Abstract
Diabetic Retinopathy is one of the hallmark microvascular diseases secondary to diabetes. Endothelial cells and pericytes are key players in the pathogenesis. Interaction between the two cell types is important in the regulation of vascular function and the maintenance of the retinal homeostatic environment. There are currently several approaches to analyze changes in morphology and function of the two cell types. Morphologic approaches include trypsin digest, while functional approaches include studying blood flow. This review explores the advantages and limitations of various methods and summarizes recent experimental studies of EC and pericyte dysfunction in rodent models of DR. An improved understanding of the role played by EC and pericyte dysfunction can lead to enhanced insights into retinal vascular regulation in DR and open new avenues for future treatments that reverse their dysfunction.
Keywords: Endothelial cell, Pericyte, Dysfunction, Rodent, Histopathology, Trypsin digest, Blood flow, Oximetry
84.1 Introduction
Diabetic Retinopathy (DR) is one of the hallmark complications of diabetes and one of the most common causes of blindness worldwide. Endothelial cells (EC) and pericytes are key players in the pathogenesis of DR, and their role in diabetes has been extensively studied. EC line the vasculature, serve as a physical barrier between blood and the surrounding tissue, and have a critical role in maintaining the retinal homeostasis. Pericytes are found in the vascular basement membrane. Their wide-ranging functions include mediating repair to the vasculature, promoting the blood-retinal barrier, and functioning as hypoxia sensors [1]. Interactions between EC and pericytes regulate vascular stabilization and function. In diabetic microangiopathy, dysfunction in either cell type leads to abnormal function of the other, which prompted the development of many techniques to study abnormalities in vascular morphology and function. This review will address the advantages and limitations of the most common methods, and summarize important conclusions from studies in rodent models of DR.
84.2 Experimental Approaches in Rodent Models of Diabetic Retinopathy
A variety of animal species have been used to study the pathogenesis of DR, which have respective advantages and limitations and are described in detail, elsewhere [2]. The rodent is the most widely available animal model, given its cost efficacy and recent availability of imaging methods to study blood flow in vivo in rodents. Currently, there are several morphological and functional approaches to study EC and pericytes in rodents (Table 84.1). It is important to note that although many important abnormalities in the pathogenesis of DR have been identified (e.g. capillary degeneration, altered blood flow), not all retinal lesions that develop in diabetic patients have been reproduced in diabetic rodents (e.g. neovascularization).
Table 84.1.
Summary of morphological/functional approaches
| Morphological approaches | Benefits | Limitations |
|---|---|---|
| Flat mount | Maintains vessel integrity | Non-specific staining, poor resolution of vasculature |
| Trypsin digest | Isolates vasculature; histopa- thology readily seen |
Technically challenging; dif- ficult to differentiate EC/ pericytes |
| Cellular markers | Identifies EC/pericytes | Not 100 % specific; dynamic marker expression |
| Functional approaches | ||
| Imaging FA Hydrogen clearance Intravital microscopy OCT SLO |
Measures blood flow 1. Easy visualization of retinal vessels 2. Can measure regional/ temporal fluctuations 4. Non-invasive |
1–3. Invasive; injections may alter hemodynamics 4. Poor resolution with cataracts |
| Oximetry Microelectrode fMRI PAOM, Phosphorescence quenching |
Measures oxygen tension 1. Gold standard 2–3. Non-invasive 2. Does not require media clarity |
Invasive 1, 3. Requires media clarity |
84.3 Morphological Approaches
Whole retinal flat-mount is a commonly used method, which maintains the blood vessel integrity while vessels are highlighted by staining. However, staining is non-specific and highlights non-vascular tissue, making it difficult to differentiate vessels and to identify pathologies such as capillary degeneration. Injection of dyes has also been used to highlight vessels, but this rarely highlights the entire retinal vasculature unless given at high pressure, which risks damaging the vessels [3].
