Abstract
Setting
KwaZulu-Natal, South Africa a predominantly rural province with high burdens of TB, MDR-TB and HIV infection.
Objective
To determine the most effective model of care by comparing MDR-TB treatment outcomes at community-based sites with traditional care at a central, specialised hospital.
Design
A non-randomised observational prospective cohort study comparing community-based and centralised care. Patients at community-based sites were closer to home, had easier access to care and home-based care was available from treatment initiation.
Results
Four community-based sites treated 736 patients, while 813 were treated at the centralised hospital (a total of 1549 patients). Overall, 75% were HIV co-infected (community: 76% vs. hospitalised: 73%, p=0.45) and 86% received antiretroviral therapy (community: 91% vs. hospitalised: 82%, p=0.22).
In multivariate analysis MDR-TB patients were more likely to have a successful treatment outcome if they were treated at a community-based site (adjusted OR=1.43, p=0.01). However, there was heterogeneity in outcomes at the four community-based sites, with Site 1 demonstrating that home-based care was associated with increased treatment success of 72% compared with success of between 52 - 60% at the other three sites.
Conclusion
Community-based care for patients with MDR-TB was more effective than care in a central, specialised hospital. Home-based care further increased treatment success.
Keywords: Models of care, HIV, outcomes
BACKGROUND
Multidrug-resistant tuberculosis (MDR-TB), defined as TB resistant to isoniazid and rifampicin, is a critical threat to global TB control and is associated with high mortality in settings with HIV co-infection.1,2 MDR-TB treatment is more difficult for patients to tolerate than first-line TB therapy, due to the long duration of treatment (18–24 months), frequent medication toxicities, and daily administration of an injectable drug for at least six months. Consequently, most countries have adopted inpatient models of care at centralised, specialised hospitals.
South Africa has one of the largest drug-resistant TB epidemics in the world.1 KwaZulu-Natal Province has emerged as a global hotspot of the TB, drug-resistant TB, and HIV syndemic, with 76% of MDR-TB patients co-infected with HIV, and MDR-TB mortality rates of 71%.3-5 Local management of MDR-TB was based on hospitalisation in a centralised specialised hospital for the initial six months of treatment, to facilitate daily injections and allow close monitoring of adverse events and adherence. Following discharge, and for the remaining period of treatment (18 months or longer), patients were expected to return for monthly out-patient visits, which for some patients entailed travelling 500kms to reach the hospital. In this setting, the escalating burden of MDR-TB together with limited bed capacity resulted in long waiting lists, an average delay of 111 days for hospital admission and treatment initiation3 and in 2007, only 32% of MDR-TB patients accessed treatment.6 Furthermore, patients were discharged before the end of the injectable phase of treatment to facilities unfamiliar with MDR-TB treatment, resulting in poor treatment outcomes and high default rates. 3-5
An alternate community-based model of care could increase MDR-TB treatment capacity (currently limited by hospital bed availability), and make treatment more accessible by being available closer to patient’s homes, enhancing support to patients and their families. Lengthy arduous trips to receive health care could be limited, thus reducing patient default and improving treatment outcomes. Furthermore, shorter periods of hospitalisation would make it possible to treat more patients and reduce the time to treatment initiation. Alternate models of care have been implemented in small study samples in other countries7-11 and in other southern African settings.12 However, to our knowledge, no study has compared treatment outcomes in a community with a centralised setting. And, little is known about the potential viability and ease of implementation of these alternate models of care on a large scale by a public health service, particularly in areas bruised by TB and HIV epidemics.
In 2008, the KwaZulu-Natal provincial Department of Health began piloting community-based care at four sites. This study was designed to evaluate the community-based model of MDR-TB care at these sites, based on relative treatment success for MDR-TB patients with and without HIV co-infection, in comparison with care in a centralised setting. Here we report final treatment outcomes and predictors for patients treated in community-based versus centralised care.
METHODS
Study design, patients and procedures
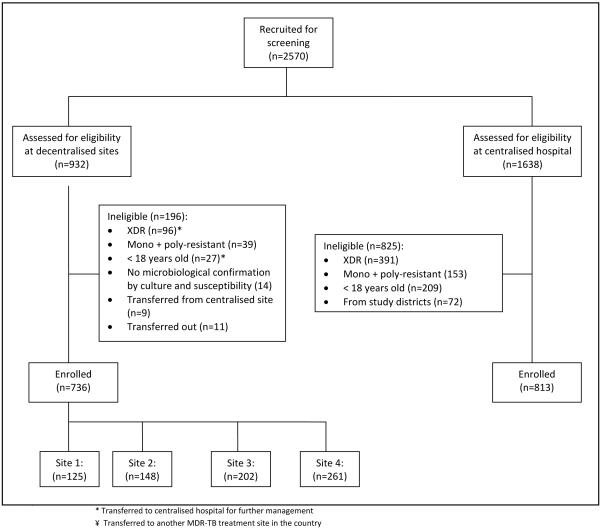
This prospective cohort study was conducted in the province of KwaZulu-Natal, South Africa. Between 1 July 2008 and 30 June 2010, 1549 patients aged ?18 years with a laboratory confirmed diagnosis of MDR-TB were enrolled (Figure 1). Patients were excluded if they were resistant to a single first-line TB drug (rifampicin, isoniazid, pyrazinamide or ethambutol), or were resistant to isoniazid and rifampicin with any second-line TB drugs. Patients who received care at both a community-based site and the centralised hospital, or who were participating in an MDR-TB clinical trial were also excluded. All patients who lived within the catchment area of each community-based site were enrolled at that site if they met the study criteria. At the centralised hospital all patients who met the study criteria were enrolled, unless they came from the catchment areas of the community-based sites.
