use (any vs. none), supplemental vitamin D use (any vs. none), cigarette use (any current vs other), alcohol use (any current vs. other) and MT stage (early perimenopausal vs. premenopausal). We tested for an interaction between isoflavone intake and MT stage. Findings are presented as multiply adjusted, least squared mean estimated BMD values in each tertile of isoflavone consumption; we conducted a test of trend across tertiles.
Longitudinal analysis
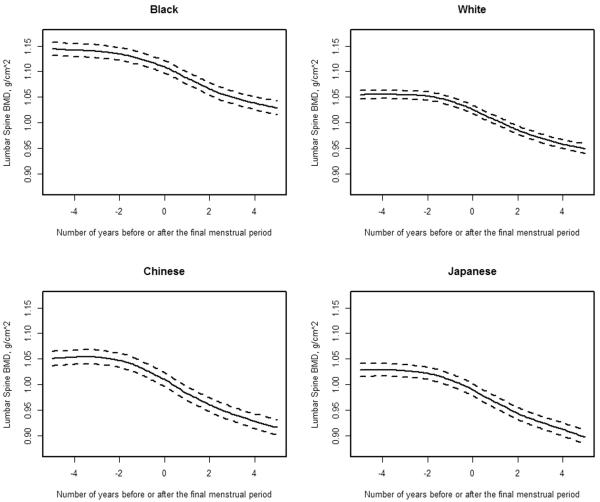
To analyze whether phytonutrient intake (categorized into tertiles) was associated with longitudinal trajectories of BMD, anchored to the date of the FMP, we used mixed effects regression. We fit piece-wise linear models to repeated measurements of baseline-normalized LS or FN BMD as functions of time before or after FMP, using linear splines with fixed knots at one year prior to and one year after the FMP. This piece-wise linear model, previously developed, identified 3 phases of bone loss in relation to the date of the FMP: pre-transmenopausal (the interval spanning from 5 years to 1 year prior to the FMP), transmenopausal (the interval between 1 year prior to and 2 years after the FMP), and postmenopausal (the interval between 2 and 5 years after the FMP) [28]. To illustrate these trajectories of bone loss relative to the FMP date, we present race-specific loess plots of baseline normalized LS BMD as a function of months prior to and after the FMP [Figure 1]. We used baseline-normalized BMD for ease of interpretation: the slope in each phase represents change in BMD per year as a percentage of baseline BMD.
Figure 1.
The longitudinal trajectory of lumbar spine (LS) bone mineral density (BMD) values relative to the amount of time prior to (negative numbers) or after (positive numbers) the final menstrual period (FMP [time zero]) in Black (N = 242), White (N = 384), Chinese (N = 117) and Japanese (N = 119) participants from the Study of Women's Health Across the Nation. These loess plots illustrate no substantive decline in LS BMD during the time span of 5 years and 1 year before the FMP, BMD loss beginning 1 year before the FMP that continued for 2 years after the FMP and a deceleration, but not termination, of BMD loss starting at 2 years after the FMP.
We modeled the slopes in each of the 3 segments of the piece-wise model as functions of the primary exposure variable (phytonutrient tertiles) estimated at the SWAN visit closest to the starting point of each segment. Models were stratified by race/ethnicity and were adjusted for age at FMP (and centered at sample mean age at FMP, 51 years). Models were also adjusted for BMI (continuous, and centered at race-specific average values), total kilocalories (continuous), supplemental calcium use (any vs. none), supplemental vitamin D use (any vs. none), cigarette use (any current vs other), alcohol use (any current vs. other); for each of these covariates, we used the values obtained at the start of each of the 3 segments of the piece-wise model. Dietary isoflavone intake and all covariates were modeled as affecting each of the 3 slopes. In addition, baseline BMD was allowed to affect the 3 slopes, to capture any independent influence of starting BMD on percentage declines in BMD during the 3 periods. We estimated the 10-year total effects of each primary exposure by linearly combining the slopes for each segment. Because the primary dietary exposure variables were dichotomized (highest tertile of phytonutrient vs. other), to test the hypothesis that higher phytonutrient intake was related to rate of bone loss in each segment, we estimated differences in slopes between the highest tertile and lower tertiles. To account for within-woman correlation between repeated observations, we included random effects for the intercept and 3 slopes (allowing the intercept and slopes to vary from woman to woman).
Interaction analyses
Racial/ethnic -stratified cross-sectional and longitudinal models disclosed apparent differences in the relation between isoflavones and BMD in Japanese and Chinese women. One reason for these differences could have been higher isoflavone intakes in Japanese women compared to the Chinese women. To explore specifically the role of racial/ethnic differences in isoflavone intakes, we created a combined sample that included both Japanese and Chinese women with comparable isoflavone intakes (based on baseline data, ranging between 963 μg and 47099 μg per day). This combined sample included 186 of the original 236 Asian women. By eliminating the extremes of exposure, this restriction eliminated the collinearity between race and isoflavones. We then formally tested for an interaction between race and isoflavone exposure in the combined sample, in both cross-sectional and longitudinal models, by including a race*MT stage*isoflavone term in the cross-sectional models and interactions of race with isoflavone for the intercept and for all 3 slopes in the longitudinal models.
