Abstract
AIM
In children with neonatal hypoxic-ischemic encephalopathy (HIE), we examined the association between 18-month functional status by parental report and disability at 6-7 years.
METHOD
Prospective observational study involving participants in the NICHD randomized controlled trial of hypothermia for HIE. Parent questionnaires-Functional Status-II (FS-II), Impact on Family (IOF) and Family Resource Scale (FRS) at 18 months were correlated with 6- to 7-year developmental assessments. Disability at 6-7 years was defined as IQ < 70, gross motor functional classification scale level III-V, bilateral blindness, deafness, or epilepsy.
RESULTS
Rates of severe HIE (32 vs. 15%), public insurance (73% vs. 47%) and IOF scales were higher and mean (SD) FS-II independence (I) {54 (SD 35) vs. 98 (SD 8)} and general health (GH) {87 (SD 14) vs. 98 (SD 6)} scores were significantly lower in children with disability (n=37) at 6-7 years, compared to those (n=74) without disability. FS-II I scores were significantly associated with disability (OR 0.92; 95% CI 0.87-0.97; p=0.003). On path analysis, severe HIE, greater IOF and public insurance were associated with poorer 18-month FS-II I scores, which, in turn, were associated with disability at 6 to 7 years.
INTERPRETATION
Poor independent functioning by parental report at 18 months in children with HIE was associated with childhood disability.
Neonatal hypoxic-ischemic encephalopathy (HIE) has an incidence of 3 to 5 per 1000 live births in the developed world and remains associated with significant mortality and neurodevelopmental sequelae.1,2 The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) whole-body cooling randomized controlled trial (RCT), involving 208 term neonates with moderate or severe HIE demonstrated a significant reduction in death versus moderate or severe disability (44% vs 62%) at 18 to 22 months of age in those neonates that were cooled compared to control participants.3 Follow-up at 6 to 7 years of age revealed a lower, although not statistically significant, rate of death or an IQ below 70 (47% vs 62%) and significantly lower rates of death (28% vs 44%) and death or severe disability (41% vs 60%) among children who underwent cooling compared to control participants.4
The NICHD workshop on follow-up of high-risk infants emphasized the need for alternative, less costly methods for follow-up assessment that could be used by community physicians and the need to measure interventions and outcomes from the family's perspective in high-risk populations.5 Standardized parental functional status questionnaires are potentially useful instruments that could address these needs.6,7 The functional status questionnaires provide information on typical performance and routine activities and can be administered easily.6 Previous studies have demonstrated the validity and correlation of such instruments with formal classifications of impairment, and have shown that parents are reliable sources regarding their child's functional status.8–10 It is also recognized that family or environmental characteristics and external support may be associated with outcomes.5
In the current study, we compared groups of surviving children with no/mild disability and those with moderate/ severe disability at 6 to 7 years of age in the following three areas: (1) functional status; (2) the impact on the family; and (3) family resources, by parental report at the 18- to 22-month follow-up visit. We also examined the association between functional status at 18 months of age and disability at 6 to 7 years of age, while controlling for neonatal and family variables and impact on the family. We hypothesized that: (1) parental report of functional status impairment and higher impact on the family at 18 to 22 months of age would be associated with moderate or severe disability at 6 to 7 years of age; and (2) HIE-related variables and impact on the family would predict functional status at 18- to 22-months of age that, in turn, would be significantly associated with childhood disability.
METHOD
This was a secondary analysis of pre-existing prospectively collected data from the 18- to 22-month and 6- to 7-year follow-up visits of infants enrolled in the NICHD NRN whole-body cooling RCT.3 The study population included all infants who met the following criteria: (1) enrolled in the NICHD NRN whole-body cooling RCT between July 2000 and May 2003; (2) who underwent follow-up testing at 18 to 22 months of age and had any of the Stein and Jessop Functional Status II (FS-II), Impact on the Family Scale (IOFS) or Family Resource Scale (FRS) questionnaires completed by the caregiver at the time; and (3) had follow-up data available at 6 to 7 years of age (between August 2006 and August 2010). Eligibility criteria for the RCT included biochemical and clinical screening criteria, and moderate or severe encephalopathy or seizures. Infants were randomly assigned to either whole-body cooling to 33.5°C for 72 hours (initiated within 6h of age, followed by rewarming over 6h) or to conventional care.
