Abstract
Fertilization triggers activation of Src-family kinases in eggs of various species including marine invertebrates and lower vertebrates. While immunofluorescence studies have localized Src-family kinases to the plasma membrane or cortical cytoplasm, no information is available regarding the extent to which these kinases are activated in different regions of the zygote. The objective of the present study was to detect the subcellular distribution of activated Src-family kinases in the fertilized zebrafish egg. An antibody specific for the active, non-phosphorylated form of Src-family PTKs was used to detect these activated kinases by immunofluorescence. The results demonstrate that Fyn, and possibly other Src family members are activated by dephosphorylation of the C-terminal tyrosine at fertilization. The activated Src-family kinases are asymmetrically distributed around the egg cortex with an area of higher kinase activity localized adjacent to the micropyle near the presumptive animal pole. Fertilization initially caused elevation of kinase activity in the cytoplasm underlying the micropyle, but this quickly spread to involve the entire zygote cortex. Later during egg activation, formation of the blastodisc involved concentration of active Src-family kinase in the blastodisc cortex. As cytokinesis began, activated Src-family kinases were no longer limited to the cortex, but became more evenly distributed in the clear apical cytoplasm of the blastomeres. The results demonstrate that the cortex of the zebrafish egg is functionally differentiated and that fertilization triggers localized activation of Src-family kinases at the point of sperm entry, which subsequently progresses through the entire egg cortex.
Keywords: Fertilization, zebrafish, Src, Fyn, protein kinase
Introduction
Fertilization triggers a series of preprogrammed biochemical steps in the egg which function to establish a block to polyspermy, activate egg metabolism, and initiate development. The signal transduction pathway activated in eggs of invertebrate and lower vertebrate species involves one or more Src-family protein tyrosine kinases (PTKs) (reviewed in (Runft et al., 2002; Sato et al., 2000)). Src-family PTK activity is thought to be required at several points during the egg activation process. Studies using dominant-negative constructs or exogenous kinases have demonstrated that Src-family PTK activation is required to trigger the sperm-induced calcium transient in invertebrates such as the starfish (Giusti et al., 1999; Shilling et al., 1994) and sea urchin (Abassi et al., 2000; Giusti et al., 2003; Kinsey and Shen, 2000;) as well as lower vertebrates such as the frog (Sato et al., 1999; 2000) and zebrafish (Kinsey et al., 2003; Wu and Kinsey, 2001). Functional studies using chemical inhibitors demonstrated a role of PTKs in pronuclear migration and initiation of DNA synthesis (Glahn et al., 1999; Moore and Kinsey, 1995; Shen et al., 1999; Wright and Schatten, 1995). The potential role of Src-family PTKs in other aspects of egg activation has been highlighted by the recent demonstration that a Src-family PTK in the Xenopus oocyte phosphorylates uroplakin III a molecule potentially involved in egg activation (Sakakibara et al., 2005). Previous studies have demonstrated that Src-family PTKs are localized to the membrane compartments of eggs including plasma membrane microdomains (Belton, et al., 2001; Kinsey, 1996; Sato et al., 2002; Wu and Kinsey, 2001), and immunofluorescence studies have indicated that Src-family PTKs were distributed more or less evenly around the cortex of the eggs which have been investigated (Sato et al., 1999; Talmor et al., 1998; Talmor-Cohen et al., 2004). However, the spatial characteristics of the activation of Src-family PTKs in fertilized eggs has not been determined. This question has special significance to fertilization which involves a highly localized stimulus at the point of sperm contact. Possible scenarios vary from a limited, highly localized activation at the point of sperm contact to a uniform, global activation in the plasma membrane or cortical membrane compartments. The zebrafish fertilization system has advantages that make it an optimal system to detect localized changes in kinase activity during egg activation. One advantage is that sperm-egg contact occurs only at the micropyle, an opening in the chorion designed to admit sperm. A second advantage is that fertilization occurs synchronously in this species under in vitro conditions so the time of sperm penetration can be established within seconds.
Src-family PTKs are initially activated by one or more specific phosphatases that remove [PO4] from the C-terminal negative regulatory tyrosine at position 530 (Superti-Furga and Courtneidge, 1995), a pathway that been shown to occur at fertilization (Wu and Kinsey, 2002). The development of phosphorylation site-specific antibodies directed against the activated (dephosphorylated) C-terminal regulatory region of Src has provided a highly sensitive way to detect activated Src family kinases (Kawakatsu et al., 1996). This monoclonal antibody recognizes the QYQPG sequence flanking Tyr 530 which is also present in the zebrafish Fyn kinase (Sharma et al., 2005) and is highly conserved among Src, Fyn, and Yes in mammals. Therefore this antibody can detect the activated form of all three kinases and possibly others (Wu et al., 2000).
In the present study, we have used the above phosphorylation site-specific antibody directed against the active (dephosphorylated) form of SFKs to detect the presence of activated kinases in different regions of the zebrafish egg before and after fertilization. The results demonstrated that active SFKs were unevenly distributed around the cortex of the unfertilized egg with a concentration of active kinases observed adjacent to the micropyle, an area which probably represents the animal pole. Fertilization triggered progressive activation of SFKs first in the cortical cytoplasm underlying the micropyle and presumptive animal pole, and then in the entire egg cortex. Formation of the blastodisc and cleavage furrow were also associated with concentrations of active kinase.
In summary, these morphological observations revealed that the distribution of activated Src-family PTKs is polarized in the zebrafish egg cortex and activation of these kinases at fertilization is propagated from the point of sperm entry.
MATERIALS AND METHODS
Buffers
Fixative: Na2HPO4, 50mM; sucrose, 4% wt/vol; paraformaldehyde, 4% wt/vol, phenylarsine oxide, 40μM; pH 7.2. Glycine blocking solution; NaH2PO4, 50mM; glycine, 10mM; sucrose, 4% wt/vol; NP40, 0.5% vol/vol; goat serum blocking solution was identical to glycine blocking solution except that goat serum, 2% vol/vol replaced the glycine; Immunoprecipitation buffer: NaCl, 150mM; Tris, 10mM; EDTA, 1mM EGTA, 1mM;Na3VO4, 100μM; NaN3, 100μg/ml; 2-mercaptoethanol, 1mM; aprotinin, 100μg/ml; phenylarsine oxide, 40μM; NP40, 1% vol/vol. pH 7.2.; TKM buffer: Tris, 50mM; KCl, 25mM; MgCl2, 5mM; EGTA, 1mM; NaN3, 100μg/ml; 2-mercaptoethanol, 1mM; pH 7.5. TTBS: Tris, 50mM; NaCl,150mM; Tween-20 0.1%, pH 7.5.
