Abstract
The oocyte is a highly specialized cell poised to respond to fertilization with a unique set of actions needed to recognize and incorporate a single sperm, complete meiosis, reprogram maternal and paternal genomes and assemble them into a unique zygotic genome, and finally initiate the mitotic cell cycle. Oocytes accomplish this diverse series of events through an array of signal transduction pathway components that include a characteristic collection of protein tyrosine kinases. The src-family protein kinases figure importantly in this signaling array and oocytes characteristically express certain SFKs at high levels to provide for the unique actions that the oocyte must perform. The SFKs typically exhibit a distinct pattern of subcellular localization in oocytes and perform critical functions in different subcellular compartments at different steps during oocyte maturation and fertilization. While many aspects of SFK signaling are conserved among oocytes from different species, significant differences exist in the extent to which src-family -mediated pathways are used by oocytes from species that fertilize externally vs those which are fertilized internally. The observation that several oocyte functions which require SFK signaling appear to represent common points of failure during assisted reproductive techniques in humans, highlights the importance of these signaling pathways for human reproductive health.
Keywords: Meiosis, maturation, fertilization, oocyte, SRC, FYN, YES, FGR, FAK, PYK2, protein kinase
Introduction
Oogenesis represents a transformation of the female primordial germ cell through intermediate growth stages during which the oocyte becomes specialized with the capacity to store metabolic precursors sufficient for the initial stages of embryo development together with a combination of meiotic cell cycle machinery, chromatin remodeling factors, and pre-assembled mRNAs (maternal factors) that encode enzymes critical for fertilization and early zygote development. Once fully grown, the oocyte may remain relatively inert for some period maintaining gap junction communication with associated follicle cells, and awaiting endocrine and paracrine signals that signal the time for meiotic maturation and subsequent fertilization. The necessity of the oocyte to respond to the diverse signals for nuclear and cytoplasmic maturation as well as fertilization is met by preassembly of signal transduction networks. These networked pathways sense inputs from the follicle cells as well as the fertilizing sperm and, in turn, control expression of maternal genes as well as the mechanical processes involved in cytoplasmic and nuclear maturation, sperm incorporation and ultimately oocyte activation. Many of these signal transduction pathways involve protein kinases at key regulatory steps and the orderly assembly and maintenance of these signaling proteins is a critical aspect of oocyte quality [1]. The protein kinases involved in meiotic maturation have been the focus of intense study for at least thirty years and that body of work propelled the oocyte into a prominent place in the cell biology field providing an important complement to the yeast system in the field of cell cycle control. For the most part, meiosis is controlled by protein kinases that phosphorylate serine or threonine (Ser/Thr kinases) and these elements have been reviewed elsewhere [2–6]. In addition, several protein tyrosine kinases (PTKs) have been found to play significant roles in the processes of oocyte maturation and fertilization [7–11] and the purpose of this chapter is to review the current knowledge in this aspect of oocyte biology.
Protein Tyrosine Kinases; Significance
The phosphorylation of proteins as a stable form of secondary modification occurs mostly at serine, threonine and tyrosine residues (phospho-histidine being relatively unstable). To be functionally significant as a signaling mechanism, a phosphorylation pathway must have enzymes to both add and remove the phosphate to control the level of signal. In addition, a substrate or product binding domain adds targeting specificity as opposed to non-specific phosphorylation. Such sophisticated mechanisms were developed for Ser/Thr phosphorylation early on and are present in all prokaryotic and eukaryotic cells. While some level of Tyr phosphorylation occurs in bacteria, plants, and single cell eukaryotes, the kinases involved are either BY kinases in bacteria [12] or are structurally related to P-loop nucleotide triphosphatases, or dual specificity kinases (TKL kinases [13;14]) and are not related to eukaryotic tyrosine kinases. The appearance of an efficient P-Tyr signaling pathway with tyrosine specific kinases, phosphatases and binding domains is thought to occur just prior to evolution of metazoans as exemplified by the choanoflagellates [15]. The appearance of this pathway has been described as a ‘game changing innovation’ [16] which provided the ability to establish new signaling pathways without the possibility of interference with pre-existing Ser/Thr kinase pathways which were by that time critical for success. The unique tyrosine specificity provided by the catalytic domain together with the P-Tyr binding specificity of the SH2 domain and opposing phosphatases with their own unique specificity enabled a vast array of signaling possibilities which could facilitate the evolution of multicellular communication and specialized tissue physiology [17]. The diversification of PTK families into cytoplasmic and transmembrane receptor classes is apparent even in these pre-metazoans [18] although the rapid expansion of the receptor PTKs among metazoans suggests that these receptors, which are thought to initially have functioned as environmental sensors, were very successfully applied in cell-cell communication.
Src-family PTKs; Structure and Capabilities
The src-family of PTKs (SFKs) appears to have evolved its characteristic domain structure in choanoflagellates (Monosiga brevicollis) where four Src kinase homologs were reported [19]. These 57–60kDa proteins consist of five conserved domains, the N-terminal unique domain, the SH3 and SH2 protein interactions domains, the catalytic domain, and the C-terminal regulatory domain (Figure 1) and this structure is retained in all metazoan species. The N-terminal unique (U or SH4) domain exhibits the most sequence divergence among Src-family members (thus the name ‘unique’). This domain contains two fatty acid acylation sites that typically become derivatized with palmitate and myristate which function in targeting the kinase to the plasma membrane rafts [20]. Phosphorylation sites at Ser17, Thr37, and ser75 are thought to antagonize membrane localization [21]. This domain also contains a lysine that is often methylated and seems to play a role during cell spreading [22]. Other functions of this domain include binding of filamentous actin bundles [23] and direct interaction with PLCγ [24;25].