RESULTS
The study sample consisted of 242 Black, 384 White, 117 Chinese and 119 Japanese participants. At baseline, average age of all participants was 46.2 years, average body mass index was 27.5 kg/m2, and 58% of women were premenopausal. The remainder was early perimenopausal by design [Table 1]. Mean values of BMI and BMD and BMI were dissimilar among the 4 racial/ethnic groups.
Table 1.
Characteristics of Study of Women's Health Across the Nation (SWAN) Phytoestrogen Study participants in the analytic sample a
| Participant Characteristics | Values in Entire Sample and in Each Racial/Ethnic Group b | ||||
|---|---|---|---|---|---|
|
| |||||
| All Participants (N=862) | Black (N=242) | White (N=384) | Chinese (N=117) | Japanese (N=119) | |
| Categorical Variables: | |||||
| Menopause transition stage | |||||
| Premenopausal | 58.4 | 55.7 | 55.1 | 68.4 | 64.9 |
| Early perimenopausal | 41.6 | 44.3 | 44.9 | 31.6 | 35.1 |
| Supplemental calcium use (% yes) | 43.8 | 35.2 | 47.4 | 33.6 | 60.3 |
| Current smoking | 16.6 | 27.7 | 16.4 | 1.7 | 9.5 |
| Continuous Variables: | |||||
| Age, years | 46.2 (2.6) | 46.1 (2.5) | 46.0 (2.6) | 46.4 (2.5) | 46.7 (2.4) |
| Age at final menstrual period, years | 51.5 (2.4) | 51.3 (2.4) | 51.5 (2.6) | 51.6 (2.3) | 52.13 (2.2) |
| Body mass index (kg/m2) | 27.5 (7.0) | 31.0 (7.3) | 28.00 (6.9) | 23.1 (4.1) | 22.7 (3.1) |
| Total dietary kilocalories | 1821.8 (628.3) | 1878.8 (730.6) | 1816.8 (572.8) | 1781.4 (648.5) | 1765.4 (560.3) |
| Bone mineral density, lumbar spine (g/cm2) | 1.078 (0.139) | 1.140 (0.146) | 1.064 (0.130) | 1.041 (0.127) | 1.019 (0.115) |
| Bone mineral density, femoral neck (g/cm2) | 0.846 (0.135) | 0.942 (0.138) | 0.827 (0.115) | 0.773 (0.101) | 0.760 (0.096) |
Except for age at final menstrual period, values in table are from SWAN cohort baseline.
For categorical variables, values shown are percents; for continuous variable, values shown are means and standard deviations.
Estimated dietary isoflavone consumption at baseline was bimodal: the lowest tertiles among Chinese or Japanese women barely overlapped with the highest of the Black or White women [Table 2]. Estimated isoflavone consumption of Asian participants was about 25 times greater than that of non-Asians. Therefore, to conduct relational analyses, isoflavone tertiles for Asian (aggregated Chinese and Japanese) and non-Asian (aggregated Black and White) groups were used.
Table 2.
Dietary intakes of isoflavones at cohort baseline: tertile medians and cut-points in ethnic/racial groups ab
| Racial/Ethnic Group | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|
| Median (Upper and Lower Bounds) | Median (Upper and Lower Bounds) | Median (Upper and Lower Bounds) | |
| Black | 78 (3 – 134) | 222 (136 – 397) | 850 (413 – 135102) |
| White | 98 (11 – 181) | 329 (182 – 654) | 1575 (668 – 72566) |
| Chinese | 1553 (27 – 3889) | 6691 (3958 – 10007) | 20518 (10129 – 118252) |
| Japanese | 3987 (288 – 8184) | 15899 (8705 – 24393) | 36358 (25884 – 89106) |
| Aggregated, Asian groups | 1751 (27 – 5633) | 8851 (5723 – 15932) | 29113 (16147 – 118252) |
| Aggregated, non-Asian groups | 88 (3 – 161) | 286 (161 – 584) | 1230 (584 – 135102) |
Values shown are in milligrams; based on the Study of Women's Health Across the Nation baseline visit.
Baseline tertile cut points were maintained throughout longitudinal analyses. Aggregated Asian and non-Asian tertiles were used for relational analyses, because the distribution of isoflavones was extremely bimodal (see Methods).
For comparability to the longitudinal results, cross-sectional analyses of between baseline levels of isoflavone intake and baseline values of LS and FN BMD were restricted to the 853 participants in the longitudinal sample [Table 3]. In multiply adjusted models, in premenopausal Japanese women only, higher tertiles of isoflavone consumption were related to higher mean FN BMD values; there was a gradient of about 17% in mean BMD ranging from the lowest higher highest tertile of isoflavone intake (p for trend = 0.0003). Among women in the remaining 3 racial/ethnic groups, no statistically significant associations were observed between isoflavone intake and BMD.
Table 3.