Embryos
Oocytes were collected from mature Danio rerio and maintained in Hank’s BSA buffer at 28°C, while sperm were maintained on ice in sperm extender solution (Lee et al., 1999). Fertilization was accomplished by mixing the sperm (5ul) with the eggs, then activating the sperm by addition of 2.5ml of aquarium water.
Immunofluorescence Microscopy
Zebrafish eggs, and zygotes were fixed for immunofluorescence in a formaldehyde fixative containing the irreversible phosphatase inhibitor phenylarsine oxide to prevent dephosphorylation of SFKs during the immunostaining process. Phenylarsine oxide was present during all manipulations of eggs up to the actual microscopy when it was replaced with sodium ortho-vanadate which was less likely to interact with UV light. Fixation was carried out overnight at 4°C, then the eggs were washed into phosphate buffered saline (PBS) and pronase 0.5% wt/vol (Calbiochem, San Diego, CA) for 6 minutes at 25°C to permeabilize the chorion. Zygotes fixed more than two minutes post-insemination also required manual dissection of the then hardened chorion. Fixed eggs were permeabilized with glycine blocking solution containing 0.5% NP40 for one hour at 25°C, then washed into goat serum blocking buffer for at least 2hrs. Antibody dilutions were made in goat serum blocking buffer and eggs were incubated overnight at 4°C. Secondary antibodies were applied after four washings with goat serum blocking buffer and eggs were incubated with the secondary antibody for 90 minutes at 25°C. The primary antibody used to detect activated Src-family PTKs by immunofluorescence was the mouse monoclonal (clone28, (cat. #AHO 0051, Biosource International. Camarillo, CA)) which was used to stain whole mount eggs at a concentration of 2.5ug/ml. The secondary antibody was goat-anti-mouse IgG-Alexa-fluor 488 (In Vitrogen, Carlsbad, CA). The eggs were then monitored by confocal fluorescence microscopy on a Nikon TE2000U microscope using a 488nm Spectra Physics (Mountainview, CA) laser and a 4X or 20X super fluor objective with pinhole settings set to obtain a theoretical 24μm optical section.
Immunoprecipitation and Western blot analysis
Eggs or zygotes (20 eggs or embryos) were washed in TKM buffer to remove unbound sperm, then homogenized in 0.5ml of TKM buffer and centrifuged at 100,000 × G for 1hr to form a particulate fraction. The particulate fraction was solubilized by homogenization in ten volumes of immunoprecipitation buffer followed by centrifugation at 10,000 × G for 10minutes. The soluble extract was incubated with anti-Fyn-3 antibody (santa Cruz Biotechnology, Santa Cruz, CA) at 0.2μg/ml for 4 hrs at 4°C. The immune complexes were collected by incubation with 25μl of a 10% suspension of Protein A-agarose, then washed three times in immunoprecipitation buffer. The immunoprecipitates were resolved by SDS-PAGE on a 10% gel, blotted to immobilon-P, and blocked with 5% casein (BioRad) in TTBS containing 100μM phenylarsine oxide and 100μM Na3VO4.
RESULTS
Detection of active SFKs in zebrafish eggs by Western blot
The clone-28 antibody has been used in a number of cell types to detect Src-family PTKs which have been activated by dephosphorylation of the C-terminal tyrosine. This antibody can be used to establish the mechanism by which the kinase is activated (dephosphorylation) and to localize the activated kinase in cells. In order to determine whether the antibody could detect activated SFKs in the zebrafish egg, we performed Western blot analysis on the particulate fraction of unfertilized eggs (Figure 1 (UF) and zygotes collected at 2.5 minutes post-insemination (Figure 1 (F)). Particular care was taken to include the PTPase inhibitor phenylarsine oxide during preparation and blotting of the samples to prevent artifactual dephosphorylation which would produce an artificially high level of kinase in the dephosphorylated configuration. The clone 28 antibody bound a closely spaced doublet at 59 and 62 KDa, an electrophoretic mobility typical of Src-family PTKs. An identical blot probed with control mouse IgG (Figure 1 (Ctrl)) demonstrated that the clone 28 antibody binding was specific. To confirm that the labeled bands included Src-family PTKs, we also performed blots of c-Fyn immunoprecipitated from an identical number of eggs and zygotes (Figure 1 (Fyn ippt.)). The clone 28 antibody detected the 59KDa Fyn protein in these immunoprecipitates, but the 62KDa band was not present indicating that it represents another Src-family member. This demonstrates that clone 28 can detect the 59KDa Fyn kinase, and possibly other kinases of similar electrophoretic mobility. The 62KDa band could represent the c-Yes kinase which is slightly larger than Fyn, but clone 28 antibody did not detect any bands in immunoprecipitates of Yes kinase using an antibody against zebrafish Yes (Tsai et al., 2005). The 62KDa band could also represent a hyperphosphorylated form of Src-family kinases previously not characterized. Figure 1 also demonstrated that the relative amount of active, dephosphorylated SFKs increased substantially after fertilization as has been previously demonstrated through the use of in vitro kinase assays(Kinsey et al., 2003). This mechanism was confirmed by probing the same samples with an antibody against the phosphorylated C-terminal tyrosine of Src family PTKS (Figure 1, [pY]-530). The fact that binding of the antibody to the phosphorylated tyrosine ([pY]-530) declined while binding of the antibody to the dephosphorylated tyrosine (clone 28) increased, demonstrated that fertilization triggers dephosphorylation of this C-terminal regulatory tyrosine.
Figure 1.
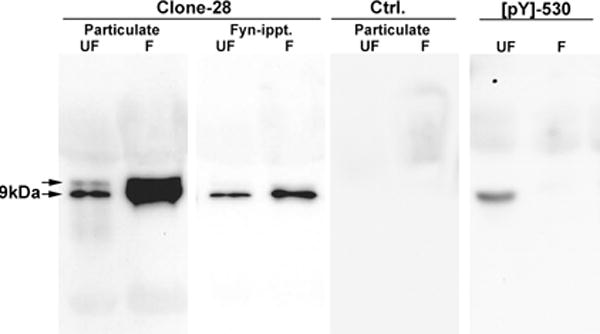
Detection of Active SFKs in zebrafish eggs and zygotes.