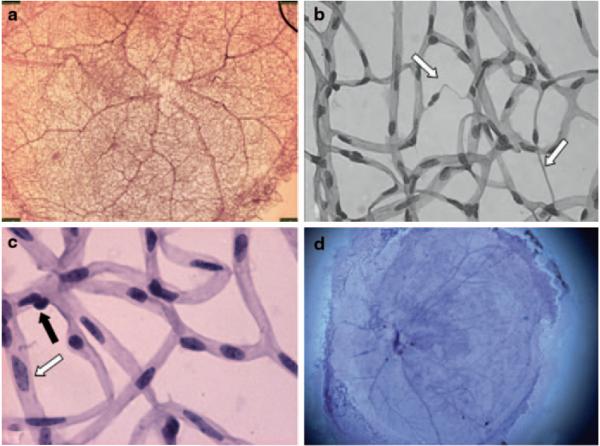
Trypsin digest has become the gold standard to analyze retinal vasculature since its description by Kuwabara and Cogan in 1960 [3]. The method removes the non-vascular tissue leaving only the vascular network intact. The retina is an ideal tissue for trypsin digest as it contains little trypsin-resistant collagen, while the EC and pericytes are protected from trypsin by a mucoproteinaceous wall. As a result, this approach provides the opportunity to study the retinal vasculature in great detail (Fig. 84.1a). Microvascular lesions such as microaneurysms, acellular capillaries, and capillary degeneration have been visualized using the technique (Fig. 84.1b). In streptozotocin-induced (STZ) rats, it has been shown that the first anatomical changes occur after 6 weeks of induction. Initially, there is the development of tortuous swollen vessels, followed by loss of EC and pericytes and subsequent microaneurysms by 28–90 weeks [4].
Fig. 84.1.
Trypsin digest. Flat mount mouse retina stained with H&E. a db/m mouse, normal retina (20 ×). b db/db mouse, capillary degeneration (white arrow) (500 ×). c db/m mouse, EC (white arrow) and pericyte (black arrow) highlighted (600 ×). d C57Bl6 mouse, insufficient removal of non-vascular tissue leading to impaired visualization of the blood vessels
EC to pericyte (E/P) ratio can be used to assess pericyte dropout, one of the earliest cellular deficiencies noted in DR [1]. The accuracy of quantifying E/P ratio rests on clear distinction between the two cell types. Pericyte nuclei are spherical, stain densely and have a protuberant position on the capillary wall, while EC nuclei are oval and lie within the vessel wall (Fig. 84.1c). Unfortunately, many cells can be intermediate that do not fit nicely into either group [5]. Another limitation of trypsin digest is that it is technically difficult and can lead to inconsistent results (Fig. 84.1d). Some of the potential technical challenges and approaches to overcome them are described by our group in detail elsewhere [6].
Immunohistochemistry has been used extensively to characterize pericytes and EC. A vast array of antibodies is available to identify and analyze pericyte and EC dysfunction (e.g. alpha-smooth muscle actin and intercellular adhesion molecule-1, respectively) [7, 8]. Unfortunately, none are 100 % specific and can unequivocally identify all pericytes or EC [9]. Furthermore, the expression of these markers is dynamic in nature and can be up- or down-regulated in various environments. For example, alpha-smooth muscle actin is not expressed under normal circumstances in mouse retina, but is expressed in pericytes during retinopathy [1]. As a result, studies that use single markers must be viewed with caution.
84.4 Functional Approaches
Regulation of retinal vascular tone is one of the most important and well-studied functions of EC and pericytes. Retinal vascular auto-regulation ensures adequate delivery of oxygen, metabolic substrates, and removal of toxic substances and hence, optimal retinal function. Improved understanding of retinal blood flow can help identify new strategies to prevent DR. STZ rats have been the most widely used animal model to study retina blood flow. Unfortunately, in contrast to the morphological methods that have yielded similar conclusions, the techniques used to evaluate retinal blood flow have shown wide variability.
