Abstract
In children, levels of play, physical activity, and fitness are key indicators of health and disease and closely tied to optimal growth and development. Cardiopulmonary exercise testing (CPET) provides clinicians with biomarkers of disease and effectiveness of therapy, and researchers with novel insights into fundamental biological mechanisms reflecting an integrated physiological response that is hidden when the child is at rest. Yet the growth of clinical trials utilizing CPET in pediatrics remains stunted despite the current emphasis on preventative medicine and the growing recognition that therapies used in children should be clinically tested in children. There exists a translational gap between basic discovery and clinical application in this essential component of child health. To address this gap, the NIH provided funding through the Clinical and Translational Science Award (CTSA) program to convene a panel of experts. This report summarizes our major findings and outlines next steps necessary to enhance child health exercise medicine translational research. We present specific plans to bolster data interoperability, improve child health CPET reference values, stimulate formal training in exercise medicine for child health care professionals, and outline innovative approaches through which exercise medicine can become more accessible and advance therapeutics across the broad spectrum of child health.
Keywords: exercise, clinical trials, pediatrics, data harmonization, cardiopulmonary exercise testing
Introduction
Physical activity in children is not merely play, but rather an essential component of healthy growth and development beginning in fetal life and lasting across the lifespan. While the idea that “exercise is good for children” seems axiomatic, translating this vague notion into specific, biological mechanisms that could be used to actually influence health has proved to be difficult. Never before has the need for such research been so great. We find ourselves in the midst of a troubling epidemic of pediatric obesity, type 2 diabetes, and the metabolic syndrome, all, in large measure, ominous consequences of unprecedented levels of physical inactivity and sedentary lifestyles in children, coupled with overnutrition.1, 2 The parallel epidemic of childhood asthma seems equally intractable, is disproportionately affecting lower socioeconomic status children,3 and is itself linked to physical inactivity and obesity.4, 5, 6, 7 At the same time, therapeutic advances have created an increasing number of childhood survivors of a wide range of conditions, including premature birth, congenital heart disease, lung disease (such as cystic fibrosis‐CF), pediatric arthritis, hematological diseases, and cancer. In these children, fitness is impaired, and physical activity is beneficial 8, 9, 10, 11, 12 only if the “exercise dose” does not exacerbate underlying inflammatory, metabolic, or physiological abnormalities. Prescribing optimal levels of exercise, identifying new clinically useful biomarkers of disease and therapy success, and/or using exercise to enhance drug and device discovery and development must be based on a better understanding of the mechanisms that link exercise with health and disease in the growing child.
Exercise testing allows dynamic evaluation of a child's response to physical challenge, which helps characterize cardiovascular, respiratory, and metabolic responses under stress and identify abnormalities that might not otherwise be evident at rest. Despite this valuable tool, as shown in Figure 1, we have identified a gap in translating new insights into the biology of exercise into clinical research targeting child health. Over the past 15 years advances in technology and data acquisition, such as the ability to capture and store large amounts of cardiovascular, respiratory, and neurosensory data, have fueled research involving two separate but related areas of interest in child health: sleep and exercise. In both of these fields, there has been a robust increase in publications focused on biological mechanisms and pathophysiology. But as seen in Figure 1, while increasing numbers of clinical trials using sleep studies accompanied the growth in mechanistic research, a parallel increase in clinical trials utilizing cardiopulmonary exercise testing (CPET) has not been nearly as robust.
Figure 1.
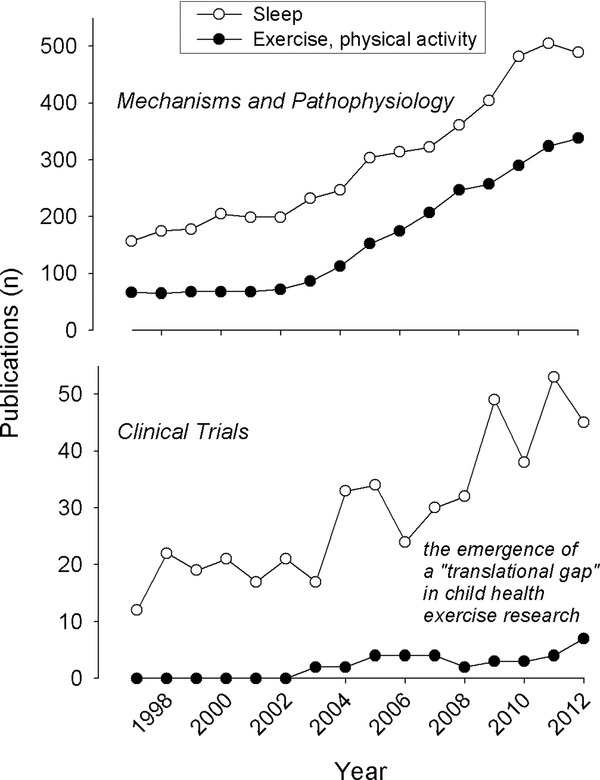
The clinical translational gap in child health exercise research. The data shown here were obtained from PubMed. As shown in the top panel, child health publications focused on biological mechanisms and pathophysiology have increased robustly for both sleep and exercise in the past 15 years. In contrast, as shown in the bottom panel, clinical trials using exercise testing in children have remained stagnant while sleep‐related clinical trials have kept pace with disease‐mechanism research.
In an effort to better understand this lack of translation and to develop a strategic action plan to address the gap, we created the Pediatric Exercise Network‐Working Group (PEN‐WG)—collaborative of physicians and scientists from across the US, Canada, and Europe. PEN‐WG was supported with funds made available through a supplemental grant to the UC Irvine Institution for Clinical and Translational Science from the Clinical Translational Science Award (CTSA) parent organization, the National Institutes of Health, in an effort to support initiatives that emanated from the CTSA‐Consortium Child Health Oversight Committee. Over an 18‐month period, PEN‐WG participated in three face‐to‐face meetings and hosted approximately 20 specific‐topic webinars and phone meetings.
Disruptive innovation is contextually defined (e.g., for industry, medicine, research) but arises from the notion that certain advances (typically, technological; sometimes, conceptual) profoundly change existing processes of “how things are done.”13 The PEN‐WG recognized that such an approach would be needed to address the child health exercise medicine translational gap that we had earlier identified. Our call for disruptive innovation in exercise medicine fits well into the transformation of child health brought about by modern pediatric research over the past century. An atmosphere of relentless questioning of the status quo and an unwillingness to accept the inevitability of morbidity and mortality of childhood illnesses have led to unimaginable progress in diseases such as polio14 and childhood leukemia,15 or conditions like premature birth.16
The PEN‐WG first highlighted a set of causes and factors that likely contributed to the translational gap. Not surprisingly, our deliberations led to a multifaceted and wide‐ranging array of probable mechanisms, shown in Table 1. We then focused on developing a series of tasks designed to address the identified causes with the ultimate goal of increasing and improving clinical trials that incorporate exercise and physical activity outcome variables. The group mapped out a set of strategies to enhance clinical research using exercise testing and physical activity assessment in children that could be, if implemented, transformative in nature. This Special Report summarizes the findings of the working group and provides recommendations for “disruptive innovation” that resulted from this concerted effort.