Particulate fractions were prepared from samples of 20 unfertilized eggs (UF) or zygotes collected 2.5 minutes after fertilization (F). Aliquots were either used directly for western blot or used for immunoprecipitation of Fyn kinase with the anti-Fyn-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) directed against the N-terminus of Fyn kinase (Fyn-ippt). The blots were incubated with the clone 28 antibody at 1μg/ml (clone 28) or with normal mouse IgG as a control (Ctrl). Aliquots of the same samples were analyzed separately and probed with an affinity purified rabbit antibody to the phosphorylated C-terminal tyrosine ([pY]530) obtained from Biosource International, Camerillo, CA (#44-912). Bound antibody was detected with goat anti-mouse IgG or goat anti-rabbit IgG coupled to horseradish peroxidase and visualized by chemiluminescence.
Detection of activated SFKs by immunofluorescence
In order to detect active SFKs by immunofluorescence, eggs or zygotes were fixed in a formalin fixative supplemented with phosphatase inhibitors. Since the chorion represents a barrier to diffusion of macromolecules, it was necessary to remove the chorion by proteolysis and microdissection so that the antibodies had access to the egg cytoplasm. After the chorion was removed, the eggs were permeabilized with nonionic detergent to increase antibody access. Analysis of unfertilized zebrafish eggs labeled with the clone 28 antibody revealed that active SFKs were highly enriched in the egg cortex and barely detectable in the central cytoplasm (Figure 2). The highest intensity of clone 28 staining was localized to the thin rim of relatively clear cortical cytoplasm which overlies the yolk-rich central region. The low level of staining in the central cytoplasm was not due to limited access of the antibody as demonstrated by examination of eggs that were cut with a scalpel prior to immunolabelling as exemplified in Figure 2. The intensity of clone-28 staining around the periphery of the entire egg was not uniform, (Figure 3a), a feature that was confirmed by rotating eggs under the microscope with a needle. In over 90% of the eggs examined, the cortical cytoplasm underlying the micropyle (Figure 3 arrows) exhibited very little active kinase, while a region of cortex adjacent to the micropyle (left of the micropyle in Figure 3) usually exhibited the highest level of fluorescence (Figure 3B,C). The highly fluorescent region adjacent to the micropyle seemed to be of normal thickness and did not reveal any specialized structures (Figure 3B,C), although it did seem to corresponded to the approximate location of the animal pole. The animal pole in zebrafish eggs is located adjacent to a shallow depression in the egg cortex which includes the smaller, funnel shaped micropyle. The term “animal pole” is a functional definition and while the region could not be positively identified until twenty to thirty minutes post insemination when the blastodisc began to form, the region of enriched kinase activity was continuously present and became more intense once the blastodisc was identifiable (see below). Since the patch of activated kinase corresponded to the animal pole once it was identifiable, we presume that it marked the animal pole prior to fertilization. However, it is possible that the pool of activated kinase is free to move independently of the animal pole. In any case, these results indicate that the distribution of active Src-family PTKs in the cortex of an unfertilized egg was polarized, and that a region near the presumptive animal pole exhibited the highest activity and the area underlying the micropyle exhibited the least activity.
Figure 2.
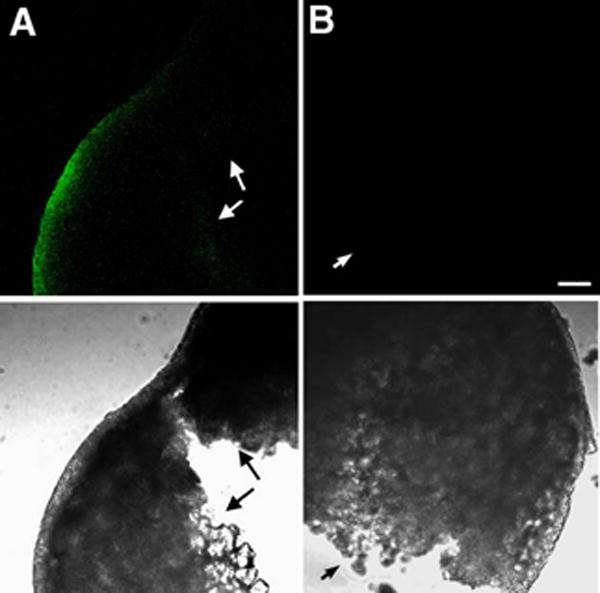
Detection of active SFKs in the unfertilized egg.
Unfertilized eggs were fixed as described in Materials and Methods, treated with pronase and the chorion removed, then cut in half with a scalpel. The samples were then blocked, permeabilized and incubated with the clone 28 antibody (A) or control mouse IgG (B) at 2.5μg/ml followed by Alexa Fluor 488-anti-mouse IgG. Samples were imaged by confocal fluorescence (above) or by transmitted light (below). The cut surfaces of the eggs are indicated by the small arrows. Magnification is indicated by the bar which represents 100μm.
Figure 3. Localization of active Src-family PTKs in the unfertilized egg.
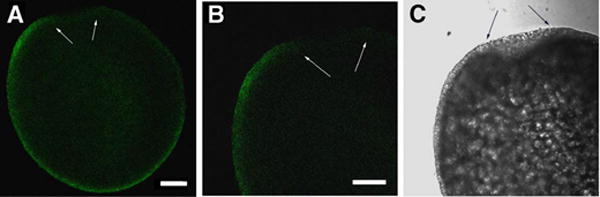
An unfertilized egg labeled with the clone 28 antibody as in Figure 2 was visualized with a 4X objective to demonstrate the entire egg periphery (A) and at higher magnification to show more detail (B,C). The arrows indicate the micropylar region while the micropyle itself was removed with the chorion by proteolysis and dissection. Magnification is indicated by the bar which represents 100μm.