Multiply-adjusted, baseline, mean bone mineral density (BMD, in g/cm2), by fertile of dietary isoflavone intake at the lumbar spine and femoral neck, stratified by racial/ethnic group.abcde
| Menopause Stage, Race/Ethnicity Isoflavone Tertiles | Least Square Mean | Least Square Mean |
|---|---|---|
| Lumbar Spine BMD | Femoral Neck BMD | |
|
| ||
| Premenopausal, Black | ||
| High | 1.17 (1.12, 1.22) | 0.93 (0.888, 0.97) |
| Medium | 1.13 (1.09, 1.18) | 0.94 (0.900, 0.97) |
| Low | 1.13 (1.08, 1.18) | 0.96 (0.925, 1.00) |
| P for trend | 0.25 | 0.25 |
|
| ||
| Early Perimenopausal, Black | ||
| High | 1.14 (1.09, 1.20) | 0.90 (0.86, 0.94) |
| Medium | 1.09 (1.04, 1.15) | 0.90 (0.86, 0.94) |
| Low | 1.12 (1.06, 1.18) | 0.96 (0.91, 1.01) |
| P for trend | 0.53 | 0.08 |
|
| ||
| Premenopausal, White | ||
| High | 1.056 (1.03, 1.083) | 0.82 (0.80, 0.84) |
| Medium | 1.039 (1.01, 1.069) | 0.81 (0.79, 0.83) |
| Low | 1.064 (1.03, 1.101) | 0.84 (0.81, 0.87) |
| P for trend | 0.88 | 0.33 |
|
| ||
| Early Perimenopausal, White | ||
| High | 1.061 (1.03, 1.090) | 0.82 (0.80, 0.85) |
| Medium | 1.040 (1.01, 1.073) | 0.80 (0.77, 0.82) |
| Low | 1.019 (0.98, 1.062) | 0.80 (0.77, 0.84) |
| P for trend | 0.10 | 0.22 |
|
| ||
| Premenopausal, Chinese | ||
| High | 1.05 (0.99, 1.10) | 0.76 (0.71,0.80) |
| Medium | 1.04 (0.99, 1.09) | 0.79 (0.75, 0.82) |
| Low | 1.06 (1.01, 1.10) | 0.77 (0.74,0.81) |
| P for trend | 0.73 | 0.62 |
|
| ||
| Early Perimenopausal, Chinese | ||
| High | 0.991 (0.90, 1.08) | 0.71 (0.65, 0.78) |
| Medium | 1.070 (0.99, 1.16) | 0.76 (0.70, 0.83) |
| Low | 1.023 (0.97, 1.08) | 0.76 (0.72,0.81) |
| P for trend | 0.72 | 0.27 |
|
| ||
| Premenopausal, Japanese | ||
| High | 1.04 (0.99, 1.07) | 0.81 (0.77, 0.84) |
| Medium | 1.03 (0.98, 1.07) | 0.75 (0.71, 0.79) |
| Low | 1.01 (0.94, 1.07) | 69 (0.64, 0.75) |
| P for trend | 0.48 | 0.0003 |
|
| ||
| Early Perimenopausal, Japanese | ||
| High | 0.98 (0.92, 1.04) | 0.75 (0.70, 0.79) |
| Medium | 1.02 (0.95, 1.09) | 0.75 (0.69, 0.80) |
| Low | 1.02 (0.95, 1.08) | 0.73 (0.68, 0.78) |
| P for trend | 0.40 | 0.61 |
Sample sizes: Black = 242 (134 premenopausal); White = 384 (207 premenopausal); Chinese = 117 (79 premenopausal); Japanese = 119 (76 premenopausal).
Isoflavone tertiles defined for Asian (Chinese and Japanese) and non-Asian (African-American and Caucasian) women.
Model is adjusted for age, body mass index, menopause transition stage (premenopausal or early perimenopausal), alcohol use (any vs. none), calcium use (any vs. none), vitamin D use (any vs. none), current smoking (any vs. none), study site and total kilocalories.
Values shown in table are adjusted least square mean estimates (95% confidence intervals) for each menopause stage among women in each tertile of intake.
Our longitudinal analysis was framed on previously published longitudinal trajectories of BMD in SWAN: there was no detectable change in LS or FN BMD until 1 year before the FMP; bone loss began 1 year before the FMP and decelerated, but did not stop, 2 years after it; trajectories of bone change were linear within each of 3 time intervals in relation to the FMP date [28]. The first linear segment spanned between 5 and 1 year prior to the FMP (pre-transmenopause). The second segment included the time interval between 1 year prior to and 2 years after the FMP (transmenopause). The final linear segment covered 2 years to 5 years after FMP (postmenopause). Loess plots illustrated that trajectories of LS BMD relative to the FMP date were similar in each ethnic/racial group [Figure 1]. FN BMD exhibited the same trajectory pattern as does the LS (data not shown). Therefore, for the current longitudinal analyses of isoflavone intake and BMD change, we again used piece-wise regressions, dividing LS and FN BMD trajectories into 3 linear segments in relation to FMP date. We tested the effects of tertiles of isoflavone intake on the slopes (changes in BMD) during each of the 3 time segments.