Fluorescein angiography (FA) and radioactive tracers (RT) are techniques to visualize the retinal circulation via injections. After injection, they can be quantified as they provide optical or radioactive contrast in the vasculature, which can be analyzed to quantify blood flow. These methods have demonstrated that blood flow decreases at 1–6 weeks after STZ-induction [10, 11]. The use of microsphere tracers is based on similar principles. Hydrogen clearance is a method that determines blood flow based on the principle that the washout rate of a non-metabolized, diffusible gas from a tissue is proportional to the blood flow [12]. This provides the opportunity to measure regional and temporal fluctuations. Contrary to FA and RT, the latter two methods have shown increases in blood flow at 3–6 weeks after STZ-induction [4, 12]. Intravital microscopy is a technique that measures flow via fluorescein injection and labeled red blood cells [13]. Using this method, it has been shown that at 1 week, there are minimal changes in blood flow, contrary to FA studies, but did support the finding that blood flow decreases by 3 weeks [14, 15].
The different principles underlying these methods may contribute to their conflicting conclusions regarding the timeline and patterns of blood flow alterations (Fig. 84.2). Furthermore, these methods are invasive and flow can be affected by the injection rate. Also, blood flow distribution throughout the retina becomes more variable with DR progression, likely due to impaired auto-regulation [4]. The combination of a lack of standardized tool to measure blood flow, coupled to the overall heterogeneity of blood flow in DR has contributed to our continued limited understanding of the course of blood flow changes in DR.
Fig. 84.2.

Blood flow changes in STZ- rats based on different techniques: Radioactive tracer (RT), Intravital microscopy (IVM), Fluorescein Angiography (FA), Hydrogen Clearance (HC), and Microsphere Tracer (MT). Notice conflicting data at 1 week and 3–6 weeks
Non-invasive imaging modalities are being developed to allow improved measurement of total blood flow in animal models. One example is Optical Coherence Tomography (OCT), a revolutionary technology developed in the 1990s that allows high-resolution imaging of the retina [16]. One obstacle limiting use of optical imaging in diabetic animals is the development of cataracts, which in STZ-rats begins by 5 weeks with progression to mature cataracts by 8 weeks [17]. There are various treatments that can delay cataract formation up to 3 months (e.g. glycine) after STZ-induction [17]. However, these treatments do have the potential to alter the blood flow. Interestingly, mice with congenital hyperglycemia (e.g. db/db) do not develop cataracts [18].
Measurements of retinal vascular oxygen is another important method of examining EC and pericyte function, as it allows direct quantification of oxygen delivery to the retina. Although a detailed analysis is beyond the scope of this chapter, a brief review is presented. Oxygen-sensitive microelectrodes are considered the gold standard. At 5–6 weeks after STZ-induction, rats have smaller arteriovenous oxygen tension differences suggesting vascular leakage [4]. However, interpretations need to be approached with caution as measurements are often made at only one time point and sample only a small retinal area. The procedure is also highly-invasive and hindered by cataract formation. Unlike the former, functional magnetic resonance spectroscopy (fMRI) is non-invasive, unaffected by cataracts, and is able to survey the entire retina. fMRI can measure retinal oxygenation response to hyperoxic provocation (ΔPO2), which has been shown to be a predictor of therapeutic efficacy. In STZ rats, ΔPO2 is significantly lower than control and these changes occur well before retinal histopathologic changes. STZ rats treated with aminoguanidine had restoration of normal ΔPO2 and did not develop subsequent retinal pathology [19]. Two novel techniques for measuring oxygenation are photoacoustic ophthalmoscopy (PAOM) and phosphorescence imaging [20, 21]. More research needs to be done to see whether they will become useful tools in rodent models of DR.
84.5 Treatment of Endothelial Cell and Pericyte Dysfunction
The importance of EC and pericytes in DR has led to research into therapies to prevent their dysfunction, before the onset of clinically overt vascular abnormalities, which become resistant to therapy over time [10]. An improved understanding of the role played by pericyte and EC dysfunction can open new avenues for treatments that reverse their dysfunction and lead to enhanced insights into retinal vascular regulation in DR.
Acknowledgments
Support This work was partly supported by the Illinois Society for Prevention of Blindness (JC, AAF), NIH (EY019951, AAF), Research to Prevent Blindness, NY (JC, and Northwestern Department of Ophthalmology).
