Abstract
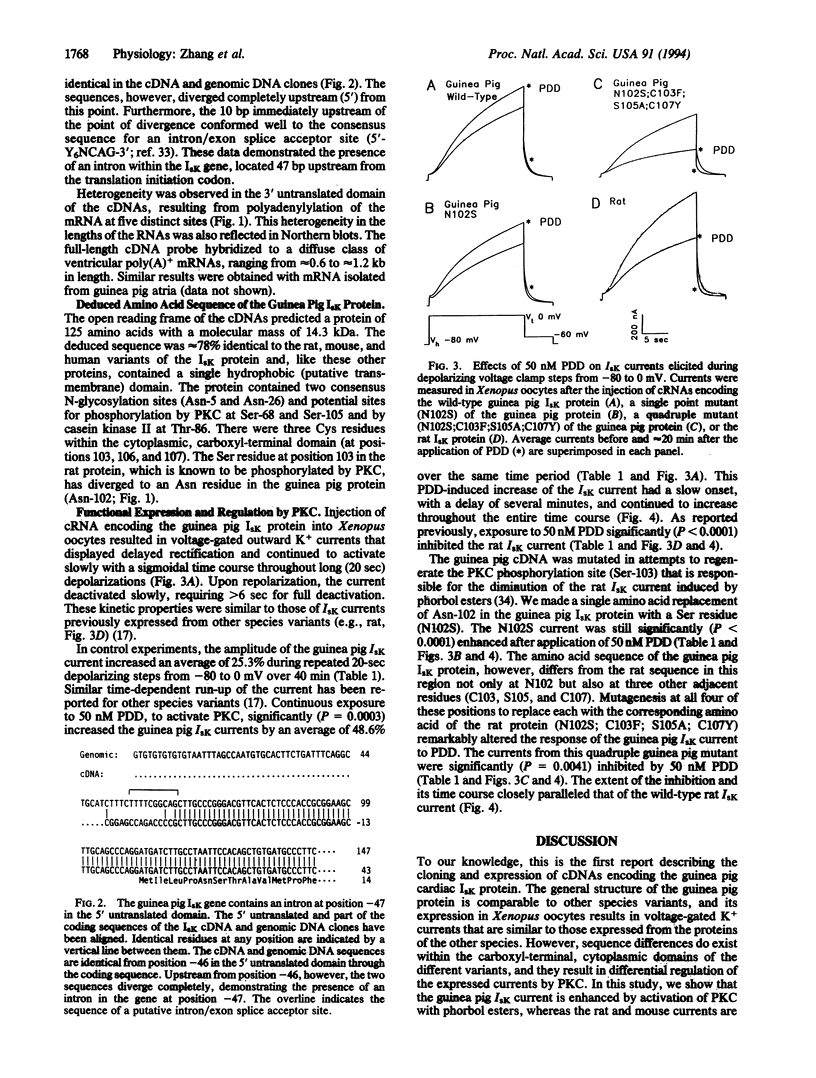
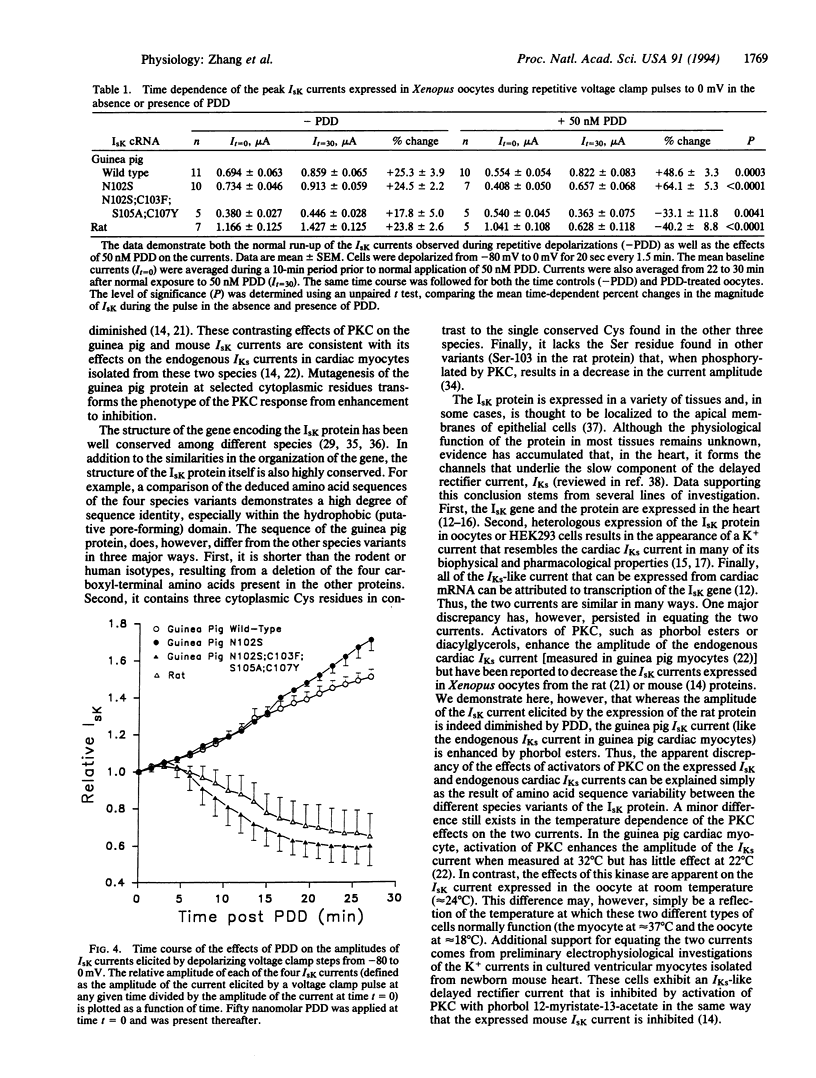
We have isolated cardiac cDNA and genomic clones encoding the guinea pig IsK protein. The deduced amino acid sequence is approximately 78% identical to the rat, mouse, and human variants of this channel, and the structure of the gene encoding the protein is also similar to that in other species. For example, the gene is present only once in the haploid genome, the protein-coding sequence is present on a single uninterrupted exon, an intron exists in the 5' untranslated domain, and multiple alternative polyadenylation sites are used in processing the transcript. Expression of the guinea pig protein in Xenopus oocytes results in a slowly activating, voltage-dependent K+ current, IsK, similar to those expressed previously from the rat, mouse, and human genes. However, in sharp contrast to the rat and mouse currents, activation of protein kinase C with phorbol esters increases the amplitude of the guinea pig IsK current, analogous to its effects on the endogenous IKs current in guinea pig cardiac myocytes. Mutagenesis of the guinea pig cDNA to alter four cytoplasmic amino acid residues alters the phenotype of the current response to protein kinase C from enhancement to inhibition, mimicking that of rat and mouse IsK currents. This mutation is consistent with reports that phosphorylation of Ser-102 by protein kinase C decreases the current amplitude. These data explain previously reported differences in the regulatory properties between recombinant rat or mouse IsK channels and native guinea pig IKs channels and provide further evidence that the IsK protein forms the channels that underlie the IKs current in the heart.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. E., Kavanaugh M. P., Varnum M. D., Adelman J. P., North R. A. Regulation by second messengers of the slowly activating, voltage-dependent potassium current expressed in Xenopus oocytes. J Physiol. 1992 May;450:491–502. doi: 10.1113/jphysiol.1992.sp019138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch A. E., Varnum M. D., North R. A., Adelman J. P. An amino acid mutation in a potassium channel that prevents inhibition by protein kinase C. Science. 1992 Mar 27;255(5052):1705–1707. doi: 10.1126/science.1553557. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christie M. J., North R. A., Osborne P. B., Douglass J., Adelman J. P. Heteropolymeric potassium channels expressed in Xenopus oocytes from cloned subunits. Neuron. 1990 Mar;4(3):405–411. doi: 10.1016/0896-6273(90)90052-h. [DOI] [PubMed] [Google Scholar]
- Covarrubias M., Wei A. A., Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991 Nov;7(5):763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Dirksen R. T., Sheu S. S. Modulation of ventricular action potential by alpha 1-adrenoceptors and protein kinase C. Am J Physiol. 1990 Mar;258(3 Pt 2):H907–H911. doi: 10.1152/ajpheart.1990.258.3.H907. [DOI] [PubMed] [Google Scholar]