Figure 1.
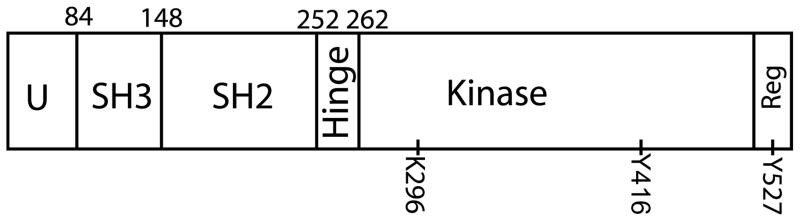
Domain structure of Src-family protein kinases
Src-family PTKs contain a small N-terminal unique (U or SH4) domain followed by the adjacent SH3 domain and SH2 domain. A short hinge region separates the N-terminal domains from the kinase domain which contains the catalytic site with its critical lysine 296 which is in the ATP binding pocket. An important tyrosine phosphorylation site (Y416) in the A loop. The C-terminal regulatory domain contains the second regulatory phosphorylation site (Y527).
The SH3 and SH2 domains are important for protein-protein interactions that relate to modification of kinase activity as well as targeting specific signal transduction pathways. The SH3 domain binds proline-rich sequences and is involved in docking interactions with other signaling proteins such as PI-3 kinase, BLK, Shc, and WASP [24;26–28], BLK [29]. Other diverse functions include Interaction with voltage gated sodium channels also occurs via this domain [30], and the integrin B3 protein binds to and is thought to activate Src though interaction between a RGT sequence and the SH3 domain[31]. The SH2 domain of SFKs exhibits binding specificity for phosphorylated tyrosine within the following motif (Y-E-E-I/L/V/P) [32]. A primary significance of this domain is that in multicellular organisms, it functions in an intra-molecular binding interaction with a C-terminal tyrosine to stabilize a ‘tight’ or ‘closed’ tertiary structure that obscures the catalytic domain and renders the kinase inactive [33;34]. The SH2 domain also participates in interactions with other proteins such as the TRPC6 channel protein [35], growth factor receptors such as the PDGF receptor and discoidin domain receptor DDR1 [36;37], receptor guanyl cyclase C [38], other kinases such as FAK and FLT3 [39;40], as well as cytoskeletal elements such as cortactin [41].
The catalytic domain of Src-family PTKs is based on a catalytic structure and mechanism common among most protein kinases including serine and threonine kinases such as protein kinase A. The domain exhibits an N-terminal lobe (N-lobe), a C-terminal lobe (C-lobe) and a central activation loop (A-loop). The catalytic site where the gamma phosphate of ATP is transferred to substrate tyrosine is located between the N and C lobes and the configuration of the A-loop controls access to the active site [42;43]. Phosphorylation of tyrosine 416 in the A-loop [44] favors a conformational change of the N and C-lobes that increases access to the catalytic site and increases catalytic activity.
The C-terminal domain of SFKs contains a number of Serine residues that can be phosphorylated as well as a phosphorylation site at tyrosine 527. While the size of the C-terminal domain might suggest several important functions, the observation that the C-terminal domain can be replaced with the syntropin PDZ domain and it ligand to produce a functional SFK [45] suggesting that the predominant requirement is that the domain function to establish the ‘closed’ configuration typical of the inactive state.
Regulation
The SFKs are held in an inactive configuration in most cells and are only activated transiently by a multi-step process involving dephosphorylation of the C-terminal tyrosine (Y527) and activation-loop phosphorylation [44]. In the absence of stimuli, the inactive configuration is maintained by phosphorylation of Y527 by CSK kinase and, once phosphorylated, the flexibility of the SH2-linker region allows the SH2 domain to bind Y527, locking the kinase into a ‘closed’ configuration. Appropriate stimulation by growth factors, cell-cell contact, or cell cycle events causes a phosphatase such as rPTPα [46] to dephosphorylate Y527 of the C-terminal domain (‘unlatching’ shown in Figure 2) allowing the kinase to unfold into the ‘open’ configuration exposing the catalytic site (‘unclamping ’ as in figure 2). The catalytic site itself requires the proper orientation of the N and C-lobes as well as the activation loop (A-loop). The activation loop alternates between the active configuration, where all elements of the active site are in the correct position, and an inactive configuration that does not support phosphorylation of tyrosine. The active conformation of the A-loop is stabilized by a second phosphorylation event, trans-autophosphorylation of Y416 in the A-loop, which switches the kinase into full catalytic activity.
Figure 2.