Table 4 summarizes the results of piece-wise linear models that quantified the changes in LS and FN BMD (slopes during each interval), during the pre-transmenopausal, transmenopausal and postmenopausal intervals. During the transmenopausal and postmenopausal periods, women of all racial/ethnic groups lost bone at both the LS and FN, irrespective of their levels of isoflavone intake. This is evidenced by bone loss rates (slopes) in all groups that were statistically significantly less than zero. In addition, isoflavone intake was related to change in BMD during in Japanese women during one time interval, the transmenopause. In Japanese women, at the LS, during the transmenopausal phase, those with higher isoflavone intake lost more bone than did those with lower intake (annualized rates of loss of were −2.80%, −2.24% and −1.94% in women in highest to lowest isoflavone intake tertiles, p for trend = 0.01). Japanese women with higher isoflavone intake also lost more bone at the FN during the transmenopausal phase. Annualized rates of loss were −2.16%, −1.89% and −1.04% among those with the highest to lowest tertiles isoflavone intake, p for trend = 0.04. In all other cases, BMD loss rates did not differ among isoflavone intake groups.
Table 4.
Multiply-adjusted, annualized rates of lumbar spine (LS) and femoral neck (FN) BMD loss by fertile of dietary isoflavone intake in each of 3 time intervals relative to the final menstrual period (FMP) date, by ethic/racial groupabc
| Annual LS BMD slopes during each time interval relative to the FMP in high and low intake groups (95% Confidence Interval)de | 10-y LS BMD change (95% Confidence Interval) | |||
|---|---|---|---|---|
|
| ||||
| Race/Ethnicity and Isoflavone Intake Level | Pre-Transmenopause | Transmenopause | Postmenopause | |
| 5y to 1 y before FMP | 1y before to 2 y after FMP | 2y to 5y after FMP | ||
|
| ||||
| Black (N=239) | ||||
| High | −0.12% (−0.59, 0.35) | −2.09% (−2.57, −1.62) | −1.18% (−1.74, −0.63) | −10.29% (−12.90, −7.68) |
| Medium | −0.15% (−0.57, 0.27) | −2.18% (−2.65, −1.72) | −0.95% (−1.49, −0.41) | −10.00% (−12.47, −7.53) |
| Low | −0.07% (−0.55, 0.42) | −2.11% (−2.80, −1.43) | −1.52% (−2.50, −0.54) | −11.16% (−14.83, −7.49) |
| P for trend | 0.81 | 0.92 | 0.93 | |
|
| ||||
| White (N=381) | ||||
| High | 0.05% (−0.14, 0.24) | −2.65% (−2.90, −2.40) | −1.16% (−1.42, −0.90) | −11.24% (−12.48, −10.00) |
| Medium | 0.05% (−0.13, 0.23) | −2.65% (−2.91, −2.40) | −1.24% (−1.50, −0.98) | −11.49% (−12.72, −10.26) |
| Low | 0.13% (−0.10, 0.37) | −2.58% (−2.92, −2.23) | −0.91% (−1.25, −0.57) | −9.93% (−11.54, −8.33) |
| P for trend | 0.54 | 0.75 | 0.30 | |
|
| ||||
| Chinese (N=117) | ||||
| High | −0.28% (−0.52, −0.05) | −2.32% (−2.72, −1.92) | −1.36% (−1.86, −0.86) | −12.16% (−13.78, −10.55) |
| Medium | −0.18% (−0.44, 0.07) | −2.57% (−2.94, −2.20) | −1.65% (−2.09, −1.21) | −13.41% (−14.96, −11.86) |
| Low | −0.13% (−0.37, 0.11) | −2.37% (−2.79, −1.94) | −1.62% (−2.25, −0.98) | −12.47% (−14.53, −10.41) |
| P for trend | 0.17 | 0.89 | 0.27 | |
|
| ||||
| Japanese (N=118) | 0.06% (−0.32, 0.44) | −2.80% (−3.30, −2.30) | −1.04% (−1.67, −0.41) | −11.31% (−13.68, −8.93) |
| High | 0.03% (−0.36, 0.43) | −2.24% (−2.81, −1.67) | −1.23% (−2.05, −0.41) | −10.25% (−13.15, −7.35) |
| Medium | 0.12% (−0.28, 0.53) | −1.94% (−2.70, −1.18) | −1.59% (−2.95, −0.24) | −10.10% (−14.40, −5.80) |
| Low | 0.75 | 0.01 | 0.36 | |
| P for trend | ||||
|
| ||||
| Annual FN BMD slopes during each time interval relative to the FMP in high and low intake groups (95% Confidence Interval)de | 10-y FN BMD change (5 y before to 5 y after FMP) | |||
|---|---|---|---|---|
|
| ||||
| Race/Ethnicity and Dietary Isoflavone Intake Level | Pre-Transmenopause | Transmenopause | Postmenopause | |
| 5 to 1 yr before FMP | 1 yr before to 2 yr after FMP | 2 yr to 5 yr after FMP | ||
|
| ||||
| Black (N=242) | ||||
| High | 0.16% (−0.23, 0.54) | −1.72% (−2.17, −1.28) | −1.14% (−1.65, −0.64) | −7.97% (−10.15, −5.79) |