Table 1.
Causes and factors contributing to the clinical translational gap in child health exercise research
| Key factor | Impact on the translational gap |
|---|---|
| Semantic, syntactic, and data interoperability and harmonization | Words matter—As the electronic medical record becomes universally adopted the need for formal concept mapping of commonly used variables such as “VO2max” or “peak VO2” will be essential for clinical applications and research. Data or reports from current CPET devices and laboratories remain inconsistent in how variables are reported and scaled. Lack of standardization in data element details can lead to costly errors and misinterpretations in multicenter trials or the inability to use the data altogether. |
| The need for norms | There is substantial challenge in establishing normative CPET data in children for a number of reasons, including: (1) accounting for dynamic changes occurring during growth and development; (2) gender dimorphism; (3) ethnic, racial, socioeconomic, and geographic effects; and (4) finding useful normative data for children with chronic diseases and disabilities. |
| Training in exercise medicine and science for the next generation of child health researchers and clinicians | Despite widespread interest in the topic of exercise and physical activity among child health clinical trainees, few formal didactic programs exist that can provide clinicians with evidence‐based formative knowledge in exercise medicine. |
| Exercise testing protocols, calibration, equipment. | There are not as yet existing approaches to ensure that the results of an exercise test performed, for example, on a child at Boston Children's Hospital are truly comparable to results obtained at UC Irvine. Such approaches are necessary if clinical trials using exercise are to increase. |
| Recognition of exercise as a biomarker of health and disease, as therapy, and as a tool for drug and device discovery and development. | Beyond the vague notion that exercise benefits child health, basic science concepts of exercise physiology, assessment of physical activity, and exercise as therapy have yet to be fully incorporated into practice guidelines. This lack of clinical application exists despite the wealth of published data demonstrating the utility of CPET in assessing treatment effects across the child health spectrum. Moreover, the idea that exercise and/or exercise testing can be used to promote drug and device discovery and development in child health is only beginning to be incorporated into research design. |
Causes and Factors Contributing to the Clinical Translational Gap in Child Health Exercise Research
Semantic interoperability and data harmonization—the key to multisite success
We first focused on key concepts of medical informatics, namely, data harmonization and semantic interoperability. This particular focus on semantics may at first glance appear to be remote and arcane for the majority of clinicians, physiologists, and scientists who work in the field of pediatric exercise medicine and research. In truth, the working group soon realized that “data harmonization”—creating robust definitions of key concepts and variables that facilitate interoperability—is of critical importance in bridging the translational research gap in child health exercise medicine and in creating a baseline to which variation of concepts in use by clinicians and researchers can be compared. Combining data collected at multiple institutions is fraught with numerous challenges, most notably ensuring that data elements and measurements represent the same concepts across institutions. Interoperability is defined in the context of health care as the ability of different information technology systems, software applications, and networks to communicate and exchange data accurately, effectively, and consistently, so clinicians can use the information as they care for patients.17 Closely related is “harmonization,” the adjustment of differences and inconsistencies among different measurements, methods, procedures, schedules, specifications, or systems to make them uniform or mutually compatible.
An example of the rewards of interoperability and harmonization is the recent Global Lung Initiative, which pooled data obtained in children from various parts of the world. This effort yielded robust new prediction equations for lung function across age‐groups and revealed novel insights into the maturation of lung function during adolescence.18 Moreover, our efforts toward harmonization echo a new era of multiinstitutional synergy. Newly developed data networks will study pediatric diseases and outcomes across disparate health delivery models and care settings creating an innovative collaborative rapid improvement paradigm called the Learning Health System. 19 We highlight below a number of areas in which novel approaches to data harmonization are needed if we are to advance clinical research utilization of exercise testing in children.
The VO2max conundrum—a plan for semantic and syntactic interoperability in child health exercise medicine and research
Maximal oxygen uptake (VO2max) [3] and its related peak VO2 were considered by our working group to be the most widely used and informative outcome variables currently available from CPET (Figure 2 ). Both variables are derived from the complex integration of cellular and systemic, physical‐chemical, and biological factors that sustain muscular work. The VO2max and peak VO2 are single values that reflect heterogeneous data elements with many interrelationships, external contextual dependencies, and widely varying data collection protocols. Consequently, it is not surprising that despite its widespread acceptance, many, sometimes conflicting, definitions for what constitutes “maximal” exist in the current literature as shown in Table 2 . The PEN‐WG identified other concepts and phrases in need of semantic harmonization in child health exercise medicine and research. Included in this list are: physical fitness, habitual physical activity, fatigue, exhaustion, gas exchange kinetics, dynamic slopes of gas exchange and CPET variables, exercise prescription, anaerobic threshold, heart rate variability, and oxygen debt and deficit.
Figure 2.
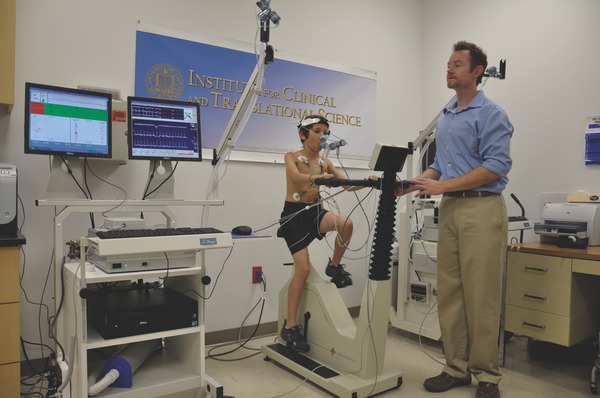
Cardiopulmonary exercise testing (CPET) in an 11‐year‐old boy. CPET remains the predominant testing approach to determine gas exchange during exercise in children and adults. Tests are typically performed in laboratory using a cycle ergometer (shown here) or treadmill. CPET provides invaluable clinical insight both during the exercise protocol (submaximal) and at the limit of the child's tolerance (VO2max or peak VO2). CPET requires sophisticated equipment, precise calibration, skilled technicians, and robust data analysis and storage capabilities, and, consequently, is not readily accessible for field or most primary care assessments of exercise responses in children.
Table 2.