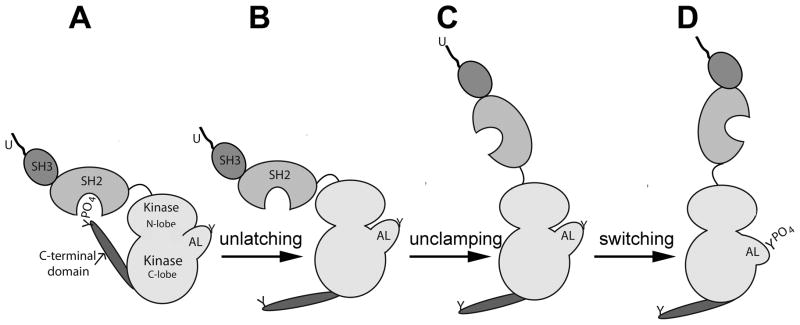
Src-family kinase activation mechanism
Src-family PTKs are held in a perdominantly inactive form in most cells and are activated only in response to stimuli via a common mechanism. The inactive kinase (left) is maintained in the closed conformation by the binding interaction between SH2 domain and the phosphorylated C-terminal tyrosine (A). Dephosphorylation of the C-terminal tyrosine ‘unlatches’ the protein (B) allowing it to assume an open configuration which allows catalytic activity ‘unclamping’ (C). Autophosphorylation of the tyrosine phosphorylation site within the activation loop (AL) stabilizes the open configuration ‘switching’ the enzyme to full catalytic activity (D) (after Roskoski [44]).
Expression
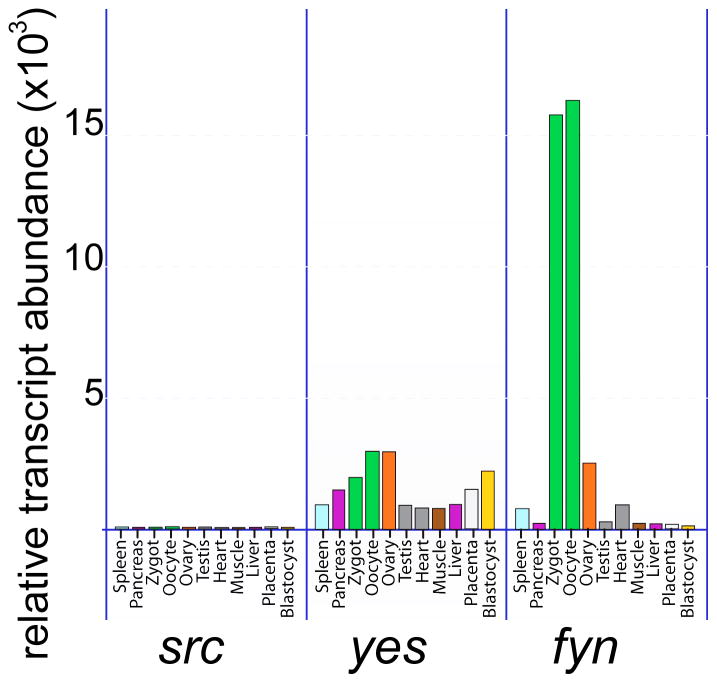
SFKs are expressed in oocytes of species as diverse as drosophila melanogaster [47], asteria miniata [48;49], S. purpuratus [50], cerebatulus [51], Xenopus [52;53] and mammals. While most of the typical Src-family domains are reasonably well conserved, significant sequence differences among the insect and marine invertebrate species that have complicated classification of individual SFKs in these species. For example, while early immunoprecipitation assays using antibodies directed against vertebrate src-family members detected PTK activities resembling Src and Fyn [54;55] later sequence analysis of individual SFKs cloned from the sea urchin and starfish [48;50] identified several novel PTKs which were clearly members of the SRC-family based on homology, but which could not be directly linked to known vertebrate SFKs (reviewed in [11]). The number of Src-family members expressed in these marine invertebrate oocytes suggests that these oocytes face requirements that are best suited by a diversity of protein tyrosine kinase properties and functional studies have demonstrated that the different Src-family members do perform different roles during fertilization. The presence of SFKs in vertebrate oocytes was initially demonstrated by cDNA cloning of mRNA isolated from Xenopus laevis oocytes [56] and was later confirmed at the protein level in Xenopus laevis where the Xyk kinase was purified and described [57;58]. Proteomic analysis revealed that three src-family members (Src1, Src2, and xSrc) are expressed in proteins in the Xenopus oocyte [59]. In the zebrafish system, FYN was detected by immune-complex assay and subsequently cloned and sequenced [60;61]. The first demonstration of SFK expression in mammalian oocytes was performed on rat and mouse oocytes [62–65] and was greatly facilitated by the availability of well-characterized antibodies specific for the different src-family members which made possible western-blot and immune-complex assays as well as immunofluorescence techniques. Later, array-based mRNA expression data of different stage mouse oocytes revealed that most members of the SFKs (fgr, hck, lyk, lyn, blk) are barely detectable in murine oocytes while fyn and yes [66] are expressed at very high levels in oocytes (Figure 3). This result seems to conflict with the situation in marine invertebrate oocytes where a number of different src-family members are expressed and play different roles during fertilization. It suggests that the mammalian oocyte does not need the diversity of SFK signaling mechanisms that are critical for marine invertebrates either because of the evolutionary distance or because of the fact that species that fertilize externally face unique challenges that the mammalian oocyte does not experience. Given the fact that fyn expression levels are much higher in oocytes than even neurons and T-cells, one might even refer to FYN kinase as an ‘oocyte-specific kinase’. At least it is clear that the oocyte is highly specialized biochemically with a large commitment to signaling pathways involving the FYN kinase. The biology of the oocyte is such that it must establish and maintain a pool of the protein kinases in order to remain ready for signals to begin meiotic maturation and later for fertilization which will trigger rapid zygote development. FYN appears to be an essential component of the oocyte signaling machinery and proper subcellular localization must be an important aspect of oocyte quality. Once the blastula stage has been reached, the high levels of FYN kinase appear to be no longer required as evidenced by the relatively low expression levels typical of the blastocyst (Figure 3).