| Medium | 0.21% (−0.12, 0.55) | −1.74% (−2.27, −1.40) | −1.34% (−1.73, −0.85) | −8.67% (−10.72, −6.63) |
| Low | 0.14% (−0.25, 0.53) | −1.42% (−2.08, −0.76) | −0.56% (−1.44, 0.31) | −5.37% (−8.52, −2.23) |
| P for trend | 0.99 | 0.55 | 0.56 | |
|
| ||||
| White (N=379) | ||||
| High | −0.01% (−0.23, 0.22) | −1.80% (−2.07, −1.52) | −1.04% (−1.37, −0.72) | −8.54% (−9.97, −7.10) |
| Medium | −0.09% (−0.30, 0.13) | −1.74% (−2.02, −1.46) | −1.08% (−1.40, −0.77) | −8.82% (−10.25, −7.40) |
| Low | 0.12% (−0.17, 0.41) | −1.88% (−2.25, −1.50) | −1.11% (−1.53, −0.69) | −8.48% (−10.39, −6.59) |
| P for trend | 0.58 | 0.82 | 0.77 | |
|
| ||||
| Chinese (N=117) | ||||
| High | −0.31% (−1.01, 0.38) | −1.71% (−2.27, −1.14) | −1.01% (−1.74, −0.28) | −9.40% (−13.10, −5.69) |
| Medium | −0.27% (−1.02, 0.49) | −1.62% (−2.15, −1.10) | −1.14% (−1.79, −0.49) | −9.37% (−13.11, −5.63) |
| Low | −0.11% (−0.77, −0.55) | −1.77% (−2.37, −1.17) | −1.43% (−2.36, −0.49) | −10.02% (−14.07, −5.97) |
| P for trend | 0.57 | 0.92 | 0.51 | |
|
| ||||
| Japanese (N=118) | ||||
| High | 0.22% (−0.31, 0.74) | −2.16% (−2.83, −1.48) | −0.85% (−1.60, −0.11) | −8.17% (−11.21, −5.12) |
| Medium | 0.28% (−0.25, 0.80) | −1.89% (−2.64, −1.13) | −1.22% (−2.17, −0.28) | −8.23% (−11.78, −4.68) |
| Low | 0.53% (−0.01, 1.08) | −1.04% (−2.05, −0.03) | −1.97% (−3.55, −0.38) | −6.89% (−12.12, −1.66) |
| P for trend | 0.31 | 0.04 | 0.11 | |
Participant numbers: Black = 242; White = 384; Chinese = 117; Japanese = 119. Total number of observations in the analyses for the LS: Black = 2068; White = 3466; Chinese = 1094; Japanese = 1053. Total observations in the analyses for the FN: Black = 2080; White = 3453; Chinese = 1096; Japanese = 1048.
For relational analyses, isoflavone tertiles were defined for Asian (Chinese and Japanese) and non-Asian (African-American and Caucasian) women (see Methods).
The levels and slopes in each of the 3 phases were modeled as functions of the primary exposure (tertiles) and covariates. Models were stratified by race/ethnicity and were adjusted for age at FMP, baseline BMD, and the following time-varying covariates: BMI (continuous), total kilocalories (continuous), calcium supplement use (yes/no) vitamin D supplement use (yes/no)and cigarette use (current). Age at FMP was centered at the sample mean (51 years), and BMI was centered at race-specific means.
Values shown are multiply adjusted mean slopes during each time interval (pretransmenopause, transmenopause, postmenopause or cumulative) and 95% confidence intervals.
During the transmenopausal segment and the postmenopausal segment all slopes were statistically significantly different from zero (p<0.05), indicating that women were losing bone during those time periods.
In stratified models, we observed a positive association between baseline FN BMD and tertiles of isoflavone intake in Japanese, but not Chinese, women. In longitudinal stratified models, only Japanese women manifested higher rates of LS and FN bone loss during the transmenopause in association with high isoflavone intake. However, Japanese women also had the highest isoflavone consumption in our study. To test formally for a possible race-isoflavone interaction, we generated a subsample consisting of Chinese and Japanese women with overlapping isoflavone intakes (between 963 μg and 47099 μg daily). In this subgroup, an isoflavone dose effect could be distinguished from a race/ethnicity effect. In the combined Japanese and Chinese sample at baseline, higher isoflavone intake was associated with higher baseline BMD only in premenopausal Japanese women; no relation was found among the premenopausal Chinese women (p for interaction = 0.0067, data not shown). In the longitudinal models, the isoflavone-ethnicity interaction was also statistically significant during the transmenopausal interval at the LS (p for interaction= 0.0194, data not shown) and of borderline significance at the FN (p for interaction= 0.1344, data not shown).