Effect of fertilization on the distribution of activated SFKs
Biochemical analysis has demonstrated that fertilization triggers activation of SFKs in eggs of marine invertebrates, frogs, and zebrafish (Runft et al., 2002). In order to visualize the subcellular distribution of these active kinases, we prepared samples for immunofluorescence analysis before and at different times after fertilization. Samples included over twenty eggs or zygotes at each stage of egg activation and were collected from approximately fifty different females as part of three replicate experiments. In order to monitor changes in fluorescence intensity during egg activation, it was necessary to remove the chorion by microdissection to ensure consistent antibody access. As seen at low magnification in Figure 4, fertilization triggered rapid activation of Src-family PTKs through the cortical cytoplasm underlying the micropylar region and the animal pole (Figure 4A,B). Kinase activation progressed toward the vegetal pole and, by eight minutes post-insemination, the entire egg cortex exhibited elevated Src-family kinase activity (Figure 4C,D). Examination at higher magnification demonstrated that the intensity of antibody labeling under the micropylar region was originally very low but increased abruptly within 30 seconds of fertilization (Figure 5). The progressive activation of kinase activity seemed to originate from the micropylar region raising the possibility that this event might have been initiated by contact or fusion with the fertilizing sperm.
Figure 4.
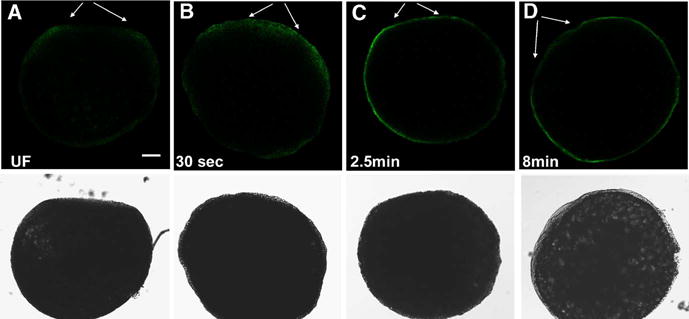
Effect of fertilization on the distribution of active Src-family PTKs in the zebrafish egg. Eggs were fixed before (A), and at 30 seconds (B), 2.5minutes (C), and 8 minutes (D) after insemination and the chorion was removed by dissection after protease treatment to ensure complete access for immunostaining. The eggs were immunolabelled with the clone 28 antibody as described in Figure 2. Conditions of illumination and signal amplification in the fluorescence channel were maintained at a constant level to allow comparison of immunofluorescence intensity fro egg to egg. Eggs were oriented with the animal pole toward the top and the micropylar region is indicated by arrows. Magnification is indicated by the bar which represents 100 μm.
Figure 5.
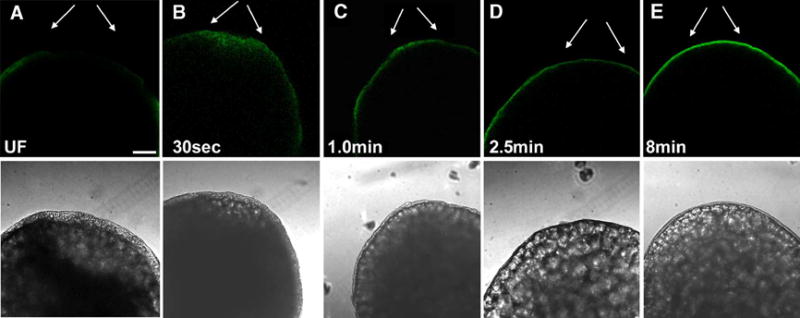
Effect of fertilization on the distribution of active Src-family PTKs at the animal pole.
Eggs were fixed before (A) and at 30 seconds (B), 1.0minute (C), 2.5minutes (D), and 8 minutes (E) after insemination and the chorion was removed by dissection after protease treatment to ensure complete access for immunostaining. The eggs were immunolabelled with the clone 28 antibody as described in Figure 2. Conditions of illumination and signal amplification in the fluorescence channel were maintained at a constant level to allow comparison of immunofluorescence intensity. Arrows indicate the position of the micropylar region and magnification is indicated by the bar, which represents 100μm.
In order to relate changes in kinase activation to the position of the fertilizing sperm, it was necessary to leave the chorion in place since its removal by microdissection usually dislodged the fertilizing sperm. Therefore, we used pronase to permeabilize the chorion and allow antibody access but did not dissect the chorion away. This method was better suited to visualization of the pattern of immunofluorescence than for comparison of immunofluorescence intensity from egg to egg. Prior to fertilization, the micropyle appeared as a funnel-like depression in the chorion that extended into a region of yolk-free cortical cytoplasm (Figure 6). The intensity of clone-28 immunofluorescence in the vicinity of the micropyle was consistently lower than in the adjacent cortical cytoplasm, though it was still detectable. Upon fertilization, the fertilizing sperm could be detected in the cortical cytoplasm underlying the micropyle where it appeared as a blue dot due to DAPI staining (white arrows). During this period, the intensity of clone-28 immunofluorescence under the micropyle increased in a diffuse area around the fertilizing sperm. By sixty seconds, post-insemination, the intensity of clone 28 immunofluorescence in the clear cytoplasm underlying the micropyle had increased further. In addition, fluorescence also became concentrated as a layer in the cortex deep to the layer of filamentous actin (Hart and Collins, 1991; Hart and Donovan, 1983; Hart and Fluck, 1995) which was visualized with Alexa Fluor 568-phalloidin (red). After the first minute, the region of more intense clone 28 immunofluorescence began to expand laterally as seen in Figure 4 and 5 above.
Figure 6.
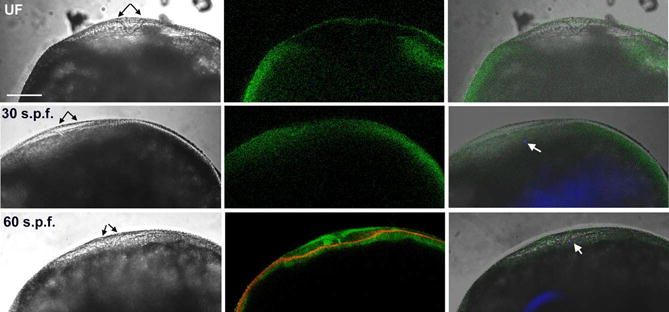
Effect of Fertilization on the distribution of active Src-family PTKs near the point of sperm entry.