The need for definitions, harmonization, and interoperability in child health exercise medicine and research. This table demonstrates through a few examples the diverse and sometimes conflicting understandings in the literature of CPET‐related phrases and concepts that are all seemingly derived from the same paradigm, namely, maximal or peak efforts. Superscripts refer to the reference source of each of the phrase or concept referred to in the Table
| Phrase/Concept from the published literature | Need for data harmonization, and syntactic and semantic operability |
|---|---|
| VO2max, oxygen uptake, plateau. “The highest oxygen uptake obtainable for a given form of ergometry despite further work rate increases and effort by the subject. This is characterized by a plateau of oxygen uptake despite further increases in work rate.”40 | This is the classic definition for VO2max but assumes that a plateau in VO2 is obtained which occurs only infrequently in children. |
| VO2peak, peak work rate peak (Wpeak) in children with cystic fibrosis (CF). “The most important parameter of aerobic exercise capacity is peak oxygen uptake (VO2peak) commonly defined as the highest oxygen uptake attained during a single progressive cardiopulmonary exercise test (CPET) […] in order to optimize the use of clinical exercise testing, the objectives of this study was to predict VO2peak [in children with CF] without gas analyses from Wpeak on a cycle ergometer.”41 | The medical community has yet to agree that VO2peak is the most important parameter of CPET in children. The authors quoted do rightfully recognize that the ability to measure gas exchange may prove challenging and expensive in clinical pediatrics. |
| VO2peak in people with spinal cord injury or abnormality. “There are no prediction equations available that can be used to estimate values for maximum oxygen uptake (VO2max) […] in adolescents with disabilities using upper extremity exercise. Therefore, peak oxygen uptake (VO2peak) […] was determined for each subject by arm crank ergometry (ACE) using a standard ramp protocol on a magnetically braked arm ergometer.”42 | The type of exercise testing performed will vary widely when attempting to gauge exercise responses in children with disease or disability. In such cases, phrases such as VO2peak must be precisely defined with regard to the protocol. |
| VO2max for hockey players. “The purpose of this study was to investigate a protocol for the determination of VO2max utilizing a motor‐driven skate treadmill (ST). The ST protocol replicates a hockey stride, which may provide more applicable information for the development of training programs.”43 | The optimal measurement of exercise responses will also be determined by the type of activity the child may be habitually engaged in. For example, assessing peak VO2 from cycle ergometry may not be useful in the context of measuring fitness for hockey players. |
| Aerobic exercise capacity. “In the present study, we compared the aerobic exercise capacity (peak VO2, anaerobic threshold, peak pulmonary ventilation (VE), peak heart rate (HR), and time to exhaustion) between normal adolescents and those with T1DM.”44 | The phrase “aerobic exercise capacity” is used frequently but no standard definition exists. In this paper, it is loosely formulated as an unspecified distribution among several variables derived from CPET. |
| Regional or national specificity. “The Canadian Aerobic Fitness Test (CAFT) (a submaximal step test) was used to estimate aerobic fitness. Because of underestimation of predicted maximal aerobic power for fit and older adults, a modified Canadian Aerobic Fitness Test (mCAFT) was subsequently developed and employed to measure aerobic fitness. Both the CAFT and the mCAFT have been validated for 15‐ to 69‐year‐olds but not for younger children.”45 | Without some broader international data harmonization, it will be difficult to achieve the transformative goals embodied in child focused global health research.46,47 |
The PEN‐WG recognized that clinicians, researchers, and informaticists must be brought together in a timely manner to define terms and create ontologies relevant to child health exercise medicine and research. Such cooperation is the only way to ensure that (1) modern informatics approaches are employed; (2) dichotomies between research‐based and clinically based terms and concepts are eliminated; and (3) the electronic medical record, when populated with CPET data, will be a useful and minable source for clinical research in children involving CPET. As noted, a primary barrier to the optimal use of clinical and research exercise testing and fitness variables is a lack of uniformity in the language of testing, data collection, and reporting, which impedes data exchange and interpretation across testing sites and over time. To overcome this barrier, we will need infrastructure to support syntactic interoperability—the means to exchange and articulate the testing data across computing platforms, operating systems, and networks.
Semantically interoperable CPET data that can be transported and stored is the foundation of a research registry. Defining the syntax of data transport and the semantics that support interoperability is the role of standards development organizations. Currently, one of the most relevant of the standards development organizations is Health Level 7 (HL7). HL7 is a not‐for‐profit, American National Standards Institute‐accredited standards developing organization dedicated to providing a comprehensive framework and related standards for the exchange, integration, sharing, and retrieval of electronic health information that supports clinical practice and the management, delivery, and evaluation of health services.
The Clinical Interoperability Council (CIC) is an HL7 working group whose mission is the development of standard data definitions that can be used by computer scientists and engineers to develop systems that are based on the clinical and research domain knowledge of health and disease specialties (e.g., broad categories such as cardiovascular disease, or specific disease categories, such as tuberculosis or schizophrenia). The CIC has a development framework that allows specialty groups such as ours to define data elements and how the elements interact with researchers, clinicians, and information systems in and beyond their own work environment. The resulting framework of detailed data elements and how they function in research and clinical environments is called a Domain Analysis Model (DAM). Developing a DAM for child health exercise medicine and research would provide definitions to facilitate and enhance multicenter trials, the creation of data registries, and lifespan research. Developing an Exercise Medicine DAM (EMDAM) can be accomplished in concurrent or sequential phases as outlined in Table 3 .
Table 3.
Developing an exercise medicine domain analysis model (DAM) with HL7 Clinical Interoperability Council (CIC)
| Phase of analysis | Objectives |
|---|---|
| Phase 0: Engage CIC | Project description and scope statement accepted and approved by CIC |
| Phase I: Data element, story board, and activity diagram development | Develop detailed data element definitions that include the context of creation and use of these data elements in the clinical or research environments as represented by activity diagrams and story boards. A story board is narrative text that explains the work flow, inputs, and outputs of exercise testing. Story boards establish a common understanding of the data elements in the environment and workflow in unambiguous, nontechnical, easily understood language. Activity diagrams are detailed analyses of the flow of work and data in the testing or clinical environment. |
| Phase II: Vetting the work with professional societies | Phase I work products brought to key stakeholders' meetings for review and approval. The purpose of this step is to establish broad‐based consensus of the foundational DAM elements before proceeding. The output of this phase will be an approved data element list definitions, activity diagrams, and story boards. |
| Phase III: Developing HL7 standards | Develop detailed class models and structured documents to support automatic data exchange and aggregation. The results of this phase would include a fully specified DAM and clinical document architecture (CDA) specifications consistent with HL7 processes. The work products will become an ANSI‐standard and provide systems designers and application developers enough detailed information to be able to develop databases, transactions and applications that are uniform and support interoperability. |
Harmonization issues—data heterogeneity
To better understand the sources of data heterogeneity in CPET, we obtained IRB approval and collected de‐identified CPET data sets from 18 participating institutions along with necessary meta‐data. Two PEN‐WG members were asked to interpret the data as received, and to then record what additional information they required to best complete the task. We collected the findings into common themes and associated these findings with possible solutions that would enable data sets to be fully interpretable at another institution. Finally, to access the impact of data acquisition on data harmonization, we reviewed breath‐by‐breath gas exchange and heart rate data generated from 1,540 cardiopulmonary exercise tests performed at a single laboratory. For these tests, a ramp protocol was used in which the work rate on a cycle ergometer increased linearly until the participant reached the limit of his or her tolerance. The recurring barriers identified by investigators as they attempted to interpret data sets provided by other institutions are shown in Table 4. Despite the diversity in data sets, formats, and exercise protocols, investigators frequently cited the same issues as listed in the table.