DISCUSSION
Using SWAN's 3-phase model of bone loss in relation to the FMP as a starting point, we evaluated the impact of consumption (in tertiles) of dietary isoflavones on longitudinal change in BMD. The pre-transmenopausal segment spans the period from 5 years to 1 year prior to the FMP; during this time no statistically significant change in LS or FN BMD was observed. During the transmenopause (1 year prior through 2 years after the FMP) and during postmenopause (2 years through 5 years after the FMP) women from all racial/ethnic groups lost bone. To assess whether isoflavone intake affected change of BMD during each of the 3 phases, we tested the hypothesis that rates of bone loss (slopes) would differ across isoflavone tertiles. That hypothesis was supported in one case: during the transmenopause, among Japanese women, greater LS and FN BMD loss was seen in association with greater isoflavone intake. Otherwise, tertiles of isoflavone intake were not related to rates of BMD loss.
That higher isoflavone intake was associated with higher FN peak bone mass in premenopausal, but not early perimenopausal, Japanese women agreed with the results of our first cross-sectional report, which relied upon the limited phytonutrient data base available to us at the time [21]. Concordant with our null cross-sectional results in Chinese women, a cross-sectional study of southern Hong Kong Chinese women reported no association between isoflavone intake and BMD in premenopausal women [19]. The mean intake in that study's top tertile was 53 mg, about twice that of the SWAN Chinese sample.
We observed a greater rate of LS BMD loss in association with higher isoflavone intake during the transmenopause only in Japanese women. In SWAN, the trajectory of serum estrogen levels in relation to the FMP mirrors the trajectory of BMD change [35]. During the transitional interval, endogenous estrogens decline, but they have not yet reached the low levels characteristic of postmenopause. In the transitional phase, therefore, isoflavones may compete with endogenous estrogens, resulting in higher bone loss rates [21].
Comparator studies of dietary isoflavone intake and longitudinal change in BMD are rare. In one longitudinal analysis of data from Hong Kong Chinese women aged 30–40 years (roughly comparable to our pre-transmenopause phase), higher soy isoflavone intake was related to lower rates of LS, but not FN, BMD loss during about 3 years of observation [36]. Allowing for differences in ascertainment, the Hong Kong study's top isoflavone quartile's mean intake of 15 mg was reasonably comparable to our definition of high Chinese intake and the food sources were similar to those in SWAN [27]. The dissimilarity between the Hong Kong and SWAN study results may be due to inter-study differences in bone loss patterns, enabling the detection of an effect in the former but not the latter. In the Hong Kong study, the premenopausal LS bone loss rate averaged 3.5%, strikingly higher than the 0.6% per year observed for SWAN's premenopausal Chinese sample.
Both positive (higher peak BMD) and negative (greater transmenopausal loss) effects of high isoflavone intake were confined to Japanese participants. Several mechanisms may account for these unique associations in Japanese women, including racial/ethnic variations in amount of isoflavone consumed, food sources of isoflavones and/or isoflavone metabolic capacity. Intakes were vastly greater in Asian than in non-Asian women, but isoflavone consumption was even higher in Japanese than in Chinese women. Our sub-analysis of Chinese and Japanese women who had comparable isoflavone levels suggested that higher dose alone did not account for the observed Japanese-Chinese difference. Higher concentrations of isoflavones typify food items in the Japanese compared to the Chinese diet [37, 38]. Unlike the Chinese diet, the Japanese diet is rich in fermented soy food, such as tempeh, miso and soy paste. Fermentation removes the isoflavone sugar moiety, creating an aglycone, which is absorbed faster and in greater amounts than are the original glycoside (with sugar attached) form [39]. This potential explanation would need to be explored with a food-level, rather than isoflavone-level, analysis. The ability to metabolize isoflavones to active forms (e.g., equol and p-ethyl phenol), which characterizes roughly 30% of Whites and 50% of Asians, may also underlie differences in the biological effects of isoflavones [40–42]. However, we know of no data that support a higher rate of isoflavone metabolic capacity in Japanese compared to Chinese women.
Limitations of our study included the inescapable error in estimating nutrient intakes. Even our expanded phytonutrient database was not exhaustive, causing underestimation, and isoflavone concentrations in soy crops vary, leading to inaccuracies [27, 29]. Nonetheless, relative rankings of nutrient intakes are robust; importantly, differential misclassification is minimized by the SWAN diet assessment because it accommodates mixed dishes and accounts for ethnic foods [27]. We conducted multiple statistical tests of our a priori hypotheses. The isolated benefit of isoflavones on peak bone mass in the Japanese women may have been due to chance; but we observed similar Japanese-only benefits of isoflavones on peak BMD in our prior study, providing some support for the current findings [21]. Our finding of a higher rate of BMD loss in higher-isoflavone consuming Japanese women during the transmenopausal phase must also be viewed cautiously, given the many statistical comparisons. We could have used a simpler biological framework --for example, we could have examined the effect of isoflavones over the entire 10-year transition period, rather than breaking the transition into three intervals. While this simpler approach would have resulted in fewer statistical tests, it would have ignored what we know of the underlying biology: SWAN and other studies have demonstrated that bone loss and estrogen decline patterns are non-linear during the 10-year interval surrounding the FMP [28, 35, 43, 44]. The ways in which isoflavones affect BMD may differ during these very different biological phases [3, 4, 6–10]. Large differences in consumption of high-isoflavone foods in Eastern and Western diets resulted in statistical methodological challenges. Collinearity between race and intake would have occurred if we created isoflavone tertiles based on the entire sample. We constructed Asian and non-Asian tertiles and conducted analyses in models stratified by race/ethnicity, recognizing that the definition of “high” isoflavone intake in Black and White women differs greatly from that in Chinese and Japanese women.