Eggs were fixed before and at 30 seconds or 60seconds post-insemination. The chorion was permeabilized by proteolysis but not removed so that the micropyle could be accurately visualized. Eggs were immunolabeled with the clone 28 antibody to detect active Src-family PTKs followed by goat anti-mouse Alexa 488 (green). Sperm chromatin was labeled with DAPI (blue) and in some cases, actin was detected by binding of alexa Fluor 568-phalloidin (red). Conditions of illumination and fluorescent signal amplification were adjusted to optimize visualization of the pattern of immunofluorescence and are not intended for comparison of the intensity of immunofluorescence. All eggs were oriented with the micropyle (black arrows) toward the top of each panel. The sperm head, stained with DAPI, is indicated by a white arrow. Magnification is indicated by the bar which represents 100μm.
Changes in Src-family PTK activity during late egg activation
The later stages of egg activation in zebrafish include extensive redistribution of cytoplasm to form the blastodisc as well as cell cycle events associated with the initiation of mitosis. As seen above, the area of intense clone 28 immunofluorescence in the zygote cortex progressively increased from the animal pole to the vegetal pole during the first few minutes after fertilization. Fluorescence intensity appeared to reach a maximum at eight to ten minutes post-insemination, then decreased somewhat between eight and 20 minutes after insemination (Figure 7A). This pattern resembled previous results obtained by biochemical analysis of PTK activity (Kinsey et al., 2003; Wu and Kinsey, 2001). The blastodisc formed as clear cytoplasm streamed toward the animal pole at approximately 35–40 minutes post-insemination. The blastodisc is apparent in Figure 7B as a blister-like accumulation of clear cytoplasm indicated by the arrows. Clone-28 immunofluorescence became concentrated in the cortex of the blastodisc and was less intense over the rest of the zygote (Fig 7B). Interestingly, no changes in SFK activity were observed in areas of cytoplasmic streaming which are responsible for formation of the blastodisc (Leung et al., 1998; Leung et al., 2000). Cytokinesis began approximately 50 minutes post-insemination and, as seen in Figure 7C, the distribution of clone 28 immunofluorescence was no longer restricted to the cortical cytoplasm and became distributed into the clear cytoplasm near the apex of the newly forming blastomeres.
Figure 7. Localization of Active SFKs between 20 and 50 minutes of development.
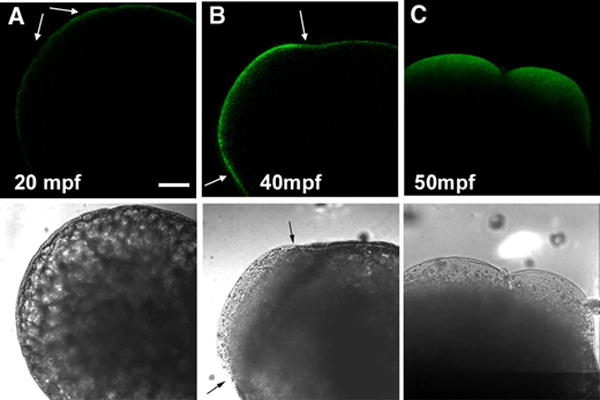
Eggs were fixed at 20 minutes (A), 40 minutes (B), and 50 minutes (C) after insemination. Immunolabeling with the clone 28 antibody was performed after chorion removal as described for Figure 2. Conditions of illumination and signal amplification in the fluorescence channel were maintained at a constant level to allow comparison of immunofluorescence intensity. The animal pole in panels (A and B) is oriented to the upper left. The animal pole in panel (C) is oriented toward the upper right. Magnification is indicated by the bar which represents 100μM.
Response of the egg to hypotonic activation
The unfertilized zebrafish egg is normally maintained in an iso-osmotic environment in the female oviduct and, when exposed to a hypotonic environment upon spawning, it becomes activated by the hypotonic conditions. This activation process normally occurs over 30 to 45 seconds and sperm must fertilize the egg during this period or the micropyle will be obstructed by glycoproteins in the perivitelline space. Hypotonic activation triggers a calcium transient that appears to mimic that induced at fertilization and results in apparently normal blastodisc expansion (Lee et al., 1999; Webb and Miller, 2000) although subsequent development is arrested. Since hypotonic activation mimics some aspects of fertilization, it is important to determine whether biochemical activation also occurs without a fertilizing sperm. Biochemical analysis demonstrated previously that Fyn kinase is not fully activated by hypotonic activation (Wu and Kinsey, 2001). In order to evaluate the effect of hypotonic treatment on the spatial pattern of SFK activation as visualized by clone 28 immunostaining, we compared eggs fertilized with sperm to those activated by water only. The results in Figure 8 show that the response to activation by sperm (A–C) was greater than that to water only (D–F) during the first eight minutes post insemination. This indicates that the sperm delivers unique signals to the egg that are not fully duplicated by hypo-osmotic activation.
Figure 8. Response to activation by sperm or hypotonic conditions.
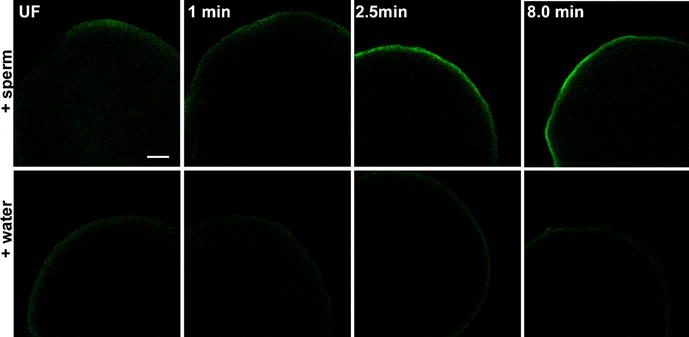
Eggs were fixed for immunofluorescence analysis before (UF) or at 60 seconds, 2.5 minutes, or eight minutes after activation by sperm and water (+sperm) or water alone (+water). Immunolabeling with the clone 28 antibody was performed after chorion removal and conditions of illumination and signal amplification in the fluorescence channel were maintained at a constant level to allow comparison of immunofluorescence intensity. All zygotes were oriented with the animal pole toward the top of each panel. Magnification is indicated by the bar, which represents 100μM.