Table 4.
Barriers related to data and semantic harmonization–results of the data architecture survey
| Barrier | Impact on translational, multicenter research |
|---|---|
| Variable name and interpretation | Different investigators or institutions use different names for the same variable. For instance the “AT” (anaerobic threshold) is semantically identical to “VT” (ventilatory threshold) or “VAT” (ventilatory anaerobic threshold). |
| Unit ambiguity or preference | Units are different for the same variable (e.g., VO2 expressed as either mL/min or L/min). There is lack of clarity around (1) relative units such as “metabolic equivalents” (METS) and (2) unitless variables such as respiratory exchange ratio (RER). |
| Lack of consistency in the selection of outcome variables in CPET reports | There is little consistency regarding which outcome variables should be included in CPET reports. Comments included: “Baseline VO2 and VCO2 data not needed, only an indication that the system was calibrated”; “Eliminate the METs, KCal, FEO2 and FECO2 columns as they are not needed in a summary report”; and “I would have liked to see VE as well as ventilatory equivalents for O2 and CO2 (VE/VO2 and VE/VCO2) and maybe O2‐pulse on a breath‐by‐breath basis.” |
| Missing exercise and environmental information | Essential environmental information such as temperature, barometric pressure, humidity, etc., is frequently missing. Commonly absent as well is specification of CPET system, i.e., breath‐by‐breath or mixing chamber; the specific mode of exercise, i.e., treadmill or cycle, etc.; and the type of testing protocol, (e.g., progressive ramp, progressive incremental, Wingate Anaerobic test). |
| Lack of numerical standardization | For example, heart rate is reported as 70.4 or 70. There is no unanimity on whether exercise data over time should be provided as “raw” breath‐by‐breath, smoothed breath‐by‐breath, or time‐averaged. |
| Data quality | Many instances where individual data points are reported but are clearly nonphysiological (e.g., HR of 0, resting VO2 data of 12.4 mL/kg/min). Patterns of data are suspect as well (e.g., HR does not increase despite increases in cycle ergometer work rate or treadmill rate and incline). |
The need for norms
There is growing recognition among researchers and clinicians that “children are not just miniature adults” and that, consequently, renewed resources are needed and in many cases have been mobilized to support the development of drugs and devices focused specifically on children.20, 21 Clinical investigators are increasingly aware of regulatory incentives to perform clinical trials on drugs used specifically for childhood diseases, rather than relying on studies that had been done in adults. Moreover, clinical researchers understand that effective clinical trials focused on childhood disease must be accompanied by improved laboratory reference standards developed for children.
In a recent comprehensive review, Shaw et al.22 outlined the challenges facing the development of laboratory reference values in pediatrics. Although focused primarily on blood biomarkers, the following summary particularly resonated with the PEN‐WG,
“Reliable and accurate reference intervals for laboratory analyses are integral for correct interpretation of clinical laboratory test results and, therefore, for appropriate clinical decision‐making. Ideally, reference intervals should be established based on a healthy population and stratified for key covariates including age, gender, and ethnicity. However, establishing reference intervals can be challenging as it requires the collection of large numbers of samples from healthy individuals. This challenge is further augmented in pediatrics, where dynamic changes due to child growth and development markedly affect circulating levels of disease biomarkers.” The PEN‐WG recognized that much work needs to be done in CPET to ensure robust and useful reference values that can facilitate clinical investigation. As shown in Figure 3, maximal and peak VO2 reference values remain quite variable.
Figure 3.
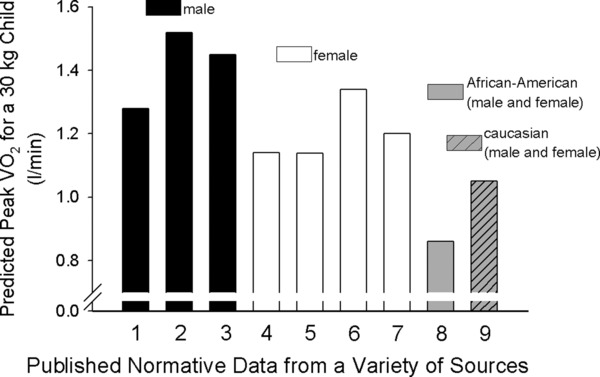
Variability in reference values for VO2 peak. We used predictive equations based on weight and calculated the expected VO2 peak for a 30 kg boy or girl. Data taken from representative selected published studies [1 – Cooper et al. (1984)49; 2–Cooper et al. (2014)31; 3–Dencker et al. (2010)50; 4–Cooper et al. (1984)49; 5–Cooper et al. (2014)31; 6–Dencker et al. (2010)50; 7–Rowland et al. (2000)51; 8 and 9–Andreacci et al. (2004)52]. We found substantial differences among the predicted values. Whether this variability arises from real sample population differences and/or from confounding technical or methodological causes is not known.
Shaw and colleagues additionally highlighted the need to establish “emerging biomarkers of cardiovascular and metabolic diseases.” They cited the fairly recent American Academy of Pediatrics guidelines of screening for cholesterol and triglyceride, and echoed the sentiment that while well‐intentioned, substantial controversy surrounds the implementation of large‐scale lipid screening in children.23 With mounting literature suggesting that fitness (assessed as peak VO2) can help predict cardiovascular disease risk in children,24 the PEN‐WG views the development of robust reference values for CPET as a potential powerful tool in the clinicians ability to (1) identify children in whom the “exercise prescription” might be most beneficial, and (2) assess the success of exercise interventions across the child health spectrum. Of equal importance is the larger public health question of the degree to which fitness and physical activity levels are decreasing in children and adolescents in the United States and throughout the world, and the extent to which these serious threats to health across the lifespan25 are related to environmental, socioeconomic, and genetic/epigenetic factors. Absent the development of robust CPET normal values, these critically important questions cannot be answered.