CONCLUSION
In aggregate, in most instances, our study indicated no relation between dietary isoflavone intake and peak bone mass or rate of bone loss during the MT; thus, overall results do not support the hypothesis that usual levels of dietary isoflavone intake affect peak bone mass or bone loss during the MT. However, these analyses, and our prior published work, do suggest that higher dietary isoflavone intake has a fairly large, positive effect on peak FN bone mass in Japanese women, and we newly report that higher isoflavone intake may have a modest, detrimental effect on both LS and FN BMD loss during the transmenopause in Japanese women, leading to more bone loss in this phase [21]. These findings must be interpreted in the context of the multiple tests that were necessary to evaluate the complex biological framework we proposed. That we have found an ethnic/racial-specific effect of isoflavones suggests that there may be environmental and genetic variation in bone accretion and loss during the life-course; the cumulative influence of isoflavones on bone may be the sum of their effects during each portion of the bone trajectory. Future work will examine the prevalence isoflavone metabolic capacity in each racial/ethnic group and whether this characteristic modifies the bone effects of dietary intake.
Acknowledgements
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 – present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 – present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
source of funding: The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The SWAN Phytoestrogen Study was supported by: AG030448. Ms. Han was also supported in part by 1P30 AG028748.
Footnotes
Conflicts of interest The authors have no conflicts of interest.
REFERENCES
- 1.Adlercreutz H, Heinonen SM, Penalvo-Garcia J. Phytoestrogens, cancer and coronary heart disease. Biofactors. 2004;22(1–4):229–236. doi: 10.1002/biof.5520220146. [DOI] [PubMed] [Google Scholar]
- 2.Cornwell T, Cohick W, Raskin I. Dietary phytoestrogens and health. Phytochemistry. 2004;65(8):995–1016. doi: 10.1016/j.phytochem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Magee PJ, Rowland IR. Phytoestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91(4):513–531. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen A, Kulling SE, Schwartz H, et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. 2009;53(Suppl 2):S266–309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 5.Sharan K, Siddiqui JA, Swarnkar G, Maurya R, Chattopadhyay N. Role of phytochemicals in the prevention of menopausal bone loss: evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr Med Chem. 2009;16(9):1138–1157. doi: 10.2174/092986709787581806. [DOI] [PubMed] [Google Scholar]
- 6.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi S, Takahashi T, Sawada Y, Lida M, Matuda T, Kojima H. Comparative study on the nuclear hormone receptor activity of various phytochemicals and their metabolites by reporter gene assays using Chinese hamster ovary cells. Biol Pharm Bull. 2009;32(2):195–202. doi: 10.1248/bpb.32.195. [DOI] [PubMed] [Google Scholar]
- 8.Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8(1):89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilšáková L, Riečanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59(5):651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- 10.Turner JV, Agatonovic-Kustrin S, Glass BD. Molecular aspects of phytoestrogen selective binding at estrogen receptors. J Pharm Sci. 2007;96(8):1879–1885. doi: 10.1002/jps.20987. [DOI] [PubMed] [Google Scholar]
- 11.Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008;46(11):1550–1555. doi: 10.1515/CCLM.2008.302. [DOI] [PubMed] [Google Scholar]
- 12.Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci. 2010;1192:391–403. doi: 10.1111/j.1749-6632.2009.05230.x. [DOI] [PubMed] [Google Scholar]
- 13.North American Menopause Society The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010) Menopause. 2011;18(7):732–753. doi: 10.1097/gme.0b013e31821fc8e0. [DOI] [PubMed] [Google Scholar]
- 14.Taku K, Melby MK, Takebayashi J, et al. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: meta-analysis of randomized controlled trial. Asia Pac J Clin Nutr. 2010;19(1):33–42. [PubMed] [Google Scholar]
- 15.Wong WW, Lewis RD, Steinberg FM, et al. Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial. Am J Clin Nutr. 2009;90(5):1433–1439. doi: 10.3945/ajcn.2009.28001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alekel DL, Van Loan MD, Koehler KJ, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in post menopausal women. Am J Clin Nutr. 2010;91(1):218–230. doi: 10.3945/ajcn.2009.28306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vupadhyayula PM, Gallagher JC, Templin T, Logsdon SM, Smith LM. Effects of soy protein isolate on bone mineral density and physical performance indices in postmenopausal women – a 2-year randomized, double-blind, placebo-controlled trial. Menopause. 2009;16(2):320–328. doi: 10.1097/gme.0b013e3181844893. [DOI] [PubMed] [Google Scholar]
- 18.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011;171(15):1363–1369. doi: 10.1001/archinternmed.2011.330. [DOI] [PubMed] [Google Scholar]