Discussion
Biochemical methods used to quantitate Src-family kinase activity have several advantages including ease of quantitation and the possibility of discriminating among Src-family PTK members. However, this type of analysis provides limited information as to the part of a cell that is involved in a particular signal transduction event. For example, biochemical analysis of eggs, from several species has demonstrated that fertilization triggers activation of Src-family PTKs that fractionate in the membrane compartment (Kinsey, 1984; Wu and Kinsey, 2001) and, in some cases, to microdomains in the egg plasma membrane (Belton, et al., 2001; Sato et al., 2002;). The advent of highly specific antibodies that recognize the C-terminal regulatory phosphorylation site of Src-family PTKs has provided a method to study the relative activation state of these kinases by immunofluorescence. The clone 28 antibody used in this study has also been used to demonstrate highly localized changes in Src-family PTK activity in tissues ranging from kidney, peripheral nerve, endometrium, and various cancers (Takikita-Suzuki et al., 2003; Zhao et al., 2003; Ito et al., 2002; Yamada et al., 2000; Yamamoto et al., 2002). One limitation is that, since the sequence of amino acids flanking the C-terminal tyrosine is highly conserved among Src-family members, this antibody cannot differentiate among the different family members. However, only a limited number of Src-family PTKs have been detected in eggs of different species. For example, in starfish oocytes, three Src family PTKs are expressed and are activated with different kinetics, but only two are required for calcium signaling at fertilization (O’Neill et al., 2004). In mouse oocytes, only Fyn, and Yes PTKs were found to be expressed at high levels (Mehlmann and Jaffe, 2005), while Fyn, Yes, and Src were detected in rat oocytes (Talmor-Cohen et al., 2004). In zebrafish eggs, we have detected Fyn (Wu and Kinsey 2001) and Yes kinases (Tsai et al., 2005), but have been unable to detect Src, Fgr, and Hck proteins by Western blot or immune complex assays using antibodies directed against the mammalian homologs (unpublished). Therefore, the kinases detected by the clone 28 antibody in the zebrafish egg probably include Fyn and Yes although other Src family PTKs may contribute to our results.
Our present findings indicate that Src-family PTKs are highly enriched in the cortical region of the zebrafish egg. This appeared to involve not only the plasma membrane but also the thin layer of clear cytoplasm deep to the plasma membrane. Since we have previously found that Fyn and Yes kinases are mostly bound to membranous subcellular fractions of the zebrafish egg, it is likely that these kinases are located in the plasma membrane and the cortical endoplasmic reticulum. The distribution of active Src-family PTKs was not uniform around the egg periphery, with one half to two thirds of the egg periphery usually exhibiting slightly higher activity than the other. However, the region that consistently exhibited the highest concentration of active Src-family PTKs was at or near the presumptive animal pole and adjacent to the micropyle. The micropylar region where the fertilizing sperm would make first contact had barely detectible levels of active SFKs. Together these observations demonstrate that the polarized nature of the zebrafish egg is reflected even in the signal transduction machinery involving Src-family PTKs.
Fertilization triggered activation of Src-family PTKs in most species where it has been examined biochemically (Kinsey, 1996; Runft et al., 2002; Sato et al., 2000). In zebrafish eggs, elevated Src-family PTK activity was detected biochemically as early as thirty seconds post insemination, and increased four to five fold over the first eight minutes (Kinsey et al., 2003). This period of egg activation involved multiple changes in the egg that are required for sperm egg adhesion and fusion (five seconds post-insemination), sperm incorporation (5–10 seconds post-insemination), the cortical reaction (thirty -sixty seconds post-insemination), and meiosis II resumption (ten minutes post-insemination) which have been elegantly described (Hart and Yu, 1980; Wolenski and Hart, 1987; Hart, 1990). It was therefore of interest to determine which compartment(s) of the egg exhibited increased kinase activity and whether activation occurred uniformly across the egg or progressively from the site of sperm contact. Our immunofluorescence results indicate that the initial response of Src-family PTK activation occurred in a diffuse region of the cortical cytoplasm immediately underlying the micropyle. This activation process was apparent as early as thirty seconds post-insemination. The region of elevated Src-family PTK activity then became restricted to the deeper cortical cytoplasm associated with the actin-rich cortical cytoskeleton and spread peripherally to involve the rest of the egg cortex.
While immunofluorescence intensity is subject to many variables, the apparent activity of active Src-family PTKs detected in this study changed in a manner similar to that reported from biochemical studies (Kinsey et al., 2003; Wu and Kinsey, 2001) in that it reached a maximum approximately eight minutes post insemination and declined to a minimum at about twenty minutes post-insemination. Formation of the blastodisc resulted in a second phase of elevated kinase activity and in concentration of the active Src-family PTKs to the blastodisc cortex. No changes in activity were associated with the extensive cytoplasmic streaming mechanism that produced the blastodisc. Cytokinesis was associated with a change in the distribution of active Src-family PTKs such that immunofluorescence was distributed deeper into the cytoplasm adjacent to the cleavage plane and was not restricted to the thin cortical layer.
The data reported here provides visual demonstration of several important aspects of egg biology. First is the polarized nature of the signal transduction mechanism in the zebrafish egg. It has been known for some time that eggs from some invertebrate and vertebrate species are polarized morphologically and functionally (Albertini and Barrio, 2004) particularly in respect to the distribution of molecules involved in later embryonic development. We have recently shown that Fyn kinase is required for epiboly which begins five hours after fertilization in zebrafish, so the availability of a pool of Src family PTKs at the animal pole would facilitate this process. However, this would not explain why this pool of kinase at the animal pole would be active even in the unfertilized egg. It seems more likely that the pool of active Src family kinase at the animal pole indicates a function either in maintaining overall animal-vegetal polarity or in precisely marking the region in which the blastodisc will form. Our results add an important component to the list of signaling molecules which respond to the fertilizing sperm and may play other important roles later in development. A second important observation was the fact that the activation of Src-family PTK activity was initiated at the point of sperm incorporation, then extended into the deeper cortical cytoplasm and laterally to encompass the entire zygote. Prior to fertilization, cortical cytoplasm underlying the micropyle appears specialized in that it is relatively free of yolk and contains a filamentous actin meshwork which forms a network around the sperm head (Hart et al., 1992). This region of yolk free cytoplasm exhibited very low Src family PTK activity before fertilization, but was the first region to respond to the fertilizing sperm via increased Src family kinase activity. The function of this early burst of Src family kinase activity in the micropylar region could relate to initiation of calcium signaling or it could act as a trigger for the large scale kinase activation that follows. It is less likely to be involved with sperm incorporation since PTK inhibitor studies performed in eggs from different species (Kinsey, 1997) and in zebrafish (Kinsey, unpublished observations), did not reveal defects in sperm incorporation. The mechanism of this initial kinase activation step clearly involved dephosphorylation of the C-terminal tyrosine since binding of the clone 28 antibody requires dephosphorylation, but while rPTPα has been implicated in this process at the level of the entire egg (Wu and Kinsey, 2002), the initial trigger for this highly localized event remains unclear.