List of Abbreviations
- DR
Diabetic retinopathy
- EC
Endothelial cells
- STZ
Streptozotocin
- H&E
Hematoxylin & eosin
- FA
Fluorescein angiography
- RT
Radioactive tracers
- MT
Microsphere tracers
- HC
Hydrogen clearance
- IVM
Intravital microscopy
- OCT
Optical coherence tomography
- SLO
Scanning laser ophthalmoscope
- fMRI
Functional magnetic resonance spectroscopy
- ΔPO2
Oxygenation response to hyperoxic provocation
- PAOM
Photoacoustic ophthalmoscopy
References
- 1.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 2.Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS. Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech. 2012;5(4):444–456. doi: 10.1242/dmm.009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- 4.Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Diabetic retinopathy: early functional changes. Clin Exp Pharmacol Physiol. 1997;24(9–10):785–788. doi: 10.1111/j.1440-1681.1997.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 5.Cuthbertson RA, Mandel TE. Anatomy of the mouse retina. Endothelial cell-pericyte ratio and capillary distribution. Invest Ophthalmol Vis Sci. 1986;27(11):1659–1664. [PubMed] [Google Scholar]
- 6.Chou J, Rollins S, Fawzi A. Trypsin digest protocol to analyze the retinal vasculature of a mouse model. JoVE. 2013 doi: 10.3791/50489. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58(1):1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 8.Portillo JA, Okenka G, Kern TS, Subauste CS. Identification of primary retinal cells and ex vivo detection of proinflammatory molecules using flow cytometry. Mol Vis. 2009;15:1383–1389. [PMC free article] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Higashi S, Clermont AC, Dhir V, Bursell SE. Reversibility of retinal flow abnormalities is disease-duration dependent in diabetic rats. Diabetes. 1998;47(4):653–659. doi: 10.2337/diabetes.47.4.653. [DOI] [PubMed] [Google Scholar]
- 11.Pouliot M, Hetu S, Lahjouji K, Couture R, Vaucher E. Modulation of retinal blood flow by kinin B(1) receptor in Streptozotocin-diabetic rats. Exp Eye Res. 2011;92(6):482–489. doi: 10.1016/j.exer.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Cringle SJ, Yu DY, Alder VA, Su EN. Retinal blood flow by hydrogen clearance polarography in the streptozotocin-induced diabetic rat. Invest Ophthalmol Vis Sci. 1993;34(5):1716–1721. [PubMed] [Google Scholar]
- 13.Wang Z, Yadav AS, Leskova W, Harris NR. Attenuation of streptozotocin-induced microvascular changes in the mouse retina with the endothelin receptor A antagonist atrasentan. Exp Eye Res. 2010;91(5):670–675. doi: 10.1016/j.exer.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bursell SE, Clermont AC, Shiba T, King GL. Evaluating retinal circulation using video fluorescein angiography in control and diabetic rats. Curr Eye Res. 1992;11(4):287–295. doi: 10.3109/02713689209001782. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Morgan GA, Harris NR. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvasc Res. 2008;76(3):217–223. doi: 10.1016/j.mvr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahmani F, Bathaie SZ, Aldavood SJ, Ghahghaei A. Glycine therapy inhibits the progression of cataract in streptozotocin-induced diabetic rats. Mol Vis. 2012;18:439–448. [PMC free article] [PubMed] [Google Scholar]
- 18.Varma SD, Kinoshita JH. The absence of cataracts in mice with congenital hyperglycemia. Exp Eye Res. 1974;19(6):577–582. doi: 10.1016/0014-4835(74)90095-5. [DOI] [PubMed] [Google Scholar]
- 19.Berkowitz BA, Ito Y, Kern TS, McDonald C, Hawkins R. Correction of early subnormal superior hemiretinal DeltaPO(2) predicts therapeutic efficacy in experimental diabetic retinopathy. Invest Ophthalmol Vis Sci. 2001;42(12):2964–2969. [PubMed] [Google Scholar]
- 20.Jiao S, Jiang M, Hu J, Fawzi A, Zhou Q, Shung KK, et al. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt express. 2010;18(4):3967–3972. doi: 10.1364/OE.18.003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahidi M, Shakoor A, Blair NP, Mori M, Shonat RD. A method for chorioretinal oxygen tension measurement. Curr Eye Res. 2006;31(4):357–366. doi: 10.1080/02713680600599446. [DOI] [PMC free article] [PubMed] [Google Scholar]