At the 18- to 22-month follow-up visit or by phone at a separate time within the study window, three questionnaires (FS-II, IOFS, and FRS) were administered by trained interviewers to the primary caregiver of the child.11–16 Interviewers at each site were trained by a primary coordinator in the administration of the questionnaires.
The Functional Status II questionnaire
The revised FS-II is an instrument to measure health status in children with ongoing chronic illnesses (The Functional Status II Measure is copyright by REK Stein, CK Riessman and DJ Jessop, 1981, 1991).11,12 The FS-II has been validated on 732 children aged 0 to 16 years with and without chronic physical conditions.11 Means and standard deviations for ‘ill’ and well children are available. The FS-II addresses elements such as communication, mobility, mood, energy, sleep, and eating, and assesses parents’ perceptions of their child in four functional domains (i.e. physical, psychological, social, and cognitive functioning). The parent responds on a 3-point categorical Likert scale. If the initial response reveals difficulty with tasks, then, in the second part of the questionnaire, the parent is asked to rate the extent to which the difficulty is a result of the illness. The FS-II generates a score expressed as the percentage of points that the child obtains of the total possible points for scales of general health (18 items) and responsiveness or independence (11 items). A lower score reflects lower functional status.
The Family Resource Scale
The FRS, developed by Dunst and Leet, is an instrument with 31 questions to evaluate the resources available to households with children.13 Each question rates the adequacy of a resource on a 0 to 5 point Likert scale as ‘does not apply’ to ‘almost always adequate’.
Resources assessed include food, clothing, housing, water and heat, dependable transportation, furniture, money for various needs, and social support for the care-giver. The revised FRS analysis has a four-factor structure that includes basic needs, money, time for self, and time for family;14 lower scores reflect lower resources.
The Impact on the Family Scale, G version
This questionnaire is a 27-item scale developed for parents or caregivers of children with medical conditions.15,16 The scale assesses financial burdens on families with children with medical conditions as well as emotional concerns and positive outcomes. It uses a 4-point Likert-type scale. For example, queries include whether the respondent agrees to fatigue being a problem, traveling to hospital being a strain, and having no time for other family members. A summary score and four subscales (financial impact, familial-social impact, personal strain, and coping or mastery) are calculated. The scale demonstrated good construct validity and internal consistency varied from 0.80 for total impact, 0.68 to 0.79 for financial subscale, to 0.46 to 0.52 for coping. Higher scores indicate greater impact of the illness on the family.
Study interventions and outcomes at 6 to 7 years of age
A neurological examination and IQ testing were performed by trained examiners, blinded to the original treatment assignment status. Cerebral palsy (CP) was classified on the basis of the Surveillance of Cerebral Palsy in Europe Network decision tree.17 Functional activity was graded according to Gross Motor Function Classification System (GMFCS) levels and IQ scores were measured using the Wechsler Preschool and Primary Scale of Intelligence III or the Wechsler Intelligence Scales for Children IV. Severe disability was defined as any of the following: an IQ score more than 3SD below the mean score (i.e. <55), a GMFCS level of IV or V, or bilateral blindness.4 Moderate disability was defined as an IQ score 2 to 3SD below the mean score (i.e. 55–69), a GMFCS level of III, bilateral deafness (with or without amplification), or refractory epilepsy (defined as clinical or electroencephalographic seizure disorder requiring anticonvulsant therapy).4 Mild disability was defined as an IQ score 1 to 2SD below the mean score (i.e. 70–84) or a GMFCS level of I or II.4 No disability was defined as an IQ score of more than 84 (i.e. >1SD below the mean) with no CP, hearing or visual deficits, or epilepsy. The primary outcome for this secondary study was moderate or severe disability, in comparison to no or mild disability.