Figure 1.
Schema of enrolment
Intervention
Patients diagnosed with MDR-TB were referred to either a community-based site or the centralised hospital for initiation of MDR-TB therapy, depending on where they lived. All patients received standardized MDR anti-tuberculosis therapy and ART in accordance with national guidelines.13,14 During the initial intensive phase of treatment (usually 4-6 months), patients were started on a regimen of kanamycin (Km), pyrazinamide (Z), ethambutol (E), ethionamide (Eto), ofloxacin (Ofx), and cycloserine (Cs). This was followed by a continuation phase of at least 18 months of oral treatment (Z, E, Eto, Ofx and Cs).
The four community-based sites were attached to purposively selected rural hospitals in areas where large numbers of MDR-TB patients were being diagnosed. Non-specialist doctors provided care to MDR-TB patients at these sites, referring patients requiring specialist care to the centralised hospital. Once discharged, all patients attended monthly follow-up treatment monitoring visits at the community-based sites. Home-based care was available for patients discharged from the community-based sites during the intensive phase of treatment, with kanamycin injections administered daily either at a local clinic or by mobile injection teams that visited patients in their homes. Mobile injection teams also followed up patients who had defaulted. The guiding principles and detailed implementation of this model have been reported previously.15,16 The implementation of injection teams varied across the sites. At Site 1, 16 injection teams were mobilised in the first year of the programme (2008). Site 2 mobilised two teams in 2009; Site 3, two teams towards the end of 2011; and Site 4 none at all. Direct-observation of therapy (DOT) is included in the national TB and MDR-TB guidelines, but is seldom implemented; most patients self-administered oral treatment and adherence was seldom monitored.
At the community-based sites, home-based care from the time of treatment initiation was available, but for a number of reasons most patients (95%) were initially hospitalised: (1) many patients co-infected with MDR-TB and HIV were very ill at the time of MDR-TB diagnosis, requiring hospitalisation; (2) clinicians unfamiliar with the co-management of MDR-TB and HIV wanted to monitor patients closely; (3) the extent of implementation of home-based care varied across the community-based sites; (4) home circumstances assessed by a social worker were determined to be unsupportive of adherence. The median duration of hospitalisation was similar at the community-based sites and centralised hospital (143 versus 144 days), but varied across the four community-based sites from 96 days at Site 1, to 180 days at Site 3 (Table 3).
Table 3.
Treatment outcomes and clinical course of patients with MDR-TB in KwaZulu-Natal, South Africa
| Centralised hospital |
All community- based sites |
Site 1 | Site 2 | Site 3 | Site 4 | p-value | |
|---|---|---|---|---|---|---|---|
| n=813 | n=736 | n=125 | n=148 | n=202 | n=261 | ||
| Treatment outcomes¶ | |||||||
| Cured* | 280 (34.4) | 373 (50.7) | 78 (62.4) | 81 (54.7) | 94 (46.5) | 120 (46.0) | <0.001 0.009 |
| Treatment completed¥ | 159 (19.6) | 54 (7.3) | 12 (9.6) | 8 (5.4) | 19 (9.4) | 15 (5.8) | <0.001 0.265 |
| Treatment success** | 439 (54.0) | 427 (58.0) | 90 (72.0) | 89 (60.1) | 113 (55.9) | 135 (51.7) | 0.180 0.002 |
| Died‡ | 113 (13.9) | 133 (18.1) | 17 (13.6) | 22 (14.9) | 25 (12.4) | 69 (26.4) | 0.211 <0.001 |
| Failed^ | 29 (3.6) | 49 (6.7) | 7 (5.6) | 11 (7.4) | 12 (5.9) | 19 (7.3) | <0.001 0.872 |
| Default‡‡ | 230 (28.3) | 107 (14.5) | 9 (7.2) | 20 (13.5) | 50 (24.8) | 28 (10.7) | 0.004 <0.001 |
| Transferred out^^ | 2 (0.25) | 20 (2.7) | 2 (1.6) | 6 (4.1) | 2 (1.0) | 10 (3.8) | <0.001 0.130 |
| Clinical course of treatment | |||||||
| n Median no. of days from initial sputum collection to MDR-TB therapy initiation¶¶, (IQR) |
811 92, (69-120) |
724 72, (54-97) |
125 65, (42-91) |
141 66, (50-86) |
199 70, (52-99) |
259 83, (63-107) |
<0.001 <0.001 |
| n Median duration of hospitalization (days), (IQR) |
243 144, (83-185) |
636 143, (90-179) |
91 96, (57-132) |
133 117, (83-146) |
151 180, (120-197) |
261 154, (115-175) |
0.302 <0.001 |
| Median duration of MDR- TB treatment (days), (IQR) |
589, (285-700) | 712, (270-740) | 719, (588-735) | 723, (273-753) | 700, (341-733) | 687, (176-742) | <0.001 0.062 |
| Patients who culture converted, n/total culture- positive at treatment start (%) |
511/638 (80) | 536/672 (80) | 95/111 (86) | 118/139 (85) | 140/174 (81) | 183/248 (74) | 0.983 0.017 |
| n Median no of days to culture conversion‡‡, (IQR) |
511 83 (56, 111) |
536 81 (56, 110) |
95 63 (53, 84) |
118 90 (58, 125) |
140 87 (58, 120) |
183 80 (54, 109) |
0.651 0.003 |
| n Median no of days follow up from diagnosis to treatment outcome, (IQR) |
811 688 (386, 791) |
724 771 (357, 822) |
125 761 (696, 811) |
141 787 (363, 824) |
199 764 (423, 811) |
259 771 (287, 831) |
<0.001 0.877 |
Data are number (%), unless otherwise indicated
Cured: Cure was defined as completion of treatment and >5 consecutive negative culture results in the final 12 months of treatment. A patient was still considered cured If only one positive culture was reported during this time, was clinically well, and this positive culture was followed by at least 3 consecutive negative cultures taken at least 30 days apart.