- 19.Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86(11):5217–5221. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 20.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy intake related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97(1):109–115. doi: 10.1016/s0029-7844(00)01080-2. [DOI] [PubMed] [Google Scholar]
- 21.Greendale GA, Fitzgerald G, Huang MH, et al. Dietary soy isoflavones and bone mineral density: results from the Study of Women's Health Across the Nation. Am J Epidemiol. 2002;155(8):746–754. doi: 10.1093/aje/155.8.746. [DOI] [PubMed] [Google Scholar]
- 22.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Soy product intake and serum isoflavonoid and estradiol concentrations in relation to bone mineral density in post menopausal Japanese women. Osteoporos Int. 2002;13(3):200–204. doi: 10.1007/s001980200014. [DOI] [PubMed] [Google Scholar]
- 23.Guthrie JR, Ball M, Murkies A, Dennerstein L. Dietary phytoestrogen intake in mid-life Australian-born women: relationship to health variables. Climacteric. 2000;3(4):254–261. doi: 10.1080/13697130008500125. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165(16):1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 25.Koh WP, Wu AH, Wang R, et al. Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese Health Study. Am J Epidemiol. 2009;170(7):901–909. doi: 10.1093/aje/kwp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause Biology and Pathobiology. Academic Press; San Diego, CA: 2000. pp. 175–188. [Google Scholar]
- 27.Huang MH, Norris J, Han W, et al. Development of an updated phytoestrogen database for use with the SWAN Food Frequency Questionnaire: intakes and food sources in a community-based, multiethnic cohort study. Nutr Cancer. 2012;64(2):228–244. doi: 10.1080/01635581.2012.638434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multi-ethnic cohort: results from the Study of Women's Health Across the Nation (SWAN) J Bone Miner Res. 2012;27(1):111–118. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinli K, Block G. Phytoestrogen content of foods – a compendium of literature values. Nutr Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 30.Huang MH, Schocken M, Block G, et al. Variation in nutrient intakes by ethnicity: results from the Study of Women's Health Across the Nation (SWAN) Menopause. 2002;9(5):309–319. doi: 10.1097/00042192-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Block G, Norris JC, Mandel RM, DiSogra C. Sources of energy and six nutrients in diets of low-income Hispanic-American women and children: Quantitative data from the HHANES survey, 1982–1984. J Am Diet Assoc. 1995;95(2):195–208. doi: 10.1016/S0002-8223(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 32.Beydoun MA, Lhotsky A, Wang Y, et al. Association of adiposity status and changes in early to mid-adulthood with incidence of Alzheimer's disease. Am J Epidemiol. 2008;168(10):1179–1189. doi: 10.1093/aje/kwn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. John Wiley; New York: 2004. [Google Scholar]
- 34.Lipsitz SR, Leong T, Ibrahim J, Lipshultz S. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. The Statistician. 2001;50(Part 1):87–95. [Google Scholar]
- 35.Randolph JF, Jr., Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho SC, Chan SG, Yi Q, Wong E, Leung PC. Soy intake and the maintenance of peak bone mass in Hong Kong Chinese women. J Bone Miner Res. 2001;16(7):1363–1369. doi: 10.1359/jbmr.2001.16.7.1363. [DOI] [PubMed] [Google Scholar]
- 37.Horn-Ross PL, Barnes S, Lee M, Coward L, Mandel JE. Assessing phytoestrogen exposure in epidemiologic studies: development of a database. Cancer Causes Control. 2000;11(4):289–298. doi: 10.1023/a:1008995606699. [DOI] [PubMed] [Google Scholar]
- 38.Ritchie MR, Cummings JH, Morton MS, Michael Steel C, Bolton-Smith C, Riches AC. A newly constructed and validated isoflavone database for the assessment of total genistein and daidzein intake. Br J Nutr. 2006;95(1):204–213. doi: 10.1079/bjn20051603. [DOI] [PubMed] [Google Scholar]
- 39.Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts then their glucosides in humans. J Nutr. 2000;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 40.Akaza H, Miyanaga N, Takashima N, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34(2):86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 41.Song KB, Atkinson C, Frankenfeld CL, et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136(5):1347–1351. doi: 10.1093/jn/136.5.1347. [DOI] [PubMed] [Google Scholar]
- 42.Maskarinec G, Yamakwa R, Hebshi S, Franke AA. Urinary isoflavonoid excretion and soy consumption in three generations of Japnese women in Hawaii. Eur J Clin Nutr. 2007;61(2):255–261. doi: 10.1038/sj.ejcn.1602511. [DOI] [PubMed] [Google Scholar]
- 43.Recker R, Lappe J, Davies K, Heaney R. Characterization of the perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000 Oct;15(10):1965–73. doi: 10.1359/jbmr.2000.15.10.1965. [DOI] [PubMed] [Google Scholar]
- 44.Sowers MR, Zheng H, Jannausch ML, McConnell D, Nan B, Harlow S, Randolph JF., Jr Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010 May;95(5):2155–62. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]