Perhaps the most interesting observation resulting from these studies is the fact that activation of Src-family PTKs was propagated from the initial point of sperm-egg contact to involve the entire cortex of the zygote. Prior to this observation, we expected that sperm-egg contact would trigger only localized activation of SFKs which would stimulate a self-propagating wave of PLCγ-mediated calcium release to activate the egg (in non-mammals). The present study is the first demonstration that Src-family PTK activity can be amplified from an initial, localized stimulus in eggs. Clearly, the progressive activation of Src-family PTKs across the egg cortex raises the question of how this rapid and unique biochemical amplification is accomplished. The propagated activation of Src kinase activity has recently been described in cultured endothelial cells triggered by mechanical stimulation of integrin-cytoskeleton complexes (Wang et al., 2005). The reported activation wave was thought to be transmitted by tension applied through the cortical actin network. A similar mechanism is possible in the zebrafish egg since fertilization does trigger a series of three or four contractions during the first ten minutes post-insemination. However, other potential mechanisms could involve a positive feedback loop in which increased kinase activity in one region could result in diffusion of a soluble phosphorylated protein that would trigger activation of Src family PTKs in the adjacent cytoplasm. Future studies will hopefully identify a mechanism by which kinase activity could be propagated through the zygote cortex.
Acknowledgments
We would like to acknowledge the technical expertise of Cory L. Lewis. Supported by NIH-CHD HD 14846 to W.H.K.
Grant Support: RO1 HD014846, S10 RR019279
References
- Abassi Y, Carroll D, Giusti AF, Belton R, Foltz KR. Evidence that Src-type tyrosine kinase activity is necessary for calcium release at fertilization in sea urchin eggs. Dev Biol. 2000;15:206–219. doi: 10.1006/dbio.1999.9582. [DOI] [PubMed] [Google Scholar]
- Albertini D, Barrio S. Developmental origins of mammalian oocyte polarity. Sem in Cell Dev Biol. 2004;15:599–606. doi: 10.1016/j.semcdb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Belton RJ, Jr, Adams NL, Foltz KR. Isolation and characterization of sea urchin egg lipid rafts and their possible function during fertilization. Mol Reprod Dev. 2001;59:294–305. doi: 10.1002/mrd.1034. [DOI] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem. 1999;274:29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- Giusti AF, O’Neill FJ, Yamasu K, Foltz KR, Jaffe LA. Function of a sea urchin egg Src family kinase in initiating Ca2+ release at fertilization. Dev Biol. 2003;256:367–378. doi: 10.1016/s0012-1606(03)00043-5. [DOI] [PubMed] [Google Scholar]
- Glahn D, Mark SD, Behr RK, Nuccitelli R. Tyrosine kinase inhibitors block sperm-induced egg activation in Xenopus laevis. Dev Biol. 1999;205:171–180. doi: 10.1006/dbio.1998.9042. [DOI] [PubMed] [Google Scholar]
- Hart NH. Fertilization in teleost fishes: mechanisms of sperm-egg interactions. Int Rev Cytol. 1990;121:1–66. doi: 10.1016/s0074-7696(08)60658-0. [DOI] [PubMed] [Google Scholar]
- Hart NH, Collins GC. An electron-microscope and freeze-fracture study of the egg cortex of Brachydanio rerio. Cell Tissue-Res. 1991;265:317–328. doi: 10.1007/BF00398079. [DOI] [PubMed] [Google Scholar]
- Hart NH, Donovan M. Fine Structure of the chorion and site of sperm entry in the egg of brachydanio. J Exp Zool. 1983;227:277–296. [Google Scholar]
- Hart NH, Fluck R. Cytoskeleton in teleost eggs and early embryos: Contributions to cytoarchetecture and motile events. Curr Top Dev Biol. 1995;31:343–381. doi: 10.1016/s0070-2153(08)60233-1. [DOI] [PubMed] [Google Scholar]
- Hart NH, Yu SF. Cortical granule exocytosis and cell surface reorganization in eggs of Brachydanio. J Exp Zool. 1980;213:137–159. doi: 10.1002/jez.1402130114. [DOI] [PubMed] [Google Scholar]
- Hart NH, Becker KA, Wolenski JS. The sperm entry site during fertilization of the zebrafsh egg: localization of actin. Mol Reprod Dev. 1992;32:217–228. doi: 10.1002/mrd.1080320306. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kawakatsu H, Takeda T, Tani N, Kawaguchi N, Noguchi S, Sakai T, Matsuura N. Activation of c-Src is inversely correlated with biological aggressiveness of breast carcinoma. Breast Cancer Res Treat. 2002;76:261–267. doi: 10.1023/a:1020860221099. [DOI] [PubMed] [Google Scholar]
- Kawakatsu H, Sakai T, Takagaki YSY, Saito M, Owada H, Yano J. A new monoclonal antibody which selectively recognizes the active form of Src tyrosine kinase. J Biol Chem. 1996;271:5680–5685. doi: 10.1074/jbc.271.10.5680. [DOI] [PubMed] [Google Scholar]
- Kinsey WH. Regulation of tyrosine-specific kinase activity at fertilization. Dev Biol. 1984;105:137–143. doi: 10.1016/0012-1606(84)90269-0. [DOI] [PubMed] [Google Scholar]
- Kinsey WH. Biphasic activation of Fyn kinase upon fertilization of the sea urchin egg. Dev Biol. 1996;174:281–287. doi: 10.1006/dbio.1996.0073. [DOI] [PubMed] [Google Scholar]
- Kinsey WH. Tyrosine kinase signaling at fertilization. Biochem Biophys Res Comm. 1997;240:519–522. doi: 10.1006/bbrc.1997.7586. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Shen SS. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Dev Biol. 2000;225:253–264. doi: 10.1006/dbio.2000.9830. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Wu W, Macgregor E. Activation of Src-family PTK activity at fertilization: role of the SH2 domain. Dev Biol. 2003;264:255–262. doi: 10.1016/j.ydbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Lee KW, Webb SE, Miller AL. A wave of free cytosolic calcium traverses zebrafish eggs on activation. Dev Biol. 1999;214:168–180. doi: 10.1006/dbio.1999.9396. [DOI] [PubMed] [Google Scholar]