Treatment completed: Treatment completion referred to completion of therapy but without bacteriologic documentation of cure.
Treatment success: Treatment success has been defined as the percentage of patients in whom the treatment outcome was either cured or completed. That is, “% successful = no. of patients cured + no. of patients completed treatment /Total no. initiated treatment × 100”.has been defined as the percentage of patients in whom the treatment outcome was either cured or completed. That is, “% successful = no. of patients cured + no. of patients completed Rx/Total no. initiated Rx × 100.
Died: Death was defined as all-cause mortality during MDR-TB treatment.
Failed: Treatment failure was defined as having more than one positive culture in the final 12 months of therapy, or if any one of the final three cultures was positive, or if more than one drug in the treatment regimen was replaced, or if treatment was terminated due to adverse events or no clinical improvement.
Default: Default was defined as an interruption in treatment for > 2 consecutive months for any reason.
Transferred out: A patient with MDR-TB who was transferred to another reporting and recording unit a year after study-enrolment whose treatment outcome is unknown.
This definition is an adaptation of the WHO definition, as date of DST results was not routinely recorded.29
Culture conversion was defined as the interval between the treatment start date and the first of two consecutive negative sputum cultures taken at least one month apart.17
End points
The primary outcome variables were treatment outcome as defined by WHO in 2008 (Table 1),17,18 and survival time over course of treatment (2008 definitions were used as most treatment outcomes had been assigned by the time the 2011 revised definitions were published). Treatment initiation delay, a secondary outcome, was defined as the interval between initial sputum collection and treatment start.
Table 1.
Treatment outcome definitions*
| Treatment outcome | Definitions |
|---|---|
| Cure | Cure was defined as completion of treatment and >5 consecutive negative culture results in the final 12 months of treatment. A patient was still considered cured If only one positive culture was reported during this time, was clinically well, and this positive culture was followed by at least 3 consecutive negative cultures taken at least 30 days apart. |
| Treatment completion |
Treatment completion referred to completion of therapy but without bacteriologic documentation of cure. |
| Treatment success | Treatment success has been defined as the percentage of patients in whom the treatment outcome was either cured or completed. That is, “% successful = no. of patients cured + no. of patients completed treatment /Total no. initiated treatment × 100”. |
| Treatment failure | Treatment failure was defined as having more than one positive culture in the final 12 months of therapy, or if any one of the final three cultures was positive, or if more than one drug in the treatment regimen was replaced, or if treatment was terminated due to adverse events or no clinical improvement. |
| Default | Default was defined as an interruption in treatment for ≥ 2 consecutive months for any reason. |
| Death | Death was defined as all-cause mortality during MDR-TB treatment. |
| Unsuccessful treatment |
Unsuccessful treatment outcome has been defined as the percentage of patients in whom the treatment outcome was died, defaulted, or failed treatment. |
| Transferred out | A patient with MDR-TB who was transferred to another reporting and recording unit a year after study- enrolment whose treatment outcome is unknown. |
Statistical analysis
We reviewed medical records to collect patient-related demographic, clinical, pharmaceutical and laboratory data. Patients’ response to treatment was monitored via continuous data collection from medical records and the laboratory database for the duration of treatment. Data collection was complete by October 2012. Baseline characteristics and treatment outcomes were described using simple frequencies. Medians among the individual community-based sites were compared using the Kruskal-Wallis non-parametric test. General linear models (GLM) using the binomial or normal distribution were used to compare proportions and/or means respectively between sites; also to compare medians using rank values. Logistic regression was utilized to assess the effect of risk factors on successful vs. unsuccessful outcomes. All models utilized the GEE (generalised estimating equation) procedure to adjust for clustering from the multiple hospitals comprising the community-based sites. The Cox proportional hazards model, incorporating a robust “sandwich” variance estimate to allow for clustering of patients from the same site, was used to assess the effect of certain risk factors on time to death. Patients who did not experience the event measured were considered censored at date of final outcome. All multivariate models used a univariate threshold of p<0.25 in order for variables to be considered for inclusion. Analyses were conducted using SAS V9.2 (SAS Institute, Cary, NC, USA).