Statistical analysis
Descriptive data were expressed as median (range), mean (SD), and number (percent) as appropriate. We conducted bivariate analyses (Χ2, Student's t-tests, and non-parametric tests, as appropriate) to compare neonatal and family factors, 18-month FS-II, IOFS, and FRS scores among children with and without moderate/severe disability at 6 to 7 years of age. We conducted receiver operating curve analysis to determine the optimal cut-off points on the FS-II general health and independence scales for predicting moderate/severe disability at 6 to 7 years of age and calculated positive and negative predictive values. Logistic multilevel regression models were conducted to examine the relationship between FS-II scores at 18 months of age and moderate/severe disability at 6 to 7 years of age, after adjustment for neonatal variables (sex, severity of HIE, cooling therapy) and family variables found to be significantly different, also accounting for clustering of children within centers. Maximum likelihood estimation procedures were used to produce unbiased estimates when data were missing. A p-value of <0.05 was considered to be significant.
We also tested a structural equation model (path analysis) using the M-plus software program (Muthén & Muthén, Los Angeles, CA, USA) to explore the relationships between neonatal variables, public insurance, 18-month FRS, IOFS, and FS-II results, and disability at 6 to 7 years of age (none, mild, moderate, and severe). To test for the model fit, values for the comparative fit index, Tucker–Lewis Index and the root mean square error of approximation were calculated. To have acceptable fit, the comparative fit index and Tucker–Lewis index should have values of 0.90 or greater and the root mean square error of approximation should be 0.08 or less.
RESULTS
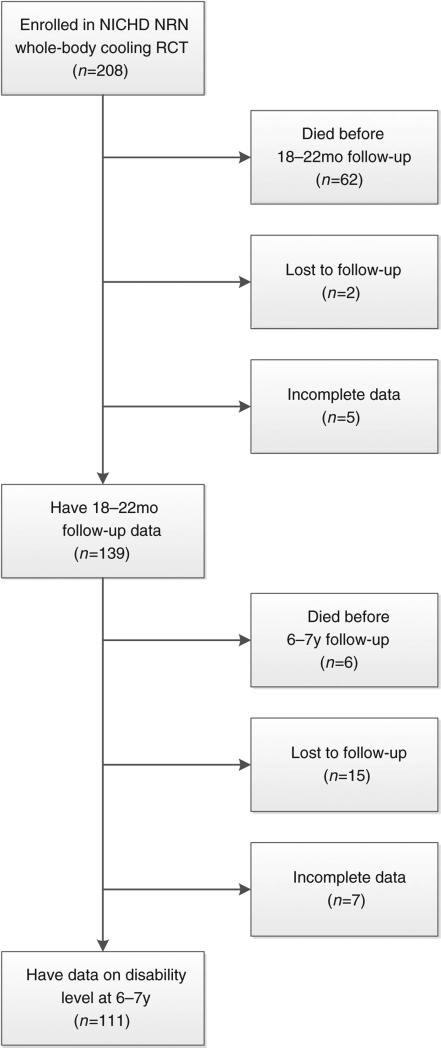
Of the 208 infants enrolled in the NICHD NRN whole-body cooling RCT from 15 participating sites, two were lost to follow-up, five had incomplete data, and 62 died before the 18- to 22-month follow-up study. Of the remaining 139 infants, six died before the 6 to 7 years follow-up visit, 15 were lost to follow-up, and seven did not have complete follow-up data. The remaining 111 children comprised the study cohort: 60 (54%) males and 51 (46%) females, with a mean gestational age of 39 weeks (SD 2wks) and birthweight of 3379g (Fig. 1). Of these, 37 (33.3%) children had severe (n=22) or moderate (n=15) disability and 74 had mild (n=25) or no (n=49) disability. Table I shows the number and proportions of participants with various neurodevelopmental outcomes at 6 to 7 years of age. There were no significant differences between the 22 children lost to follow-up or with incomplete data at 6 to 7 years and the 111 children included in the analysis in sex distribution, ethnic group, maternal education, income, public insurance, severity of HIE, birthweight, gestational age, or maternal age.
Figure 1.
Sample selection flowchart. NICHD, National Institute of Child Health and Human Development; NRN, Neonatal Research Network.
Table I.