Training in exercise medicine and science for the next generation of child health researchers and clinicians
PEN‐WG identified training in exercise medicine as essential in addressing the translational gap in child health exercise research and practice. Formal didactics (perhaps in the form of certification) would, we believe, extend beyond the immediate objective to improve the competence of clinicians in assessing physical activity, sedentary behaviors, and exercise prescriptions, all of which are necessary to promote child health. The core body of knowledge in child health‐focused exercise medicine is deeply integrative in that it encompasses: (1) systemic physiology; (2) the molecular mechanisms of growth and development; (3) the interplay of environmental factors with genomic and epigenetic function; and (4) behavioral science. In the current age of “molecular reductionism,”26 exercise medicine resonates well with the history of pediatric research and practice which uniquely recognized the interplay of biologic, behavioral, and social determinants of child health. PEN‐WG proposes that robust training in the specific area of exercise medicine will lead to a generation of child health researchers and clinicians who can exploit the richness of translational science and ultimately impact a very broad spectrum of child health research and practice.
In an effort to understand current educational expectations related to exercise in pediatrics, we reviewed certification examination content outlines in general pediatrics, as well as in the subspecialties of pediatric cardiology, pediatric pulmonology, and sports medicine through respective websites. The American Board of Pediatrics subspecialty content outlines and the American Board of Family Practice Sports Medicine Examination content outlines each include between 20 and 40 teaching points related to understanding the interactions of exercise with health and disease in children. In pediatric cardiology, pediatric pulmonology, and sports medicine there are competency requirements related to the administration and interpretation of clinical exercise tests. Interestingly, no single exercise‐related teaching point is present in the content outlines of all of these specialties, indicating that there is little or no training in pediatric exercise medicine that is common to these disciplines.
The PEN‐WG (led by co‐author Tod Olin) developed a brief survey to assess (1) current didactic opportunities related to exercise medicine in general pediatrics and pediatric subspecialties, (2) current interest in a standardized curriculum among program directors and clinical trainees, and (3) perceived barriers to curriculum implementation among a potential group of users. We electronically distributed (www.surveyshare.com) surveys to directors of all accredited training programs in general pediatrics (n = 198), pediatric cardiology (n = 54), pediatric pulmonology (n = 49), pediatric sports medicine (n = 13), and primary care sports medicine (n = 111) in the United States. Surveys were initially distributed in April 2012. The study was approved by the National Jewish Health Institutional Review Board.
One hundred forty‐nine program directors (35%) and 178 trainees (24%) completed the survey. A majority (86%) of both program directors and trainees expressed an interest in the publication of a formal pediatric exercise curriculum. About two‐thirds of trainees, regardless of subspecialty, expressed interest in pediatric exercise medicine as a dedicated track within current subspecialty training programs, while approximately one‐fifth of trainees expressed interest in pediatric exercise medicine as a stand‐alone fellowship. As identified by responding training program directors, a small percentage (14%) of training programs currently possesses a written pediatric exercise medicine curriculum. Only about one‐third of trainees expressed comfort in their training as it pertains to the evaluation of disorders related to exercise in pediatrics.
This survey demonstrated that: (1) pediatric subspecialty trainees do not feel adequately trained to evaluate disorders or make recommendations related to exercise; (2) there are currently very few clinical general pediatric or pediatric subspecialty training programs in the United States that possess a structured curriculum to teach trainees about pediatric exercise medicine; and (3) a large majority of program directors and trainees have an interest in a structured curriculum. More than half of responding trainees expressed interest in dedicated pediatric exercise medicine training as a track within existing fellowships. Despite the current length of medical training and the financial debt that accompanies training, roughly one‐fifth of fellows expressed interest in a dedicated fellowship in pediatric exercise medicine, were such a program to be developed with proper accreditation.
Exercise testing protocols, calibration, and equipment
The PEN‐WG identified a myriad of potential challenges and barriers in the realm of testing protocols, equipment, and calibration. They are briefly reviewed and referenced in Table 5. Traditional exercise testing in the pediatric population utilizes specialized technology and equipment that measure physiologic changes occurring during an acute bout of progressive exercise. The goal is to ultimately reach and measure a state of maximal physiologic exertion for the determination of maximal aerobic capacity reflected by VO2max. VO2max requires the individual to exert an effort beyond typical daily exertion, and a major tenet of clinicians, scientists, and researchers has been that achieving this physiologic limit uncovers a broad range of physiological responses not otherwise detectable.
Table 5.
Technical and methodological issues in pediatric exercise testing that require harmonization
| Technical/Methodological concern | Challenges |
|---|---|
| Testing modality | Treadmill vs. cycle ergometer |
| Testing protocol | Ramp, incremental, submaximal, Bruce, Wingate, etc. Rate of work rate increase. |
| Pre‐ and postexercise protocols and measurements | Warm‐up, 0‐watt pedaling |
| Testing equipment | Metabolic carts with different apparati for measurement and analysis of expired gases |
| Data acquisition and processing | Breath‐by‐breath, mixing chamber, n second averaging, moving averaging |
| Determination of VO2peak | Criteria for max effort: HR, RER |
| Expression of VO2peak units | mL/min, mL/kg/min, mL/kgLBM/min, mL/kgFFM/min,% predicted, mL/htb (where b is an allometric power function such as 2/3 or 3/4) |
| Criteria for maximal effort | Perceived exertion, subjective assessment |
| Calibration procedures | Metabolic cart, treadmill, ergometer, calibration of work rate |
Differences in testing protocols, testing conditions, equipment, and calibration may fundamentally affect the accuracy of deriving VO2max, the most important CPET parameter. Maximal or peak effort during progressive exercise testing is still poorly defined, with only scant physiologic criteria and consensus reported in the exercise literature. It is known that several physiologic, environmental, anatomic, metabolic, anthropometric, and behavioral factors affect an individual's ability to achieve VO2max. Despite its proven clinical and research utility, VO2max, defined as a leveling off in VO2 despite an increase in work rate, occurs only in a minority of exercise tests even in otherwise healthy children27, 28, 29 and maybe less so in children with chronic disease or disability. Consequently, the term VO2 peak is used to reflect the highest VO2 attained. Other criteria for a maximal effort are used, such as attainment of age‐predicted maximal heart rate. However, traditional criteria may not be appropriate for children with chronic disease. For example, in a large study of children and adolescents (mean age 12.3 years old) who had undergone the Fontan correction for congenital heart disease during childhood, only 166 of 411 patients (40%) achieved an acceptable VO2max using current criteria.30 Thus, the PEN‐WG believes that additional studies are needed to identify ways to optimize the interpretation of data collected at peak (or maximal) exercise and to further explore the utility of data obtained during the submaximal portion (Figure 4) of typical CPET.31
Figure 4.