Study oversight
The study protocol was approved by the Biomedical Research Ethics Committee at the University of KwaZulu-Natal (Ref: BF052/09).
RESULTS
Of the 1549 patients prospectively enrolled in the study, 736 were treated at the community-based sites and 813 at the centralised hospital (Figure 1). Ninety-four percent of all patients were tested for HIV, and co-infection rates were high at both the community-based sites and centralised hospital (76.3% vs. 73.1%), with 91.3% of patients at the community-based sites receiving ART compared with 82% at the centralised hospital (Table 2). Patients at the community-based sites were less likely to have a history of TB (55.8% vs. 95.7%, p<0.001), and had a lower median pre-treatment weight (50 vs. 53 kg, p <0.001). More patients at the community-based sites were smear-positive at diagnosis (75.7% vs. 60.4%, p<0.001).
Table 2.
Baseline demographic and clinical characteristics of patients with MDR-TB in KwaZulu-Natal, South Africa
| Characteristic | Centralised hospital | All community- based sites |
Site 1 | Site 2 | Site 3 | Site 4 | p-value |
|---|---|---|---|---|---|---|---|
| n=813 | n=736 | n=125 | n=148 | n=202 | n=261 | ||
| Female | 413 (50.8) | 390 (52.9) | 68 (54.4) | 88 (59.5) | 98 (48.5) | 136 (52.1) | 0.233 0.230 |
| Median age (years, IQR) | 34 (27-41) | 36 (28-43) | 36 (28-42) | 37 (28-42) | 34 (26-42) | 36 (29-44) | <0.001 0.531 |
| TB characteristics | |||||||
| Previous TB | 778 (95.7) | 411 (55.8) | 87 (69.6) | 81 (54.7) | 136 (67.3) | 107 (41.0) | <0.001 <0.001 |
| Previous MDR-TB | 10 (1.23) | 53 (7.2) | 6 (4.8) | 17 (11.5) | 16 (7.9) | 14 (5.4) | <0.001 0.098 |
| Sputum-smear positive at diagnosis |
491 (60.4) | 557 (75.7) | 75 (60.0) | 121 (81.8) | 152 (75.3) | 209 (80.1) | <0.001 <0.001 |
| Resistant to ≥3 drugs at diagnosis |
467 (57.4) | 410 (55.7) | 74 (59.2) | 91 (61.5) | 101 (50.0) | 144 (55.2) | 0.364 0.149 |
| Initial regimen ≥6 drugs | 717 (88.2) | 591 (80.3) | 115 (92.0) | 131 (88.5) | 91 (45.1) | 254 (97.3) | 0.492 <0.001 |
| HIV characteristics | |||||||
| HIV-infected, n/total tested (%) |
576/788 (73.1) | 528/692 (76.3) | 96/124 (77.4) | 112/144 (77.8) | 123/189 (65.1) | 197/235 (83.8) | 0.450 <0.001 |
| HIV unknown | 25 (3.1) | 44 (6.0) | 1 (0.8) | 4 (2.7) | 13 (6.4) | 26 (10) | 0.051 <0.001 |
| Patients with available CD4 count |
282 | 345 | 82 | 72 | 92 | 99 | |
| Median baseline CD4 count (cells per μL), (IQR) |
185, (106-300) |
193, (88-329) |
158, (71-286) |
230, (136-366) |
139, (64-322) |
227, (96-365) |
0.943 0.010 |
| On ART, n/total HIV-infected (%)* |
454/554 (82.0) | 440/482 (91.3) | 92/94 (97.9) | 103/106 (97.2) | 116/117 (99.2) | 129/165 (78.2) | 0.225 <0.001 |
Data are number (%), unless otherwise indicated
MDR-TB = multidrug-resistant tuberculosis
IQR = interquartile range
HIV = human immunodeficiency virus
ART = antiretroviral therapy
On ART at start of MDR-TB therapy or within two weeks of MDR-TB treatment initiation. Denominator excludes HIV+ patients with missing ART information.