Neurodevelopmental outcomes at 6 to 7 years of age
| Outcome | n (%) |
|---|---|
| Disability level | |
| None | 49 (44) |
| Mild | 25 (23) |
| Moderate | 15 (14) |
| Severe | 22 (20) |
| IQ score | |
| <55 | 22 (20) |
| 55–69 | 8 (7) |
| 70–84 | 27 (24) |
| ≥85 | 54 (49) |
| GMFCS | |
| Level I or II | 3 (3) |
| Level III | 4 (4) |
| Level IV or V | 17 (15) |
| Bilateral blindness | 3 (3) |
| Bilateral deafness | 4 (4) |
| Refractory epilepsy | 15 (14) |
GMFCS, Gross Motor Function Classification System.
Comparisons of the neonatal characteristics and 18-month FS-II, FRS and IOFS scores in the two groups of children with no/mild and moderate/severe disability are shown in Table II. Severe HIE and public insurance were significantly more frequent in children who had moderate or severe disability, compared to survivors with no or mild disability at 6 to 7 years of age. Mean (SD) FS-II general health and independence scores, total FRS scales, and the FRS subscale for money were significantly lower in this group. The IOFS reflected a greater total impact, financial impact, and caregiver and family burden, and greater disruption of planning in those who had moderate or severe disability, although coping was similar in both groups. Applying a cut-off point of 76 (i.e. scores less than 76 indicating disability), the positive predictive value of the FS-II independence scale was 92.3 (95% CI 73.5–98.1) and the negative predictive value was 84.7 (95% CI 75.3–91.0). Using a cut-off point of 92, the corresponding values for the FS-II general health scale were 76.7 (95% CI 58.2– 88.7) and 82.7 (95% CI 72.7–89.6).
Table II.
Neonatal and family factors and 18-month functional status and family resources by disability status at 6 to 7 years of age
| Variable | No/mild disability (n=74) | Moderate/severe disability (n=37) | p |
|---|---|---|---|
| Birth | |||
| Birthweight (g), mean (SD) | 3416 (611) | 3307 (559) | 0.36 |
| Gestational age (wks), mean (SD) | 39 (2) | 39 (2) | 0.67 |
| Males/Females, n | 40/34 | 2017 | 1.00 |
| Ethnic group, n (%) | |||
| Black | 24 (32) | 15 (41) | 0.54 |
| White | 32 (43) | 12 (32) | |
| Hispanic | 16 (22) | 10 (27) | |
| Other | 2 (3) | 0 (0) | |
| Severe HIE, n (%) | 11 (15) | 12 (32) | 0.031 |
| Cooling therapy, n (%) | 44 (59) | 21 (57) | 0.79 |
| Maternal age, y, mean (SD) | 27 (6) | 25 (6) | 0.09 |
| 18mo | |||
| Adjusted age at visit, mean (SD) | 20 (4) | 21 (4) | 0.19 |
| Less than high school education, n (%) | 24 (32) | 12 (33) | 0.93 |
| Public insurance, n (%) | 35 (47) | 27 (73) | 0.010 |
| Income <US$20 000/y, n (%) | 24 (32) | 17 (47) | 0.13 |
| Functional Status II, mean (SD) | |||
| Independence | 98 (8) | 54 (35) | <0.001 |
| General health | 98 (6) | 87 (14) | <0.001 |
| Family Resource Scale, mean (SD) | |||
| Total | 134 (16) | 127 (19) | 0.054 |
| Basic needs | 34 (2) | 33 (3) | 0.28 |
| Money | 23 (5) | 20 (7) | 0.04 |
| Time for self | 23 (5) | 21 (7) | 0.06 |
| Time for family | 9 (1) | 9 (1) | 0.78 |
| Impact on Family Scale, mean (SD) | |||
| Total | 26 (8) | 33 (9) | <0.001 |
| Financial impact | 4 (2) | 5 (2) | 0.034 |
| Disruption of planning | 9 (3) | 12 (3) | <0.001 |
| Caregiver burden | 6 (2) | 8 (3) | <0.001 |
| Family burden | 7 (2) | 9 (3) | <0.001 |
| Coping | 13 (2) | 13 (2) | 0.33 |
HIE, hypoxic–ischemic encephalopathy.