- Leung CF, Webb SE, Miller AL. Calcium transients accompany ooplasmic segregation in zebrafish embryos. Dev Growth, & Diffr. 1998;40:313–326. doi: 10.1046/j.1440-169x.1998.t01-1-00007.x. [DOI] [PubMed] [Google Scholar]
- Leung CF, Webb SE, Miller AL. On the mechanism of ooplasmic segregation in single-cell zebrafish embryos. Dev Growth & Differ. 2000;42:29–40. doi: 10.1046/j.1440-169x.2000.00484.x. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kinsey WH. Effects of protein tyrosine kinase inhibitors on egg activation and fertilization-dependent protein tyrosine kinase activity. Dev Biol. 1995;168:1–10. doi: 10.1006/dbio.1995.1056. [DOI] [PubMed] [Google Scholar]
- O’Neill FJ, Gillet J, Foltz K. Distinct roles of multiple Src family kinases at fertilization. J Cell Science. 2004;117:6227–6238. doi: 10.1242/jcs.01547. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: Where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Sato K, Yoshino K, Oshiro N, Hirahara S, Mahbub Hasan AK, Iwasaki T, Ueda Y, Iwao Y, Yonezawa K, Fukami Y. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J Biol Chem. 2005;280:15029–15037. doi: 10.1074/jbc.M410538200. [DOI] [PubMed] [Google Scholar]
- Sato K, Iwao Y, Fujimura T, Tamaki I, Ogawa K, Iwasaki T, Tokmakov AA, Hatano O, Fukami Y. Evidence for the involvement of a Src-related tyrosine kinase in Xenopus egg activation. Dev Biol. 1999;209:308–320. doi: 10.1006/dbio.1999.9255. [DOI] [PubMed] [Google Scholar]
- Sato K, Iwasaki T, Ogawa K, Konishi M, Tokmakov AA, Fukami Y. Low density detergent-insoluble membrane of Xenopus eggs: subcellular microdomain for tyrosine kinase signaling in fertilization. Development. 2002;129:885–896. doi: 10.1242/dev.129.4.885. [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Fukami Y. Fertilization signalling and protein-tyrosine kinases. Comp Biochem Physiol [B] 2000;126:129–148. doi: 10.1016/s0305-0491(00)00192-9. [DOI] [PubMed] [Google Scholar]
- Sato KI, Tokmakov AA, Iwasaki T, Fukami Y. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol. 2000;224:453–469. doi: 10.1006/dbio.2000.9782. [DOI] [PubMed] [Google Scholar]
- Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Shen SS, Kinsey WH, Lee SJ. Protein tyrosine kinase-dependent release of intracellular calcium in the sea urchin egg. Dev Growth Diff. 1999;41:345–355. doi: 10.1046/j.1440-169x.1999.413436.x. [DOI] [PubMed] [Google Scholar]
- Shilling FM, Carroll DJ, Muslin AJ, Escobedo JA, Williams LT, Jaffe LA. Evidence for both tyrosine kinase and G-protein-coupled pathways leading to starfish egg activation. Dev Biol. 1994;162:590–599. doi: 10.1006/dbio.1994.1112. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G, Courtneidge SA. Structure-function relationships in Src-family and related protein tyrosine kinases. BioEssays. 1995;17:321–330. doi: 10.1002/bies.950170408. [DOI] [PubMed] [Google Scholar]
- Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmor A, Kinsey WH, Shalgi R. Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004;127:455–463. doi: 10.1530/rep.1.00104. [DOI] [PubMed] [Google Scholar]
- Tsai WB, Zhang X, Sharma D, Wu W, Kinsey WH. Role of Yes kinase during early zebrafish development. Dev Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Wang Y, Botvinick E, Zhao Y, Berns M, Usami S, Tsien R, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller A. Calcium signalling during zebrafish embryonic development. BioEssays. 2000;22:113–123. doi: 10.1002/(SICI)1521-1878(200002)22:2<113::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wolenski JS, Hart NH. Scanning electron microscope studies of sperm incorporation into the zebrafish (Brachydanio) egg. J Exp Zool. 1987;243:259–273. doi: 10.1002/jez.1402430211. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil Cytoskeleton. 1995;30:1122–1135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- Wu W, Kinsey WH. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int J Dev Biol. 2001;44:837–841. [PubMed] [Google Scholar]
- Wu W, Kinsey WH. Role of PTPase(s) in regulating Fyn kinase at fertilization of the zebrafish egg. Dev Biol. 2002;247:286–294. doi: 10.1006/dbio.2002.0697. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ozaki Y, Inoue K, Satoh K, Ohmori T, Yatomi Y, Owada K. Differential activation and redistribution of c-Src and Fyn in platelets, assessed by MoAb specific for C-terminal tyrosine-dephosphorylated c-Src and Fyn. Biochim Biophys Acta Mol Cell Res. 2000;1497:27–36. doi: 10.1016/s0167-4889(00)00043-4. [DOI] [PubMed] [Google Scholar]
- Yamada T, Aoyama Y, Owada MK, Kawakatsu H, Kitajima Y. Scraped-wounding causes activation and association of C-Src tyrosine kinase with microtubules in cultured keratinocytes. Cell Struct Funct. 2000;25:351–359. doi: 10.1247/csf.25.351. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Maruyama T, Sakai N, Sakurai R, Shimizu A, Hamatani T, Masuda H, Uchida H, Sabe H, Yoshimura Y. Expression and subcellular distribution of the active form of c-Src tyrosine kinase in differentiating human endometrial stromal cells. Mol Hum Reprod. 2002;8:1117–1124. doi: 10.1093/molehr/8.12.1117. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Takagawa K, Oya T, Sata M. Active Src expression is induced after rat peripheral nerve injury. Glia. 2003;42:184–193. doi: 10.1002/glia.10223. [DOI] [PubMed] [Google Scholar]