At the community-based sites significantly more patients were cured (50.7% vs. 34.4%, p<0.001), and significantly fewer patients defaulted (14.5% vs. 28.3%, p=0.004) (Table 3). In addition, more patients achieved a successful treatment outcome (58%) than at the centralised hospital (54%). In multivariate analysis adjusting for HIV status, age, previous MDR-TB infection, and pre-treatment weight, patients were more likely to have a successful treatment outcome if they were treated at a community-based site (adjusted OR=1.43, p=0.01) (Table 4). There was no effect modification on MDR-TB outcomes of HIV status and ART. Regardless of site, HIV-positive patients were at greater risk of dying (16.3% vs. 11.4%, p=0.022) (Table 5).
Table 4.
Predictors of treatment success and death in patients with MDR-TB from the community-based sites and centralised hospital in KwaZulu-Natal, South Africa
| Predictors of treatment success | ||||
|---|---|---|---|---|
| Variables | Unadjusted Odds Ratio (95% CI) |
p-value | Multivariate Odds Ratio (95% CI) |
p-value |
| Community-based site | 1.19 (0.91 to 1.57) | 0.207 | 1.43 (1.09 to 1.88) | 0.010 |
| Female gender | 1.21 (1.09 to 1.36) | <0.001 | 1.19 (1.05 to 1.34) | 0.007 |
| Age≥30 years | 1.28 (1.01 1.63) | 0.042 | 1.40 (1.18 to 1.65) | <0.001 |
| No previous MDR-TB | 2.63 (2.05 to 3.39) | <0.001 | 2.51 (1.64 to 3.84) | <0.001 |
| HIV and ART status | ||||
| HIV positive, on ART HIV negative HIV positive, not on ART |
1.63 (1.40 to 1.89) 1.43 (1.08 to 1.90) Reference |
<0.001 0.012 | 1.5 (1.38 to 1.62) 1.35 (1.05 to 1.75) Reference |
<0.001 0.021 |
| Weight, ≥50 kg female or ≥55 kg male | 1.31 (1.06 to 1.63) | 0.013 | 1.28 (1.06 to 1.56) | 0.011 |
| Length of hospitalization^ | 1.01 (0.99 to 1.01) | 0.091 | ** | |
| Culture conversion <90 days from Rx start¶ |
1.71 (1.44 to 2.03) | <0.001 | ** | |
|
| ||||
| Predictors of death | ||||
|
| ||||
| Variables |
Unadjusted
Hazards Ratio (95%) CI) |
p-value |
Multivariate Hazards
Ratio (95% CI) |
p-value |
| Community-based Site | 1.27 (0.82 to 1.96) | 0.289 | 1.06 (0.87 to1.30) | 0.567 |
| Age, ≥30 years | 1.70 (1.03 to 2.83) | 0.039 | 1.61 (1.09 to 2.38) | 0.018 |
| Low pre-treatment weight, ≤50 kg female or≤55 kg male |
1.35 (0.88 to 2.05) | 0.161 | 1.40 (1.00 to 1.96) | 0.048 |
| Previous TB or MDR-TB | 0.82 (0.58 to 1.19) | 0.312 | 1.28 (0.78 to 2.09) | 0.324 |
| Year treatment started | ||||
| 2008 2009 2010 |
1.78 (1.35 to 2.35) 1.27 (1.09 to 1.48) Reference |
<.001 .002 | 1.77 (1.36 to 2.31) 1.53 (1.05 to 2.22) Reference |
<0.001 0.027 |
| HIV and ART status | ||||
| HIV positive, not on ART HIV positive, on ART HIV negative |
2.12 (1.54 to 2.93) 1.23 (0.93 to 1.63) Reference |
<0.001 0.154 |
1.59 (0.98 to 2.59) 1.01 (0.72 to 1.41) Reference |
0.062 1.006 |
| Median baseline CD4 count (cells per μL) |
** | |||
| <50 50-199 200+ |
1.90 (1.11 to 3.26) 1.02 (0.80 to 1.31) Reference |
0.019 0.853 |
||
| Length of hospitalization^ | 0.92 (0.88 to 0.96) | <0.001 | ** | |
| Extensive chest disease‡ | 2.54 (1.00 to 6.44) | 0.049 | ** | |
HIV = human immunodeficiency virus
ART = antiretroviral therapy
For every additional 14 day stay
culture conversion <90 days defined as two consecutive negative sputum cultures taken at least 1 month apart less than 90 days after treatment started.
Not included in multivariate model because too few non-missing values.
Extensive chest disease defined if bilateral involvement or cavities present on chest X-ray.
Table 5.