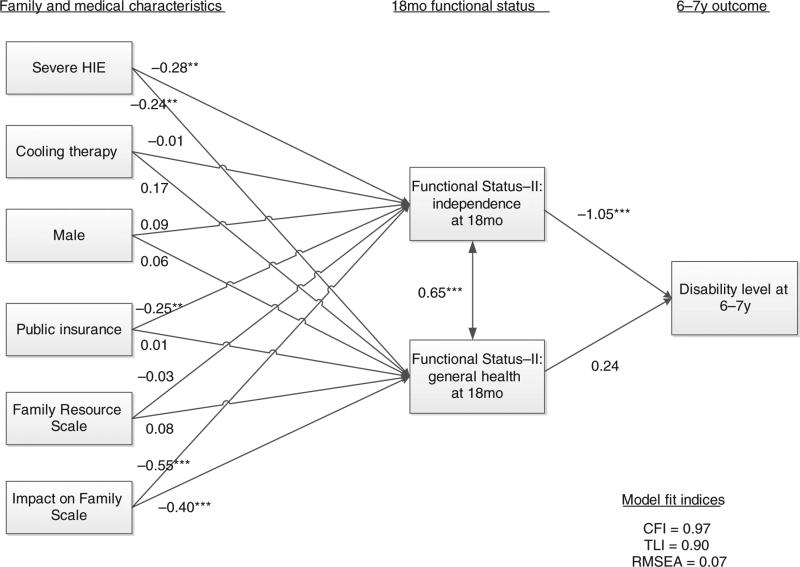
Regression models were used to explore the relationship of neonatal variables, family characteristics, IOFS, and FS-II scores to moderate or severe disability at 6 to 7 years of age (Table III). In the combined model, FS-II independence scores were significantly associated with moderate or severe disability at 6 to 7 years of age; each unit increase in the score was associated with a reduction in moderate or severe disability (OR 0.92; 95% CI 0.87–0.97). The area under the curves for the three models improved from 0.732 to 0.932 on adding FS-II, and to 0.935 when the IOFS data were included. The path analysis (Fig. 2) revealed that children with severe HIE and those whose families reported greater impact had significantly poorer functional status (FS-II independence and general health scores) at 18 months of age. Public insurance was associated with poorer FS-II independence scores. FS-II general health and independence scores at 18 months of age were strongly correlated; independence, but not general health, at 18 months of age was associated with disability at 6 to 7 years of age. The percentage of variance in the disability level accounted for by the model or R-squared was 58%.
Table III.
Logistic regression models of moderate/severe disability at 6 to 7 years of age
| Variable | Adjusted OR (95% CI) | p |
|---|---|---|
| Model 1: Demographic/medical factors | ||
| Severe HIE | 3.20 (1.14–8.97) | 0.027 |
| Cooling therapy | 1.18 (0.49–2.85) | 0.712 |
| Male | 0.92 (0.39–2.18) | 0.845 |
| Public insurance | 3.61 (1.42–9.19) | 0.008 |
| Model 2: Demographic/medical factors + 18mo IOFS and FRS | ||
| Severe HIE | 4.53 (1.20–17.14) | 0.026 |
| Cooling therapy | 1.56 (0.54–4.48) | 0.406 |
| Male | 0.53 (0.19–1.47) | 0.221 |
| Public insurance | 3.55 (1.15–11.01) | 0.029 |
| FRS score | 1.03 (0.99–1.06) | 0.143 |
| IOFS total score | 1.16 (1.07–1.26) | <0.001 |
| Model 3: Demographic/medical factors + 18mo IOF, FRS, and FS-II scales | ||
| Severe HIE | 1.72 (0.25–12.00) | 0.582 |
| Cooling therapy | 3.15 (0.66–14.96) | 0.147 |
| Male | 0.87 (0.21–3.53) | 0.844 |
| Public insurance | 3.74 (0.87–16.03) | 0.075 |
| FRS score | 1.04 (0.98–1.09) | 0.181 |
| IOFS total score | 1.04 (0.93–1.17) | 0.520 |
| FS-II independence score | 0.92 (0.87–0.97) | 0.003 |
| FS-II general health score | 0.98 (0.91–1.06) | 0.619 |
OR, odds ratio; CI, confidence interval; FRS, Family Resource Scale; FS-II, Functional Status II; HIE, hypoxic-ischemic encephalopathy; IOFS, Impact on Family Scale.