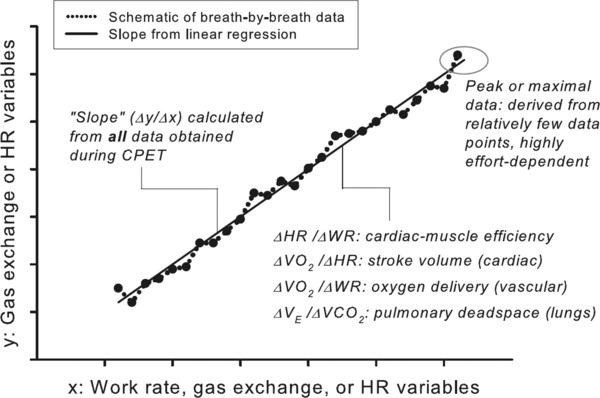
Additional uses of breath‐by‐breath data in evaluation of cardiorespiratory and metabolic disease in children. This is a schematic generalization of the determination of slopes of key CPET variables from a ramp‐type, cycle ergometry, progressive exercise protocol. Unlike the peak VO2 or VO2 max, which rely on a relatively small set of data obtained during very heavy exercise, the slopes are derived from a much larger set of data obtained throughout the exercise protocol. (See Cooper et al.31)
Recognition of exercise as a biomarker of health and disease, as therapy, and as a tool for drug and device discovery and development
In his advocacy of “disruptive innovation” in the creation of the new National Center for Advancing Translational Science (NCATS), NIH Director Francis Collins noted,32 “Through partnerships that capitalize on our respective strengths, NIH, academia, philanthropy, patient advocates, and the private sector can take full advantage of the promise of translational science to deliver solutions to the millions of people who await new and better ways to detect, treat, and prevent disease.” It was in this context that the PEN‐WG viewed our task of addressing the gap in child health research exercise medicine. Following the paradigm set by Director Collins, we outline in Table 6 novel ways in which exercise testing could be used to achieve the objectives of NCATS.
Table 6.
New ways to configure the role of exercise medicine research in reengineering child health translational science
| Key step in drug discovery | Novel use of exercise |
|---|---|
| Therapeutic target validation | Exercise profoundly perturbs homeostasis. Consequently, interactions between drug effects and key metabolic homeostatic pathways can be readily examined in controlled animal models of exercise. |
| Chemistry | From the emerging notion of muscle as a hormonally active tissue to the alterations of blood flow and redistribution of small and large molecules that accompany exercise, exercise is a model for testing emerging research from nanoparticle drug delivery to the reinvigoration of natural products chemistry. |
| Virtual drug design | As evidenced recently by Srinivasan48 computational models of exercise related key physiological processes (e.g., bone formation) lend themselves to testing in vivo and to identification, and testing, of potential therapies that interact synergistically with physical activity. |
| Preclinical toxicology | Safety is typically tested in the relatively physically inactive state. It is clear that this approach has failed to identify activity dependent adverse effects such as the potentially serious combination of receptor agonism or antagonism and exercise in patients with coronary artery disease or asthma. |
| Biomarkers | Fitness itself may prove to be a marker of efficacy in certain drugs. Moreover, new research into breath biomarker, metabolic, and immunologic responses to acute bouts of exercise suggest that a new family of activity related biomarkers of disease may be within our grasp. |
| Efficacy testing | As noted, since exercise provides a safe and controllable model of rather profound perturbation of cellular homeostasis, exercise testing at early phases of efficacy testing may hasten the process of identifying issues in efficacy and safety that have traditionally been discovered only after widespread use. |
| Phase 0 clinical Trials | The advances in metabolomics and other minimally invasive methodologies to assess cellular homeostasis can be combined with exercise testing. Consequently, exercise testing might become a tool in phase zero clinical trials of in vivo, human testing, of potentially therapeutic drugs given at very low doses. |
| Rescuing and repurposing | NIH Director Francis Collins has suggested a bold plan whereby NCATS serves as an “honest broker” for matchmaking between compounds that have been abandoned by industry before approval and new applications for which these compounds might show efficacy. Exercise testing could prove to be a screening tool for identifying metabolic, endocrinologic, and immunologic diseases in which particular drugs might be repurposed. |
| Clinical trial design | Physical activity is rarely considered in clinical trial design, but a patient's ability to resume as normal activity of daily living is often a critical endpoint of a clinical trial. Better ways to assess habitual physical activity and fitness through data harmonization, common protocols, and concept consensus could become embedded in clinical trials and improve our ability to ultimately determine the efficacy of a new therapy. |
| Postmarketing research | The evaluation of therapeutics, diagnostics, and devices does not end at the time of FDA approval. Ubiquitous computing technologies such as the ability to assess physical activity, heart rate, and/or blood pressure in the field, may prove very useful in the ongoing assessment of new drugs as they are introduced for widespread use. |
Further, PEN‐WG recognized the need for continued innovation and research into physical fitness, habitual physical activity, and exercise participation assessment tools that can expand exercise testing to a wider group of clinicians, health care professionals, and researchers. CPET is invaluable, but it is expensive and time consuming, and requires sophisticated equipment, highly trained professionals and extensive data analysis. Consequently, CPET is not generally amenable for use in the office, school, playground, and field. Rapid and ongoing advances in ubiquitous computing,33 activity monitoring,34, 35 and video technologies36 are opening new, minimally invasive ways to link laboratory measures of fitness with daily life activity. These technologies must be harnessed and integrated into standard child health assessments. Our ultimate goal is to easily measure a “physical activity health quotient” for every child and embed such metrics in the next generation of clinical trials focused on child health.
Recommendations
Based on the analysis presented earlier, we believe that the following actions will begin to address the gap.
Facilitate an Exercise Medicine DAM in partnership with organizations such as the HL7 CIC. Creating a framework for data harmonization in child health exercise science is a sina qua non to support the hoped‐for expansion of clinical trials using CPET.
- Create a network of child health focused clinical and research exercise laboratories with the ultimate goal of establishing a data consortium. The network will:
- Work to establish robust reference values in child health CPET;
- Begin to more precisely define the impact of disease and therapy on exercise responses during growth and development in children.
Engage and collaborate with existing child health and adult exercise focused groups and organizations to support lifespan research, global health initiatives, and create economies of scale in the data harmonization efforts.
Promote formal programs to train child health care professionals in essential areas of exercise physiology, physical activity assessment, and relevant concepts of fitness in children.
Utilize the network to establish common protocols for CPET to ensure, in particular, that data obtained from multicenter trials are truly comparable.