Treatment outcomes stratified by HIV status in patients with MDR-TB in KwaZulu-Natal, South Africa¶
| HIV positive | HIV negative | |||||
|---|---|---|---|---|---|---|
| Centralised Hospital |
All community- based sites |
p-value | Centralised Hospital |
All community- based sites |
p-value | |
| n=576 | n=528 | n=212 | n=164 | |||
| Cured* | 200 (34.7) | 286 (54.2) | <0.001 | 71 (33.5) | 79 (48.2) | <0.001 |
| Treatment completed¥ |
109 (18.9) | 35 (6.6) | <0.001 | 46 (21.7) | 16 (9.8) | <0.001 |
| Treatment success** |
309 (53.6) | 321 (60.8) | 0.013 | 117 (55.2) | 95 (57.9) | 0.372 |
| Died† | 82 (14.2) | 98 (18.6) | 0.114 | 24 (11.3) | 19 (11.6) | 0.970 |
| Failed^ | 23 (4.0) | 32 (6.1) | <0.001 | 5 (2.4) | 14 (8.5) | 0.008 |
| Default‡‡ | 160 (27.8) | 65 (12.3) | <0.001 | 66 (31.1) | 32 (19.5) | 0.025 |
| Transferred out^^ |
2 (0.4) | 12 (2.3) | ** | 0 | 4 (2.4) | ** |
Data are number (%), unless otherwise indicated
Cured: Cure was defined as completion of treatment and >5 consecutive negative culture results in the final 12 months of treatment. A patient was still considered cured If only one positive culture was reported during this time, was clinically well, and this positive culture was followed by at least 3 consecutive negative cultures taken at least 30 days apart.
Treatment completed: Treatment completion referred to completion of therapy but without bacteriologic documentation of cure.
Treatment success: Treatment success has been defined as the percentage of patients in whom the treatment outcome was either cured or completed. That is, “% successful = no. of patients cured + no. of patients completed treatment /Total no. initiated treatment × 100”.has been defined as the percentage of patients in whom the treatment outcome was either cured or completed. That is, “% successful = no. of patients cured + no. of patients completed Rx/Total no. initiated Rx × 100.
Died: Death was defined as all-cause mortality during MDR-TB treatment.
Failed: Treatment failure was defined as having more than one positive culture in the final 12 months of therapy, or if any one of the final three cultures was positive, or if more than one drug in the treatment regimen was replaced, or if treatment was terminated due to adverse events or no clinical improvement.
Default: Default was defined as an interruption in treatment for > 2 consecutive months for any reason.
Transferred out: A patient with MDR-TB who was transferred to another reporting and recording unit a year after study-enrolment whose treatment outcome is unknown.
Survival probability appeared slightly worse at the community-based sites (p=0.064), with higher mortality at Site 4 influencing overall mortality (Table 3). Multivariate analysis showed significantly increased mortality for patients who were older than 30 (HR=1.64, p=0.010); had low pre-treatment weight (HR=1.42, p=0.041); were HIV-positive and not on ART (HR=1.77, p=0.018, referent HIV-negative) (Table 4).
There was heterogeneity in treatment outcomes across the four community-based sites with treatment success varying from 72% (Site 1) to 51.7% (Site 4) (p<0.01). Seventy patients (10%) at the community-based sites received exclusive home-based care. There was no difference in successful treatment outcomes in these patients compared with those who were initially hospitalised at the community-based sites (59% vs. 61%, p=0.511).
Treatment initiation delay was shorter at the community-based sites than the centralised hospital (median=72 vs. 92 days respectively, p<0.001) (Table 3) - but delay was not associated with treatment outcomes or mortality.
DISCUSSION
Our study shows that community-based care is more effective than centralised care as evidenced by higher cure (50.7% vs. 34.3%, p<0.001), lower default (14.5% vs. 28.3%, p=0.004) and earlier treatment initiation (72 vs. 92 days, p<0.001). Confirmatory logistic regression models adjusted for HIV status and receipt of ART, showed that patients at community-based sites were more likely to have a successful treatment outcome (adjusted OR=1.43, p=0.01) (Table 4).
WHO, together with others, is now promoting ambulatory models of care for MDR-TB patients.19-21 Our study findings support these calls as the introduction of community-based care increased the capacity of the public health system to provide treatment to approximately 900 more MDR-TB patients.22 Furthermore, higher successful treatment outcomes and lower default rates at community-based sites suggest that community-based care may address patients’ needs more successfully as care closer to home is easier to access, convenient, allows family support and eliminates long and costly trips to a centralised hospital.
Other studies reporting outcomes for community-based MDR-TB care have documented similar success rates, but have involved small numbers of patients and been implemented with support of an external organisation.7,8,12 In contrast, our study reports findings of a programme implemented and funded entirely by the Department of Health. This increases the generalizability of our findings to other resource-limited situations.