Figure 3.
Oocytes express fyn and yes transcripts are high levels relative to somatic cells.
The relative abundance of the most common Src-family kinase mRNAs reported for mouse oocytes in the BioGPS expression array database (Novartis BioGPS, http://biogps.gnf.org; Su et al., 2002a) is presented for several different tissues and cell types. Values in green represent oocyte and zygote material.
Subcellular localization
The membrane targeting (U) and protein interaction domains (SH3,SH2) of src-family PTKs direct physical association of these kinases with the subcellular structures where they function. Specific targeting of FYN and YES kinases to structures within the oocyte has been detected with antibodies specific for individual kinases and the activation state of SFKs can be detected with phosphorylation site-specific antibodies such as the clone 28 mouse monoclonal which recognizes the dephosphorylated form of the C-terminal regulatory tyrosine(Y527)[67]. Also, antibodies specific for the phosphorylated and non-phosphorylated regulatory tyrosine located in the catalytic domain (Y416) of SFKs can differentiate between active and inactive forms but cannot distinguish among the different family members due to the highly conserved sequence of the phosphorylation site. Early subcellular fractionation studies demonstrated that Fyn and possibly other SFKs were concentrated in the plasma membrane and cortex of sea urchin and zebrafish oocytes [61;68;69]. Confocal immunofluorescence analysis has been used to examine the subcellular localization of SFKs in oocytes from sea urchins, zebrafish, and mice. In the sea urchin, the SpSFK1/7 kinase was detected in the cortical actin layer and in the sub-adjacent cortical cytoplasm which is typically enriched in endoplasmic reticulum [50]. A similar distribution of active SFKs was detected in the zebrafish oocyte [70] and mouse oocyte [71] suggesting that this kinase family plays some role in regulation of cortical cytoskeletal components. This supposition is supported by the fact that SpSFK1/7 [50;50] and activated SFKs detected with the clone 28 antibody were found to be highly concentrated in the fertilization cone of sea urchin and zebrafish oocytes [72]. In the mammalian oocyte, a characteristic localization pattern of SFKs has been demonstrated as early as the germinal vesicle (GV) stage where FYN has been reported to preferentially localize in the cortical region while YES was distributed uniformly in the ooplasm [62;71;73]. During the GV stage, a subpopulation of FYN is also concentrated within the nuclear envelope raising the possibility that it might play some role in chromatin remodeling or nuclear envelope dynamics in addition to having functions within the oocyte cortex. This property was unique to FYN as the YES kinase did not concentrate in the GV of wild type oocytes [11]. After germinal vesicle breakdown (GVBD), FYN and YES were both localized to the cortex of the mature oocyte in zebrafish, rat, and mouse systems [62;64;66;72]. Once the meiotic spindle was formed, FYN and possibly other active SFKs detected with the clone 28 antibody, were found to be highly concentrated in close association with microtubules of the meiotic spindle or residual body [10;64;74–76]. The tight association of FYN with spindle components has also been observed in studies of somatic cells [77;78] at least some fraction of the spindle-associated SFKs are in the active state as demonstrated with the clone 28 antibody [67;79;80]. A separate antibody to (Y416) of SFKs has also been used to detect activated SFKs that were closely associated with the meiotic spindle of mouse oocytes [76;81].
Functions during oocyte maturation
The first analysis of PTK activation during oocyte maturation was done with the starfish Marthasterias glacialis where induction of oocyte maturation with 1-methyl adenine induced activation of PTK activity detected via accumulation of P-Tyr-containing proteins in the oocyte [82]. This study also detected a 68 KDa PTK activity in autophosphorylation assays performed on purified cortex preparations suggesting a possible role of src-family PTKs during oocyte maturation. However, the first definitive proof that a src-family PTK was involved in oocyte maturation was revealed in Xenopus laevis oocytes where SFK activation represents one of the earliest responses to progesterone treatment of the oocyte [83;84]. The progesterone receptor is known, in some cases, to activate SRC kinase activity through an SH3 displacement interaction [85] which raises the possibility that the progesterone receptor in the oocyte or in tightly associated follicle cells might be a key element of meiosis regulation. The potential function of SRC during oocyte maturation was shown by injection of active SRC kinase into GV stage Xenopus oocytes which resulted in accelerated progesterone-induced GV breakdown [83] and MAPK activation [84].
In mammalian oocytes, progesterone or LH stimulation of GV stage oocytes has not been associated with elevated SFK activity, however significant changes in the subcellular distribution of active Src-family PTKs has been reported [10].