Empower the network to become a resource for review, data sharing, and innovation across the broad spectrum of physical activity research and clinical application in child health.
We contend that these six recommendations can only be accomplished with a concerted effort and strong financial as well as organizational support. The PEN‐WG firmly believes that there is no better time than now to begin this effort. For example, the Affordable Care Act places great emphasis on preventive health measures in both adults and children. A provocative “call to action” for the larger community of exercise medicine clinicians and scientists to embrace translational investigation was recently published by a group affiliated with the American College of Sports Medicine.37 Moreover, in the recent Institute of Medicine report on the Clinical Translational Science Award program,38 the reviewers noted, “For too long, research examining the safety and efficacy of medications and other health interventions has focused on adults, and little has been known about health‐ and development‐related impacts of medications, devices, and preventive measures on children. Thus, clinical and translational research is urgently needed in the area of child health.”
It is time for child health clinicians and researchers who are aware of the therapeutic, diagnostic, and fundamental research opportunities and benefits of exercise medicine to work collaboratively to garner the needed support to forge new ground in advancing the field of pediatric exercise medicine and science, and ultimately the health of our nation.
Over 20 years ago, a group of pioneering clinical research leaders presciently outlined the existing gaps in knowledge in pediatric exercise medicine. They wrote,39“Physical activity and fitness appear to have a pervasive effect on health among adults and children. Much research needs to be done to clarify and capitalize on the early promise of physical activity for the health of children. The role of genetics and mechanisms of action must be clarified in responses to activity and fitness. The prognostic role of stress testing needs to be explored. Obtaining this information will take substantial funds and a number of years, but the investment should earn substantial returns in many areas.”
The bulk of these challenges still remain, but we hope this special report will stimulate a revitalized era of discovery focused on exercise medicine and child health.
Acknowledgments
This effort was supported by NIH through the Clinical and Translational Science Award (CTSA) at the University of California, Irvine (Grant 3UL1RR031985) and a program project grant, Mechanisms of Health Effects of Exercise in Children (P01HD‐048721). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1. Ode KL, Frohnert BI, Nathan BM. Identification and treatment of metabolic complications in pediatric obesity. Rev Endocr Metab Disord 2009; 10: 167–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahn JA, Huang B, Gillman MW, Field AE, Austin SB, Colditz GA, Frazier AL. Patterns and determinants of physical activity in U.S. adolescents. J Adolesc Health 2008; 42: 369–377. [DOI] [PubMed] [Google Scholar]
- 3. Stevens GD, Pickering TA, Seid M, Tsai KY. Disparities in the national prevalence of a quality medical home for children with asthma. Acad Pediatr 2009; 9: 234–241. [DOI] [PubMed] [Google Scholar]
- 4. Vahlkvist S, Pedersen S. Fitness, daily activity and body composition in children with newly diagnosed, untreated asthma. Allergy 2009; 64: 1649–1655. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Powell A, Hoskins G, Neville R. Exploring and explaining low participation in physical activity among children and young people with asthma: a review. BMC Fam Pract 2008; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lang DM, Butz AM, Duggan AK, Serwint JR. Physical activity in urban school‐aged children with asthma. Pediatrics 2004; 113: e341–e346. [DOI] [PubMed] [Google Scholar]
- 7. Peroni DG, Pietrobelli A, Boner AL. Asthma and obesity in childhood: on the road ahead. Int J Obes (Lond) 2010; 34: 599–605. [DOI] [PubMed] [Google Scholar]
- 8. Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G, Stocks J. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax 2010; 65: 165–172. [DOI] [PubMed] [Google Scholar]
- 9. Fitzgerald DA, Sherwood M. Long‐term cardio‐respiratory consequences of heart disease in childhood. Paediatr Respir Rev 2007; 8: 313–321. [DOI] [PubMed] [Google Scholar]
- 10. Wilkes DL, Schneiderman JE, Nguyen T, Heale L, Moola F, Ratjen F, Coates AL, Wells GD. Exercise and physical activity in children with cystic fibrosis. Paediatr Respir Rev 2009; 10: 105–109. [DOI] [PubMed] [Google Scholar]
- 11. Long AR, Rouster‐Stevens KA. The role of exercise therapy in the management of juvenile idiopathic arthritis. Curr Opin Rheumatol 2010; 22: 213–217. [DOI] [PubMed] [Google Scholar]
- 12. Wolin KY, Ruiz JR, Tuchman H, Lucia A. Exercise in adult and pediatric hematological cancer survivors: an intervention review. Leukemia 2010; 24: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 13. Yu D, Hang CC. A reflective review of disruptive innovation theory. Int J Manage Rev 2010; 12: 435–452. [Google Scholar]
- 14. Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine 2011; 29( Suppl 4): D80–D85. [DOI] [PubMed] [Google Scholar]
- 15. Ribatti D. Sidney Farber and the treatment of childhood acute lymphoblastic leukemia with a chemotherapeutic agent. Pediatr Hematol Oncol 2012; 29: 299–302. [DOI] [PubMed] [Google Scholar]
- 16. Gregory GA. Continuous Positive Airway Pressure (CPAP) – historical perspectives. NeoReviews 2004; 5: e1–e5. [Google Scholar]
- 17. Heubusch K. Interoperability: what it means, why it matters. J AHIMA 2006; 77: 26–30. [PubMed] [Google Scholar]
- 18. Quanjer PH, Stanojevic S, Stocks J, Hall GL, Prasad KV, Cole TJ, Rosenthal M, Perez‐Padilla R, Hankinson JL, Falaschetti E et al. Changes in the FEV(1)/FVC ratio during childhood and adolescence: an intercontinental study. Eur Respir J 2010; 36: 1391–1399. [DOI] [PubMed] [Google Scholar]
- 19. Kahn MG, Bailey LC, Forrest CB, Padula MA, Hirschfeld S. Building a common pediatric research terminology for accelerating child health research. Pediatrics 2014; 133: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kearns GL. Beyond biomarkers: an opportunity to address the 'pharmacodynamic gap' in pediatric drug development. Biomark Med 2010; 4: 783–786. [DOI] [PubMed] [Google Scholar]