Figure 2.
Path diagram of disability level at 6 to 7 years of age. **p<0.01; ***p<0.001. HIE, hypoxic-ischemic encephalopathy; CFI, comparative fit index; RMSEA, root mean square error of application; TLI, Tucker–Lewis index.
DISCUSSION
We utilized data from the NICHD NRN whole-body cooling RCT and the 18- to 22-month and 6- to 7-year follow-up data to explore the relationships between neonatal variables, socio-economic characteristics, and parental perception of functional status and disability in childhood. Severe HIE and greater impact on the family were significantly associated with FS-II general health and independence scores at 18 months of age; FS-II independence scores, in turn, were associated with childhood disability at 6 to 7 years of age.
A previous study has evaluated functional outcomes at 7 to 8 years of age in 62 of 135 surviving children with neonatal HIE who participated in the CoolCap trial of selective head cooling.18 Disability status at 18 months was strongly associated with later functional outcomes measured by WeeFIM ratings.19 Children with favorable 18-month outcomes had a mean (SD) rating of 115 (19), compared to 67 (42) in those with unfavorable outcomes.20 There was no significant effect of cooling treatment.18 Our results showing a significant difference in mean FS-II scores for general health and more so for independence at 18 months of age in groups of children who had moderate/severe disability at 6 to 7 years and others who did not, were, therefore, expected. The significant association between FS-II independence scores and childhood disability suggest that such parental questionnaires may be useful as screening instruments, to plan formal follow-up assessments of complex motor and cognitive functioning, and to identify the need for support services. The FS-II questionnaire has been used in studies on preterm infants, children with asthma, and after traumatic brain injury, among others.8,21,22 It has been shown to correlate with hospitalization, absence days from school, mobility, and morbidity status, and to not be influenced by maternal psychological adjustment and sociodemographic variables.20,23 In a previous study in the NICHD NRN involving 5100 extremely low birthweight children at 18 to 22 months corrected age, general health and independence scales demonstrated good internal consistency and were significantly lower in infants with developmental impairment on formal testing, compared to those without impairments.8 The differences were particularly pronounced for the independence scale.8
Although the social, emotional, and financial impact on the family of caring for a child with neonatal HIE, which is associated with a high likelihood of neurodevelopmental sequelae, may be considerable and crucial, it has not been previously studied. Our finding that families of children with moderate/severe disability at 6 to 7 years of age experienced a significantly greater impact on the financial, family/social, and personal strain subscales is consistent with previous data in other pediatric populations.24 The parents of 96 very low birthweight infants reported greater financial impact, family and social burden, personal strain, and mastery impact compared to term control participants.24 Families were affected more when children had a functional disability or low adaptive developmental quotient.24 In another longitudinal study of 224 preterm low birth-weight infants for the first 6 months after discharge, impact on the family decreased over time and was higher in those with re-hospitalizations.25 Coping scales were comparable in our study between groups of children with and without moderate/severe disability at 6 to 7 years of age. This finding is similar to a previous study by Wake et al.26 in which parents of 80 children with CP responded to the Child Health Questionnaire. Psychosocial health and emotional impact on parents were similar for mild and severe CP. The authors noted that, even for children with mild CP, the need for services, psychosocial problems and learning difficulties may have imposed a burden on the families.26
In children with cancer, differences in functional outcomes and quality of life have been noted in association with socio-economic and educational disparities; these differences in outcomes appear to be mediated by family factors.27 Among 30 preterm infants, higher maternal social support on the FRS scale at 4 months of age was significantly associated with better receptive language function and fewer internalizing behaviors at 36 months of age.28 We found a significant difference in the proportion of public insurance, total, and money FRS scales between the groups with and without disability at 6 to 7 years of age. FRS and income were not significantly associated with childhood outcomes using path and regression analyses. We attribute this finding to the fact that maternal education, FRS social support, and coping or resilience on the IOFS were comparable between groups and are more likely to influence outcome.
The limitations of our study are that that socio-economic data were obtained during the 18-month follow-up and may have varied over the 6- to 7-year period, and that income and education data were by self-report. Our sample size, although small, was adequate for the number of parameters and the simple models assessed.29 There were missing data for 22 children, which may have been a potential source of bias. Details of family structure, health care utilization, and support services were not collected. While our path analysis suggested that children of families that reported greater impact had poorer functional status, the relationship may well be bidirectional.