There was considerable heterogeneity in treatment outcomes across the four community-based sites as treatment success varied from a high of 72% at Site 1 to a low of 51.7% at Site 4 (p<0.002). Higher treatment success at Site 1 was as a consequence of it being in a well-functioning and supportive district, where district leadership took ownership of the MDR-TB problem, re-organised and re-aligned health service components and allocated sufficient financial resources. This translated into the provision of 16 injection teams, additional staff at the outpatients’ clinic who established systems, implementation of a locally developed patient treatment literacy programme and home-assessment by a multi-disciplinary team prior to patient discharge. These programme components were partially implemented at other decentralised sites. However, even when removing Site 1 from the analysis, treatment at the community-based sites remained as effective as treatment in the centralised site. Although the heterogeneity in treatment success across the community-based sites was greater than expected, we believe this adequately reflects variation in health service provision across different sites when services are expanded or a new programme is introduced. The variation in the number of days of hospitalisation at the decentralised sites (Site 1= 96; Site 3= 180; p<0.002) illustrates the different interpretation and implementation of guidelines, highlighting the importance of regular monitoring and support during service expansion, to ensure health systems are functional and new programmes implemented in accordance with guidelines, thereby optimising the probability of treatment success.23-25
Survival rates at the community-based sites were somewhat lower than at the centralised site (Table 3). There are five possible explanations. Firstly, survival bias may play a role at the centralised hospital where the median treatment initiation delay was longer than at the community-based sites (92 vs. 72 days). Secondly, experienced clinicians at the centralised hospital with access to more sophisticated laboratory and other investigations were able to detect patients failing to respond to treatment more quickly. Thirdly, there were a number of patients from the centralised hospital whose treatment outcomes were not known as their clinical records were missing. Although we are confident that no patient with a successful outcome was incorrectly classified, we may have misclassified patients as defaulters when in fact they had died, thus underestimating mortality at the centralised hospital. A number of studies evaluating ART programmes, particularly those with large proportions of TB co-infection, have documented high mortality in patients lost to follow up.26,27 Fourthly, baseline characteristics of patients at the community-based sites differed from those at the centralised hospital, suggesting that this patient population was more physically impoverished. Fifthly, there was considerable heterogeneity in mortality across the decentralised sites. The mortality rate at Site 4 was twice that at Site 1, influencing overall mortality.
Survival improved for the duration of the study. Across all sites, MDR-TB patients starting treatment in 2008 and 2009 were – respectively – 1.77 and 1.53 times more likely to die than patients starting treatment in 2010 (Table 4). Possible reasons for this improvement include policy changes in 2009 which promoted earlier initiation of ART for patients with MDR-TB and HIV,28 new drugs introduced into the MDR-TB regimen, and improvement of patient management at the community-based sites with time and experience.
This operational study evaluated an intervention implemented by the public sector and we therefore had limited control over the design, scope and quality of implementation. Data used for programme evaluation were routinely collected by health workers, and were occasionally incomplete. Furthermore, during the study, as clinical records for some patients at the centralised site could not be found, we took several steps to determine the treatment outcomes of these patients. These included searching the national laboratory database (which demonstrated on-going care) and consulting the national registration system to verify if the patient had died. At the end of the analysis all patients had been assigned an outcome.
CONCLUSION
We conclude that community-based care is more effective than care in a centralised setting, based on similar treatment success rate, lower defaulter rate and shorter time to treatment initiation at the four community-based sites. Even in the presence of HIV co-infection, community-based care increased treatment success. Still to be determined is whether exclusive home-based care can achieve the same treatment success.
As alternate models of care for patients are introduced or expanded, we recommend regular monitoring and support of district and facility managers and individual health workers to ensure that services are equitable, guidelines adhered to, quality of care is optimal and the chance of treatment success optimised.
Acknowledgements
We acknowledge and thank Nonhlanhla Yende-Zuma for her help with statistical interpretation. We also acknowledge the KwaZulu-Natal Department of Health and thank facility level managers, doctors, nurses and data capturers at the study sites for their assistance. We gratefully acknowledge the participants in the study.
Financial support: The work was funded by the Medical Research Council of South Africa, Izumi Foundation and Eli-Lilly Foundation. Marian Loveday is supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP), Implementation Science Traineeship Program funded by the United States President’s Emergency Plan for AIDS Relief (PEPFAR) through the Fogarty International Center, National Institutes of Health (grant # D43TW00231). JB is supported by the National Institute of Allergy and Infectious Diseases (K23AI083088).
Role of the funding source: The funders had no role in study design, in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. All researchers were independent of funders and sponsors.
Footnotes
Ethics approval: The study protocol was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (Ref: BF052/09), and by the KwaZulu-Natal Department of Health. Only secondary data, the data routinely collected by health workers for clinical care was used in this study. To protect patient confidentiality and anonymity the data bases were de-identified and access strictly limited. Informed consent was waived by the ethics committee, since all data used were previously collected during the course of routine medical care and did not pose any additional risks to the patients.
Conflicts of interest: There are no potential conflicts of interest relevant to this article. All authors reported no conflict of interest.