GV stage oocytes are characterized by concentration of active SFKs at cytoplasmic microtubule arrays and in the region surrounding the nucleus [10]. After GVBD, active kinase was detected only on the meiotic spindle of the MI and MII oocyte. The function of SFK members during oocyte maturation has been studied with chemical inhibitors such as SKI-606, PP2 and SU6656 as well as by siRNA knockdown, dominant-negative constructs, and single gene knockout models. Each approach has its own drawbacks. The chemical inhibitors cannot distinguish among different Src-family members very well and usually inhibit the closely related Abl kinase [86–89]. Dominant-negative constructs provide better specificity toward SFKs and can block scaffolding interactions, but require injection of cRNA and adequate expression in the oocyte to exert their effect. Knockdown and gene knockout studies provide better specificity toward individual SFKs but allow compensation due to increased expression of other SRC-family members [90]. As might be expected given the limitations of the different methods used, experimental analysis of the role of SFKs in initiation of meiosis (GVBD) has produced conflicting results. Inhibitors such as PP2 [76] and SU6656 [73] block GVBD in culture, while SKI-606 does not block GVBD, but instead stimulates GVBD even in the presence of phosphodiesterase inhibitors [10]. Functional studies in which the role of FYN was tested by injection of cRNA encoding a dominant-negative FYN construct, partially blocked GVBD in culture [73], while siRNA knockdown and fyn-null oocytes revealed no inhibition of GVBD [91]. This type of conflict might result from compensation by other SFKs if chemical inhibitors and dominant-negative constructs were more effective against compensation by other kinases due to the similarity of the catalytic sites or the protein interaction domains. Single gene knockdown or knockout would leave the possibility of compensation entirely open as demonstrated by up-regulation of Yes kinase in the fyn-null oocyte [91].
While SFK activity may or may not be required for initiation of GVBD, suppression of SFK activity with SKI-606 or by siRNA knockdown or knockout of fyn caused significant disruptions in the spindle and chromatin organization that resulted in failure to complete MI and reach normal metaphase-II [10;74;90;91]. In addition to the effects on spindle function, suppression of SFKs (particularly FYN) had additional effects on the cortical actin layer polarity. During oocyte maturation, the metaphase-I spindle must remain in close proximity to the egg cortex while the spindle rotates and the polar body is extruded at telophase-I. However, chemical inhibition by SKI-606 or knockout of fyn leads to abnormal cortical polarity seen as reduced filamentous actin content near the spindle and enlarged polar bodies which correlated well with a failure of the meiotic spindle to maintain a position close to the oocyte cortex [91]. It is likely that FYN and other SFKs act directly on the cortical actin layer since injection of active viral-SRC into oocytes was shown to have dramatic effects on the cortical cytoskeleton [92], and moesin, a known component of the oocyte cortex, was identified as a SFK target in Xenopus oocytes [93]. In any case, the suppression of Fyn kinase through gene knockout, knockdown, or chemical inhibition resulted in loss of developmental competence with high failure rates during MI and MII stages of maturation [90;94].
Functions during fertilization
SFKs function in a variety of signaling pathways including several that have found application in the oocyte during fertilization. As mentioned above, highly reactive cells such as platelets and T-cells use SFKs such as SRC and FYN to stimulate phospholipase Cγ activation either through direct phosphorylation or through PI-3 kinase activation [95;96] as part of a rapid IP3-mediated calcium signal and or mitogenic signals. SFKs also play an important cell cycle control events at the G2/M transition [97;98] and, together with FAK, can function during actin remodeling events [23;99–101]. Since the biology of fertilization involves some basic functions that are shared between invertebrate and vertebrate animals (ex. gamete binding-fusion, block to polyspermy, metabolic activation, and cell cycle resumption) it is not surprising that most oocytes studied rely heavily on a similar complement of protein kinases. However, since fertilization strategies can differ significantly depending on the environment where reproduction occurs (ex. external fertilization vs internal fertilization) the relative importance of different pathways can also vary.
Early events during external fertilization
Among species that fertilize externally, the functional significance of SFKs during fertilization has been found to center primarily in the area of regulating PLCγ mediated calcium signaling during the earliest phases of fertilization. Marine invertebrate, amphibian, and fish species fertilize large numbers of oocytes externally which facilitated biochemical analysis of large numbers of synchronously fertilized eggs. As a result, SFK activity could be measured directly by immune-complex assays and autophosphorylation assays which were not generally feasible for mammalian oocytes. The most thoroughly studied species include sea urchins, starfish, frogs and zebrafish which typically exhibit rapid activation of SFK activity within the oocyte cortex or plasma membrane compartment [55;57;58]. The significance of the SFKs to fertilization in externally fertilizing species is particularly obvious in the sea urchin and starfish systems since these oocytes expresses multiple different SFKs simultaneously. In the sea urchin S. purpuratus, four SRC-family members SpFRK, SpSFK1, 3, and 7 are expressed as proteins in oocytes [50], while in the starfish A. miniata, AmSFK1, AmSFK2, and AmSFK3 are expressed as proteins [48]. SpSFK1 and SpSFK7 as well as AmSFK 1, 2, and 3 are known to be concentrated in the plasma membrane or cortex of oocytes as determined by subcellular fractionation or immunofluorescence microscopy. Upon fertilization, AmSFK1 was activated transiently within 20–40 seconds post fertilization followed by AmSFK3 which exhibited a slower time course [48]. The timing of kinase activation correlates well with the high amplitude fertilization-induced calcium transient typical of marine invertebrate oocytes and functional studies using recombinant-SH2 domain fusion proteins specific for AmSFK1 and 3 demonstrated that these two kinases play a role in initiation or amplification of this important calcium signaling event. Given the fact that PLCγ-mediated IP3 production was known to play a major role in the fertilization-induced calcium transient of oocytes which were fertilized externally [102–104] the potential role of specific SRC-family PTKs to stimulate PLCγ-mediated IP3 production was of intense interest. Additional mechanistic analysis demonstrated that SpSFK1 binds directly to the SH2 domain of PLCγ, suggesting that this kinase might be primarily responsible for activating PLCγ during fertilization [50]. This work culminated a series of studies from several different labs which demonstrated a functional relationship between SFKs and PLCγ-mediated calcium signaling at fertilization [105–108] reviewed in [109;110]). This has led to a model for the initiation of calcium release at fertilization in sea urchins where sperm-egg binding or fusion leads to the activation of SpSFK3 which quickly triggers activation of SpSFK1. SpSFK1 directly interacts with and activates PLCγ, leading to the production of diacylglycerol and IP3. Calcium would then be released from the endoplasmic reticulum through the IP3 receptor as occurs in a wide variety of cells types. Interestingly, evidence also supports an alternative mechanism involving NAADP-mediated calcium signaling during the initial ‘cortical flash’ that precedes the high amplitude calcium transient in marine invertebrate oocytes. The ‘cortical flash’ is then thought to be amplified by the above IP3-mediated mechanism and prolonged by cADPR signaling [111;112]. In fact, both a plasma membrane and an endoplasmic reticulum associated ADP-ribosyl cyclase was demonstrated in the sea urchin egg [113] indicating that cADPr may play a role in calcium flux across the plasma membrane as well as the endoplasmic reticulum or other intracellular vesicles. Taken together, it is clear that the PLCγ/IP3 mechanism plays a dominant role in producing the high amplitude calcium transient that occurs in most externally fertilizing species and appears to rely on SRC-family PTKs for activation, but the significance and regulation of the NAADP, and cADPR-mediated calcium events needs to be resolved before a full understanding of fertilization in externally fertilizing species can be achieved.