- Folander K., Smith J. S., Antanavage J., Bennett C., Stein R. B., Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. C., Kass R. S. Expression of a minimal K+ channel protein in mammalian cells and immunolocalization in guinea pig heart. Circ Res. 1993 Nov;73(5):968–973. doi: 10.1161/01.res.73.5.968. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E., Attali B., Romey G., Heurteaux C., Ricard P., Lesage F., Lazdunski M., Barhanin J. Cloning, expression, pharmacology and regulation of a delayed rectifier K+ channel in mouse heart. EMBO J. 1991 Oct;10(10):2805–2811. doi: 10.1002/j.1460-2075.1991.tb07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Evidence for the formation of heteromultimeric potassium channels in Xenopus oocytes. Nature. 1990 Jun 7;345(6275):530–534. doi: 10.1038/345530a0. [DOI] [PubMed] [Google Scholar]
- Iwai M., Masu M., Tsuchida K., Mori T., Ohkubo H., Nakanishi S. Characterization of gene organization and generation of heterogeneous mRNA species of rat ISK protein. J Biochem. 1990 Aug;108(2):200–206. doi: 10.1093/oxfordjournals.jbchem.a123181. [DOI] [PubMed] [Google Scholar]
- Lesage F., Attali B., Lazdunski M., Barhanin J. ISK, a slowly activating voltage-sensitive K+ channel. Characterization of multiple cDNAs and gene organization in the mouse. FEBS Lett. 1992 Apr 20;301(2):168–172. doi: 10.1016/0014-5793(92)81240-m. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- McCormack K., Lin J. W., Iverson L. E., Rudy B. Shaker K+ channel subunits from heteromultimeric channels with novel functional properties. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1361–1371. doi: 10.1016/0006-291x(90)90836-c. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T., Kakizuka A., Takumi T., Ohkubo H., Nakanishi S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem Biophys Res Commun. 1989 May 30;161(1):176–181. doi: 10.1016/0006-291x(89)91577-5. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Propranolol-insensitive effects of epinephrine on action potential repolarization in electrically driven atria of the guinea pig. J Pharmacol Exp Ther. 1971 Apr;177(1):85–95. [PubMed] [Google Scholar]
- Po S., Roberds S., Snyders D. J., Tamkun M. M., Bennett P. B. Heteromultimeric assembly of human potassium channels. Molecular basis of a transient outward current? Circ Res. 1993 Jun;72(6):1326–1336. doi: 10.1161/01.res.72.6.1326. [DOI] [PubMed] [Google Scholar]
- Pragnell M., Snay K. J., Trimmer J. S., MacLusky N. J., Naftolin F., Kaczmarek L. K., Boyle M. B. Estrogen induction of a small, putative K+ channel mRNA in rat uterus. Neuron. 1990 May;4(5):807–812. doi: 10.1016/0896-6273(90)90207-v. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Knoth K. M., Po S., Blair T. A., Bennett P. B., Hartshorne R. P., Snyders D. J., Tamkun M. M. Molecular biology of the voltage-gated potassium channels of the cardiovascular system. J Cardiovasc Electrophysiol. 1993 Feb;4(1):68–80. doi: 10.1111/j.1540-8167.1993.tb01214.x. [DOI] [PubMed] [Google Scholar]