- 21. Field MJ, Ellinger LK, Boat TF. IOM Review of FDA–approved biologics labeled or studied for pediatric use. Pediatrics 2013; 131: 328–335. [DOI] [PubMed] [Google Scholar]
- 22. Shaw JL, Binesh MT, Colantonio D, Adeli K. Pediatric reference intervals: challenges and recent initiatives. Crit Rev Clin Lab Sci 2013; 50: 37–50. [DOI] [PubMed] [Google Scholar]
- 23. Gillman MW. Changing the conversation regarding pediatric cholesterol screening: the rare disease paradigm. Arch Pediatr Adolesc Med 2012; 166: 1097–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisenmann JC, Welk GJ, Ihmels M, Dollman J. Fatness, fitness, and cardiovascular disease risk factors in children and adolescents. Med Sci Sports Exerc 2007; 39: 1251–1256. [DOI] [PubMed] [Google Scholar]
- 25. Myer GD, Faigenbaum AD, Stracciolini A, Hewett TE, Micheli LJ, Best TM. Exercise deficit disorder in youth: a paradigm shift toward disease prevention and comprehensive care. Curr Sports Med Rep 2013; 12: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Joyner MJ. Giant sucking sound: can physiology fill the intellectual void left by the reductionists? J Appl Physiol (1985) 2011; 111: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowland TW, Cunningham LN. Oxygen uptake plateau during maximal treadmill exercise in children. Chest 1992; 101: 485–489. [DOI] [PubMed] [Google Scholar]
- 28. Barker AR, Williams CA, Jones AM, Armstrong N. Establishing maximal oxygen uptake in young people during a ramp cycle test to exhaustion. Br J Sports Med 2011; 45: 498–503. [DOI] [PubMed] [Google Scholar]
- 29. Robben KE, Poole DC, Harms CA. Maximal oxygen uptake validation in children with expiratory flow limitation. Pediatr Exerc Sci 2013; 25: 84–100. [DOI] [PubMed] [Google Scholar]
- 30. Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J. A cross‐sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008; 52: 99–107. [DOI] [PubMed] [Google Scholar]
- 31. Cooper DM, Leu SY, Galassetti P, Radom‐Aizik S. Dynamic interactions of gas exchange, body mass, and progressive exercise in children. Med Sci Sports Exerc 2014; 46: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins FS. Reengineering translational science: the time is right. Sci Transl Med 2011; 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baca A, Dabnichki P, Heller M, Kornfeind P. Ubiquitous computing in sports: a review and analysis. J Sports Sci 2009; 27: 1335–1346. [DOI] [PubMed] [Google Scholar]
- 34. Olesen LG, Kristensen PL, Korsholm L, Froberg K. Physical activity in children attending preschools. Pediatrics 2013; 132: e1310–e1318. [DOI] [PubMed] [Google Scholar]
- 35. Janssen I, Wong SL, Colley R, Tremblay MS. The fractionalization of physical activity throughout the week is associated with the cardiometabolic health of children and youth. BMC Public Health 2013; 13: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burkow‐Heikkinen L. Non‐invasive physiological monitoring of exercise and fitness. Neurol Res 2011; 33: 3–17. [DOI] [PubMed] [Google Scholar]
- 37. Bamman MM, Cooper DM, Booth FW, Chin ER, Neufer PD, Trappe S, Lightfoot JT, Kraus WE, Joyner MJ. Exercise biology and medicine: innovative research to improve global health. Mayo Clin Proc 2014; 89: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The CTSA Program at NIH : Opportunities for advancing clinical and translational research‐institute of medicine of the national academies report In: Leshner AI, Terry SF, Schultz AM, Liverman CT, eds. Washington, D.C: The National Academies Press; 2013: 1–166. [PubMed] [Google Scholar]
- 39. Baranowski T, Bouchard C, Bar‐Or O, Bricker T, Heath G, Kimm SY, Malina R, Obarzanek E, Pate R, Strong WB et al. Assessment, prevalence, and cardiovascular benefits of physical activity and fitness in youth. Med Sci Sports Exerc 1992; 24: S237–S247. [PubMed] [Google Scholar]
- 40. Wasserman K, Hansen JE, Sue DY, Whipp BJ, Sietsema KE, Sun XG, Stringer WW. Principles of Exercise Testing and Interpretation. 5 ed Philadelphia: Walters Kluwer, Lippincot Williams and Wilkins, 2012; 547–548. [Google Scholar]
- 41. Werkman MS, Hulzebos EH, Helders PJ, Arets BG, Takken T. Estimating peak oxygen uptake in adolescents with cystic fibrosis. Arch Dis Child 2014; 99: 21–25. [DOI] [PubMed] [Google Scholar]
- 42. Widman LM, Abresch RT, Styne DM, McDonald CM. Aerobic fitness and upper extremity strength in patients aged 11 to 21 years with spinal cord dysfunction as compared to ideal weight and overweight controls. J Spinal Cord Med 2007; 30 Suppl 1: S88–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dreger RW, Quinney HA. Development of a hockey specific, skate treadmill VO2 max protocol. Can J Appl Physiol 1999; 24: 559–569. [DOI] [PubMed] [Google Scholar]
- 44. Komatsu WR, Gabbay MA, Castro ML, Saraiva GL, Chacra AR, de Barros Neto TL, Dib SA. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes 2005; 6: 145–149. [DOI] [PubMed] [Google Scholar]
- 45. Craig CL, Shields M, Leblanc AG, Tremblay MS. Trends in aerobic fitness among Canadians, 1981 to 2007–2009. Appl Physiol Nutr Metab 2012; 37: 511–519. [DOI] [PubMed] [Google Scholar]
- 46. Kantomaa MT, Stamatakis E, Kankaanpaa A, Kaakinen M, Rodriguez A, Taanila A, Ahonen T, Järvelin MR, Tammelin T. Physical activity and obesity mediate the association between childhood motor function and adolescents' academic achievement. Proc Natl Acad Sci USA 2013; 110: 1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cappelleri JC, Hwang LJ, Mardekian J, Mychaskiw MA. Assessment of measurement properties of peak VO2 in children with pulmonary arterial hypertension. BMC Pulm Med 2012; 12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Srinivasan S, Ausk BJ, Prasad J, Threet D, Bain SD, Richardson TS, Gross TS. Rescuing loading induced bone formation at senescence. PLoS Comput Biol 2010; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cooper DM, Weiler Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol 1984; 56: 628–634. [DOI] [PubMed] [Google Scholar]
- 50. Dencker M, Bugge A, Hermansen B, Froberg K, Andersen LB. Aerobic fitness in prepubertal children according to level of body fat. Acta Paediatr 2010; 99: 1854–1860. [DOI] [PubMed] [Google Scholar]
- 51. Rowland T, Miller K, Vanderburgh P, Goff D, Martel L, Ferrone L. Cardiovascular fitness in premenarcheal girls and young women. Int J Sports Med 2000; 21: 117–121. [DOI] [PubMed] [Google Scholar]
- 52. Andreacci JL, Robertson RJ, Dube JJ, Aaron DJ, Balasekaran G, Arslanian SA. Comparison of maximal oxygen consumption between black and white prepubertal and pubertal children. Pediatr Res 2004; 56: 706–713 [DOI] [PubMed] [Google Scholar]