Evidence that SFK activation of PLCγ plays a role in the fertilization-dependent calcium transient other external fertilization models has been developed in Xenopus laevis and in Danio rerio where pharmacological and dominant-negative suppression of SFKs was also found to suppress the fertilization-induced calcium transient [102;114;115]. In Xenopus, the src-related kinase XYK was shown to associate with PLCγ and stimulate calcium release when injected into mature oocytes [7;116;117]. In Xenopus, cADPr is thought to promote IP3-mediated calcium signaling through its effect on SERCA pumps which maintain calcium stores in the endoplasmic reticulum [118]. As yet, a direct effect of cADPR or NAADP pathways on the fertilization-induced calcium transient has not been demonstrated. The zebrafish oocyte is characterized by a large central yolk mass that is surrounded by a more active cortical cytoplasm and extensive imaging studies have documented that IP-3 mediated calcium release is primarily responsible for the fertilization-induced calcium transient in this oocyte[119;120]. The cortical cytoplasm is enriched in SFKs and in IP3r channels relative to the central cytoplasm and confocal-based calcium imaging indicates that the fertilization-induced calcium transient propagates through the cortical cytoplasm faster than through the central cytoplasm [72]. The importance of SFK signaling in the fertilization-induced calcium transient through the cortical cytoplasm was shown by injection of a FYN-SH2 domain fusion construct which suppressed the calcium response in the cortex, but had little effect in the yolk mass.
The fact that the extracellular coat or chorion of fish oocytes is specialized to exclude sperm except for a single pore (the micropyle) through which sperm can pass presented the opportunity to reliably predict where the fertilizing sperm would contact the oocyte. This feature enabled detection of the initial effect of sperm-oocyte contact or fusion on SFK activation which revealed that activation of SFKs (as well as PYK2 kinase activation) occurred initially in the immediate vicinity of sperm-oocyte contact/fusion[121]. Kinase activation then progressed through the oocyte cortex from animal pole to vegetal pole eventually involving the entire oocyte cortex. The fact that the progression of SFK activation through the egg cortex correlated with the progress of the high amplitude calcium transient raised the possibility that SFK activation might function to amplify and propagate PLCγ mediated calcium release through the relatively large distances required to traverse the fish oocyte, which in the case of zebrafish, represents a diameter of 700um. This was supported to some extent by the fact that the central yolk mass (largely devoid of FYN kinase and IP3r exhibited a slower calcium response to fertilization. However, additional work to demonstrate that a propagated wave of PLCγ phosphorylation occurred coincident with the SFK activation would further strengthen this model.
Early events during internal Fertilization
The characteristics and role of SFKs in fertilization of the mammalian oocyte appear significantly different from that in species which fertilize externally. Mouse and rat oocytes express FYN, YES, and perhaps SRC proteins [62;63]. FYN is concentrated in the oocyte cortex [62;64] as were some of the SFKs expressed in marine invertebrate oocytes described above. However, while SFK activation could be demonstrated in the cortex of sea urchin, frog, and zebrafish oocytes by biochemical analysis of membrane fractions [55;61] [58] or through the use of phosphorylation site-specific antibodies [70], mammalian oocytes were nearly impossible to analyze biochemically and immunofluorescence failed to detect activated SFKs in the mouse egg cortex [81;122]. While a negative result proves nothing, the fact that exogenous Fyn-eGFP constructs modified to remain in the ‘open’ configuration preferentially localized at the oocyte cortex suggests that if fertilization had resulted in activation of endogenous FYN, it would have become localized in the cortex as well [123]. The failure to detect endogenous activated SFKs in the cortex of fertilized mouse oocytes by immunofluorescence may indicate that it does not normally occur and can be detected only by over-expressing the FYN protein. In any case, functional studies using chemical inhibitors, dominant-negative constructs, and single gene knockout have consistently demonstrated that suppression of SFKs in the mammalian oocyte has, at best, a minor impact on the fertilization-induced calcium oscillations [49;64;65]. The subtle changes in oscillation frequency that do occur in fyn-null oocytes seem more likely to reflect disruption of the normal distribution of IP3 receptors and cortical cytoskeletal organization which results from fyn suppression rather than any effect of phospholipase C activity [90]. In mammalian oocytes, SFK activity seems to be important before fertilization to maintain organization of cytoskeletal components in a fertilizable state and during the later stages of fertilization including pronuclear congression and the entry into mitosis.