Our data cannot resolve this issue because the IOFS and FS-II were measured only once and contemporaneously. The questionnaires were administered when the child was 18 months of age, when severe disability may have become apparent and support services may have been instituted. Whether the FS-II is predictive at younger ages, when the potential utility may be greater, remains to be determined. Nonetheless, our study has several strengths. The study population comprised a homogenous group of children diagnosed with moderate or severe HIE using stringent criteria, data were prospectively collected, and follow-up evaluations were performed using certified neurologists and psychologists trained to high reliability. The questionnaires used were previously validated instruments. Most importantly, this is the first systematic exploration of the complex paradigm of disease-related factors, family socioeconomic status and impact, parental perception of functional impairment, and eventual childhood outcomes in the context of neonatal HIE. Our findings support the socioecological systems theory that disease-related outcomes of children may be affected by the stressful impact on families.27 Understanding and mitigating the family burden in high-risk pediatric populations may potentially modify functional outcomes. Such information is important from a policy perspective and could potentially facilitate family-level interventions.
Supplementary Material
What this paper adds
Children with a history of neonatal hypoxic-ischemic encephalopathy (HIE) and moderate/severe disability at age 6 to 7 have lower functional status scores by parental report at 18 months.
Severe HIE and greater impact on the family are significantly associated with lower functional status scores at 18 months.
Lower independent functioning scores at 18 months are associated with childhood disability.
ACKNOWLEDGEMENTS
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provided grant support for the Neonatal Research Network's Whole-Body Hypothermia Trial and its 18- to 22-month and 6-to 7-year follow-up studies through cooperative agreements. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD. Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (data coordinating center Principal Investigator) and Mr Scott A McDonald (data coordinating center Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study: a detailed acknowledgement of all involved investigators is included in Appendix S1.
ABBREVIATIONS
- FRS
Family Resource Scale
- FS-II
Functional Status II
- HIE
Hypoxic-ischemic encephalopathy
- IOFS
Impact on the Family Scale
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
Footnotes
DISCLOSURES
Dr Cotten reports having served on the data and safety monitoring board for the Inhibitex phase III study of Veronate for the prevention of infections in preterm infants. Dr Carlo reports having served on the advisory board of Pediatrix Medical Group and ParadigmHealth and holding stock options at the Pediatrix Medical Group. Dr Stevenson reports having received research support from Pfizer.
SUPPORTING INFORMATION
The following additional material may be found online:
Appendix S1: Additional investigators.
REFERENCES
- 1.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RE, et al. Whole body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Shankaran S, Pappas A, McDonald S, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–92. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Child Health and Human Development. Vohr B, Wright LL, Hack M, Aylward G, Hirtz D. Follow-up care of high-risk infants. Pediatrics. 2004;114(Suppl):1377–97. [Google Scholar]
- 6.Glascoe FP, Dworkin PH. The role of parents in the detection of developmental and behavioral problems. Pediatrics. 1995;95:829–36. [PubMed] [Google Scholar]
- 7.Hack M. Consideration of the use of health status, functional outcome, and quality of life to monitor neonatal intensive care practice. Pediatrics. 1999;103(Suppl. E):319–23. [PubMed] [Google Scholar]
- 8.Da Costa D, Bann CM, Hansen NI, Shankaran S, Delaney-Black V, the National Institute of Child Health and Human Development Neonatal Research Network Validation of the Functional status II questionnaire in the assessment of extremely low birth weight infants. Dev Med Child Neurol. 2009;51:536–44. doi: 10.1111/j.1469-8749.2009.03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones HP, Guildea ZES, Stewart JH, Cartlidge PHT. The health status questionnaire: achieving concordance with published disability criteria. Arch Dis Child. 2002;86:15–20. doi: 10.1136/adc.86.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glascoe FP. Parents’ concern about children's development: prescreening technique or screening test? Pediatrics. 1997;99:522–8. doi: 10.1542/peds.99.4.522. [DOI] [PubMed] [Google Scholar]