REFERENCES
- 1.World Health Organisation . Global Tuberculosis Report 2013. World Health Organisation; Geneva, Switzerland: 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Gandhi N, Shah S, Andrews J, et al. HIV Co-infection in Multidrug- and Extensively Drug-Resistant Tuberculosis Results in High Early Mortality. Amer J Resp Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 3.Wallengren K, Scano F, Margot B, et al. Resistance to TB drugs in KwaZulu-Natal: causes and prospects for control. 2011 http://arxiv.org/abs/1107.1800 (Last accessed 25 July 2011)
- 4.Zager E, McNerney R. Multidrug-resistant tuberculosis. BMC Infectious Diseases. 2008;8 doi: 10.1186/1471-2334-8-10. doi:10.1186/471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brust J, Gandhi N, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000-2003. Int J Tuberc Lung Dis. 2010;14:413–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Wallengren K, Scano F, Margot B, et al. Drug-Resistant Tuberculosis, KwaZulu-Natal, South Africa, 2001-2007. EID. 2011;17:1913–6. doi: 10.3201/eid1710.100952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyieng’o D, Park P, Gardner A, et al. Community-based treatment of multidrug-resistant tuberculosis: early experience and results from Western Kenya. Public Health Action. 2012;2:38–42. doi: 10.5588/pha.12.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multi-drug resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 9.Kim H, Hong Y, Kim S, Lew W, Lee E. Ambulatory treatment of multidrug-resistant pulmonary tuberculosis patients at a chest clinic. Int J Tuberc Lung Dis. 2001;5:1129–36. [PubMed] [Google Scholar]
- 10.Van Deun A, Hamid Salim M, Kumar Das A, Bastian I, Portaels F. Results of a standardised regimen for multidrug-resistant tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2004;8:560–7. [PubMed] [Google Scholar]
- 11.Keshavjee S, Gelmanova I, Pasechnikov A, et al. Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci. 2008;1136:1–11. doi: 10.1196/annals.1425.009. [DOI] [PubMed] [Google Scholar]
- 12.Satti H, McLaughlin M, Hedt-Gauthier B, et al. Outcomes of Multidrug-Resistant Tuberculosis Treatment with Early Initiation of Antiretroviral Therapy for HIV Co-Infected Patients in Lesotho. PLoS ONE. 2012;7:e46943. doi: 10.1371/journal.pone.0046943. doi:10.1371/journal.pone.0046943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.South African Department of Health Management of drug-resistant tuberculosis: Policy Guidelines. Pretoria. 2008 Jun; [Google Scholar]
- 14.South African Department of Health . Clinical Guidelines for the Management of HIV and AIDS in Adults and Adolescents. Pretoria; Department of Health: 2010. http://www.doh.gov.za/docs/facts-f.html (accessed 30 July 2010) [Google Scholar]
- 15.Loveday M, Wallengren K, Voce A, et al. Comparing early treatment outcomes of MDR-TB in decentralised and centralised settings in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2012;16:209–15. doi: 10.5588/ijtld.11.0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brust J, Shah N, Scott M, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012;16:998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organisation . Emergency Update. World Health Organisation; Geneva: 2008. 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2008.402. [Google Scholar]
- 18.Laserson K, Thorpe L, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–5. [PubMed] [Google Scholar]
- 19.Falzon D, Jaramillo E, Schunemann H, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 20.Toczek A, Cox H, du Cros P, Cooke G, Ford N. Strategies for reducing treatment default in drug-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2013;17:299–307. doi: 10.5588/ijtld.12.0537. [DOI] [PubMed] [Google Scholar]
- 21.Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. INT J TUBERC LUNG DIS. 2014;18:441–8. doi: 10.5588/ijtld.13.0742. [DOI] [PubMed] [Google Scholar]
- 22.Margot B. Management of M/XDR-TB in KwaZulu-Natal: How are we doing?. South African TB conference.2011. [Google Scholar]
- 23.Loveday M, Padayatchi N, Wallengren K, et al. Association between Health Systems Performance and Treatment Outcomes in Patients Co-Infected with MDR-TB and HIV in KwaZulu-Natal, South Africa: Implications for TB Programmes. PLoS ONE. 2014;9:e94016. doi: 10.1371/journal.pone.0094016. doi:10.1371/journal.pone.0094016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atun R. Health systems, systems thinking and innovation. Health Policy and Planning. 2012;27:iv4–iv8. doi: 10.1093/heapol/czs088. doi:10.1093/heapol/czs088. [DOI] [PubMed] [Google Scholar]
- 25.Tkatchenko-Schmidt E, Atun R, Wall M, Tobi P, Schmidt J, Renton A. Why do health systems matter? Exploring links between health systems and HIV response: a case study from Russia. Health Policy and Planning. 2010;25:283–91. doi: 10.1093/heapol/czq001. [DOI] [PubMed] [Google Scholar]
- 26.Brinkhof M, Pujades-Rodriguez M, Egger M. Mortality of Patients Lost to Follow-Up in Antiretroviral Treatment Programmes in Resource-Limited Settings: Systematic Review and Meta-Analysis. PLoS ONE. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. doi:10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassett I, Chetty S, Wang B, et al. Loss to Follow-Up and Mortality Among HIV-Infected People Co-Infected With TB at ART Initiation in Durban, South Africa. Journal of Acquired Immune Deficiency Syndromes. 2012;59:25–30. doi: 10.1097/QAI.0b013e31823d3aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdool Karim S, Naidoo K, Grobler A, et al. Timing of Initiation of Antiretroviral Drugs during Tuberculosis Therapy. NEJM. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . A minimum set of indicators for the programmatic management of MDR-TB in national tuberculosis control programmes (WHO/HTM/TB/2010.11) World Health Organization; Geneva: 2010. Multidrug-resistant tuberculosis (MDR-TB) indicators. [Google Scholar]