Other functions of SFKs during fertilization
Additional aspects of egg activation that involve SFKs activity include maintenance of meiotic spindle and cortical actin cytoskeleton integrity. The role of SFKs in these cytoskeletal functions seems to be conserved among externally fertilizing and internally fertilizing species. SFKs are known to become concentrated in the region of both mitotic and meiotic spindle microtubules [74;80;81;124] so it is not surprising that suppression of this kinase family has an impact on spindle organization. Early experiments in the sea urchin system revealed that SFKs inhibitors caused disruption of the spindle microtubules [125] which was interpreted to indicate a role in microtubule dynamics. Later work in the mouse oocyte revealed that chemical inhibition of SFKs and knockout of fyn caused disorganization of the MII spindle with loss of the metaphase plate structure, as well as frequent displacement of individual chromosomes from the spindle, and failure to maintain the spindle in close proximity to the oocyte cortex [126]. The phenotype resulting from SFK suppression in oocytes appears more severe than that which occurs in somatic cells where chemical SFK suppression and triple src/fyn/yes (SYF cells) resulted in a reduced rate of spindle assembly and a high frequency of mis-oriented spindles, but loss of chromosomes from the spindle were not reported [127]. The fact that oocytes rely very heavily on SFK activity for the assembly and maintenance of the meiotic spindle is particularly significant since errors in spindle function during meiosis lead to chromosomal abnormalities that will impact the new embryo and subsequent generations. The question of whether SFK suppression of microtubule function is responsible for the observed high rate of pronuclear congression failure [64;90;125;128;129] remains open at present.
The potential role of SFKs in regulation of the cortical actin cytoskeleton was first demonstrated in Xenopus oocytes where constitutively active mutant forms of SRC and FYN were found to cause patches of enhanced actin filament density that co-localized with accumulation of phosphotyrosine-containing proteins and cortical pigment granules [92]. Later work in the mouse oocyte demonstrated the role of FYN kinase in maintenance of the polarity of the cortical filamentous actin layer with a thickened actin cap overlying the meiotic spindle and polarized distribution of cortical secretory granules and microvilli [90;91]. Suppression of FYN activity by chemical inhibition or fyn knockout resulted in a less polarized actin layer, disorganized cortical granule distribution, failure to maintain the normally close relationship between the spindle and the actin layer, as well as displacement of chromosomes from the spindle as mentioned above. These changes in the filamentous cortical actin layer correlated well with the morphological defects exhibited by fyn-null oocytes (Figure 4). Ooyctes from fyn-null mice incorporated sperm at normal rates but exhibited reduced developmental competence which likely resulted from several mechanisms. For example, mouse oocytes normally maintain a microvilli-free zone over the MII spindle which is thought to reduce the possibility of gamete fusion near the site of second polar body emission. Suppression of SFK activity chemically or by fyn knockout allowed microvilli to form near the spindle and an increased frequency of gamete fusion near the MII spindle. This occasionally resulted in the sperm nucleus being expelled as another polar body as the cytokinesis machinery was unable to differentiate between maternal and paternal chromatin during polar body emission. In addition, SFK knockout caused disorganization of the cortical granules that normally undergo exocytosis at fertilization and subsequently establish a slow block to polyspermy as well as modifying the oocyte surface for subsequent development. Detachment of these secretory granules from the oocyte cortex likely impaired the post-fertilization wave of exocytosis although the significance of that for development is not understood at present. Other potential consequences of impaired cortical cytoskeletal structure could include disruption of the subcortical maternal complex important during post-transcriptional gene regulation prior to the maternal to zygotic transition [130] and reduced ability to establish cell-cell communication at compaction even when fertilized by wild-type sperm.
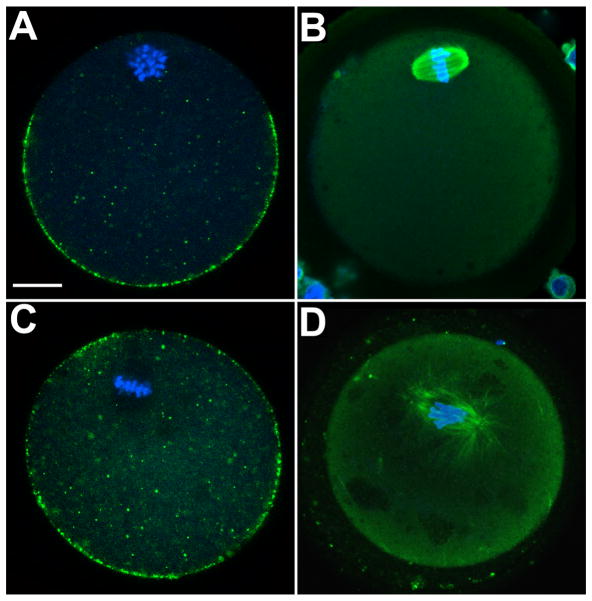
Figure 4.
Cytoskeletal changes in oocytes as a result of fyn knockout.