- Roberds S. L., Tamkun M. M. Cloning and tissue-specific expression of five voltage-gated potassium channel cDNAs expressed in rat heart. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1798–1802. doi: 10.1073/pnas.88.5.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Schröter K. H., Sakmann B., Stocker M., Sewing S., Pongs O. Heteromultimeric channels formed by rat brain potassium-channel proteins. Nature. 1990 Jun 7;345(6275):535–537. doi: 10.1038/345535a0. [DOI] [PubMed] [Google Scholar]
- Sanguinetti M. C., Jurkiewicz N. K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990 Jul;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Liao Y. J., Jan Y. N., Jan L. Y. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993 Sep 2;365(6441):72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Tanabe Y., Shigemoto R., Iwai M., Takumi T., Ohkubo H., Nakanishi S. Immunohistochemical study of a rat membrane protein which induces a selective potassium permeation: its localization in the apical membrane portion of epithelial cells. J Membr Biol. 1990 Jan;113(1):39–47. doi: 10.1007/BF01869604. [DOI] [PubMed] [Google Scholar]
- Swanson R., Folander K. In vitro synthesis of RNA for expression of ion channels in Xenopus oocytes. Methods Enzymol. 1992;207:310–319. doi: 10.1016/0076-6879(92)07020-o. [DOI] [PubMed] [Google Scholar]
- Swanson R., Marshall J., Smith J. S., Williams J. B., Boyle M. B., Folander K., Luneau C. J., Antanavage J., Oliva C., Buhrow S. A. Cloning and expression of cDNA and genomic clones encoding three delayed rectifier potassium channels in rat brain. Neuron. 1990 Jun;4(6):929–939. doi: 10.1016/0896-6273(90)90146-7. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Irisawa H. Intracellular Ca2+ and protein kinase C modulate K+ current in guinea pig heart cells. Am J Physiol. 1987 Nov;253(5 Pt 2):H1321–H1324. doi: 10.1152/ajpheart.1987.253.5.H1321. [DOI] [PubMed] [Google Scholar]
- Tohse N., Kameyama M., Sekiguchi K., Shearman M. S., Kanno M. Protein kinase C activation enhances the delayed rectifier potassium current in guinea-pig heart cells. J Mol Cell Cardiol. 1990 Jun;22(6):725–734. doi: 10.1016/0022-2828(90)91015-y. [DOI] [PubMed] [Google Scholar]
- Tohse N., Nakaya H., Kanno M. Alpha 1-adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circ Res. 1992 Dec;71(6):1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Kass R. S. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988 Oct 7;242(4875):67–69. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- Wang H., Kunkel D. D., Martin T. M., Schwartzkroin P. A., Tempel B. L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993 Sep 2;365(6441):75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang Z., Fermini B., Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993 Aug;73(2):276–285. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]