- 11.Stein RE, Jessop DJ. Functional status II®. A measure of child health status. Med Care. 1990;28:1041–55. doi: 10.1097/00005650-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Stein REK, Jessop DJ. PACTS Papers. Albert Einstein College of Medicine; Bronx, NY: 1991. Manual for the Functional Status-II® Measure. [Google Scholar]
- 13.Dunst C, Leet H. Measuring the adequacy of resources in households. Child Care Health Dev. 1987;13:111–25. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 14.Van Horn ML, Bellis JM, Snyder SW. Family Resource Scale: revised psychometrics and validation of a measure of family resources in a sample of low-income families. J Psychoeduc Assess. 2001;19:50. [Google Scholar]
- 15.Stein RE, Riessman CK. The development of an impact-on-family scale: preliminary findings. Med Care. 1980;18:465–72. doi: 10.1097/00005650-198004000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Stein RE, Jessop DJ. The impact on family scale revisited: further psychometric data. J Dev Behav Pediatr. 2003;24:9–16. [PubMed] [Google Scholar]
- 17.Cans C. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 18.Guillet R, Edwards AD, Thoresen M, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res. 2012;71:205–9. doi: 10.1038/pr.2011.30. [DOI] [PubMed] [Google Scholar]
- 19.Msall ME, DiGaudio K, Rogers BT, et al. The Functional Independence Measure for Children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clin Pediatr (Phila) 1994;33:421–30. doi: 10.1177/000992289403300708. [DOI] [PubMed] [Google Scholar]
- 20.Palermo TM, Long AC, Lewandowski AS, Drotar D, Quittner AL, Walker LS. Evidence-based assessment of health-related quality of life and functional impairment in pediatric psychology. J Pediatr Psychol. 2008;33:983–96. doi: 10.1093/jpepsy/jsn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keenan HT, Runyan DK, Nocera M. Child outcomes and family characteristics 1 year after severe inflicted or noninflicted traumatic brain injury. Pediatrics. 2006;117:317–24. doi: 10.1542/peds.2005-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy KR, Fitzpatrick S, Cruz-Rivera M, Miller CJ, Parasuraman B. Effects of budesonide inhalation suspension compared with cromolyn sodium nebulizer solution on health status and caregiver quality of life in childhood asthma. Pediatrics. 2003;112(Pt 1):e212–9. doi: 10.1542/peds.112.3.e212. [DOI] [PubMed] [Google Scholar]
- 23.Dadds MR, Stein REK, Silver EJ. The role of maternal psychological adjustment in the measurement of children's functional status. J Pediatr Psychol. 1995;20:527–44. doi: 10.1093/jpepsy/20.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Cronin CM, Shapiro CR, Casiro OG, Cheang MS. The impact of very low-birth-weight infants on the family is long lasting. A matched control study. Arch Pediatr Adolesc Med. 1995;149:151–8. doi: 10.1001/archpedi.1995.02170140033005. [DOI] [PubMed] [Google Scholar]
- 25.Gennaro S. Preterm low-birth weight infants: health and family outcomes. Fam Community Health. 1995;17:12–21. [Google Scholar]
- 26.Wake M, Salmon L, Reddihough D. Health status of Australian children with mild to severe cerebral palsy: cross sectional survey using the Child Health Questionnaire. Dev Med Child Neurol. 2003;45:194–9. doi: 10.1017/s0012162203000379. [DOI] [PubMed] [Google Scholar]
- 27.Litzelman K, Barker E, Catrine K, Puccetti D, Possin P, Witt WP. Socioeconomic disparities in the quality of life in children with cancer or brain tumors: the mediating role of family factors. Psychooncology. 2013;22:1081–8. doi: 10.1002/pon.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miceli P, Goeke-Morey M, Whitman T, Kolberg KS, Miller-Loncar C, White RD. Brief report: birth status, medical complications, and social environment: individual differences in development of preterm, very low birth weight infants. J Pediatr Psychol. 2000;25:353–8. doi: 10.1093/jpepsy/25.5.353. [DOI] [PubMed] [Google Scholar]
- 29.Bentler PM, Chou CP. Practical issues in structural modeling. Sociol Methods Res. 1987;16:78–117. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.