Oocytes from wild type (A,C) and fyn-null mice (B,D) were labeled with FITC-tagged wheat germ agglutinin to demonstrate the distribution of cortical granules (A,C) or with an antibody to tubulin to label spindle components (B,C) and imaged by confocal immunofluorescence. Chromatin was labeled with ethidium homodimer (blue). Magnification is indicated y the bar which represents 10um. Images by Jinping Luo.
Another of the events that require SFK activity during the later stages of fertilization is pronuclear congression and entry into mitosis. Once meiosis has been completed and sperm incorporation is complete, the maternal and paternal nuclei become surrounded by nuclear envelopes forming the male and female pronucleii. These pronuclei enter S phase and proceed through DNA replication. After a short G2 interval, the pronuclear envelopes break down and both maternal and paternal chromosomes commingle for the first time, a process termed pronuclear congression. The chromosomes align on the mitotic spindle and the zygote enters mitosis marking the end of the fertilization process. The formation and disassembly of the pronuclear envelope is under control of MAPK [131]. Fertilization triggers reduction of both MPF and MAPK in the egg which is necessary to allow the pronuclear envelopes to form [132]. As DNA synthesis nears completion, MAPK and MPF activities increase again and the MAPK activity triggers pronuclear envelope breakdown while MPF activity promotes entry into mitosis. SFK inhibition by chemical inhibitors, dominant-negative constructs, or fyn knockout results in a high developmental failure rate at the late pronuclear stage [64;90;125;129;133]. The specific events that require SFK activity are unknown but include cell cycle control mechanisms that may require SFK activity. For example, SFK activity is stimulated at G2/M [98] and Src-family PTK activity is required for the G2/M transition in fibroblasts [97;124]. The P62 GAP-associated protein associates with SRC via SH3 and SH2 domains and is the major SRC substrate at this point of the cell cycle [27;134] although the relationship between p62 phosphorylation and pronuclear congression has not been demonstrated. The role of SFKs during pronuclear congression could be very important relative to ART procedures as failure of pronuclear congression is reported to account for 19.2% of the failed oocytes in human IVF and 22.6% in human ICSI procedures [135].
In summary, SFK signaling plays an important role in assembly and/or maintenance of the cortical actin layer and the meiotic spindle in oocytes from externally and internally fertilizing species. In addition, oocytes from externally fertilizing species make use of an SFK-mediated pathway to drive PLCγ-mediated IP3 production while mammalian oocytes rely instead on the sperm-borne PLZζ to initiate IP3 production [136]. We have proposed that this difference may reflect the need for very rapid establishment of the block to polyspermy during external fertilization. As mentioned above, SFK-induced PLCγ signaling often occurs in highly reactive cells such as platelets where a rapid, high amplitude calcium transient is used to trigger secretory granule release in response to some stimulus, a feature that oocytes share. Species that fertilize internally would likely have less need of such a rapid polyspermy block and could rely on the slower plasma membrane block to polyspermy [137] or the even slower zona reaction that follows cortical granule release in mammals.
Mechanism of SFK activation at fertilization
The above functional consequences of SFK signaling in oocytes highlight the obvious question of how these kinases are activated at different points during the response to fertilization. As described above, SFKs are activated in a multistep process that is initiated by removal of a phosphate from the C-terminal tyrosine by a phosphatase. However, the signals that trigger this dephosphorylation event and the phosphatase that accomplishes it may be different at different points in the oocyte life. The question is complicated by the fact that many other non-src-related PTKs are regulated by PTPases including Wee1 kinase which is regulated by Cdc25 to activate cyclin-dependent kinase [138]. In other, well studied tissues, receptor-type PTPases such as CD45 [139], the leukocyte antigen receptor family (LAR) of PTPases, rPTPepsilon, and the orphan receptor phosphatase rPTPα are implicated in regulation of Fyn [140–142] and other SFKs. Cytosolic PTPases such as PTP1B [143] have been shown to regulate SFKs in many somatic cells.
The importance of PTPase activity in activation of SFK activity in the zebrafish oocyte was demonstrated with PTPase inhibitors that delayed the cortical reaction, cleavage, and FYN kinase activation [144]. The rPTPα phosphatase was observed to complex with FYN in zebrafish oocyte plasma membranes, but our subsequent unpublished studies indicate that rPTPα is not critical for successful fertilization in that species. More functional information regarding the role of PTPases during fertilization has been developed in the c. elegans oocyte where the EGG3 phosphatase [145], EGG 4 and 5 [146;147] have been shown to play a role in cortical actin cytoskeletal organization. These related phosphatases share a complex interdependence with EGG-4 and EGG-5 being required to properly coordinate redistribution of EGG-3 away from the cortex during meiotic anaphase I and are thought to link events during egg activation with the advancing cell cycle. This fertilization system has obvious advantages for the study of egg activation, but at present, the possible role of the EGG PTPases in PTK activation is entirely unknown.
An alternative mechanism for the activation of SFKs at fertilization makes use of Focal Adhesion Kinase which can complex with SFKs resulting in conformational changes in both kinases. This interaction occurs between phosphorylated tyrosine 397 of FAK and the SH2 domain of SFKs. This SH2 displacement mechanism forces the C-terminal negative regulatory domain of the SFK into the open configuration rendering it available for dephosphorylation. The end result is that the active configuration of the SFK is favored resulting in increased SFK activity [148;149]. Interestingly, SFK activity in turn promotes the activity of FAK or PYK2 [150] leading to a reciprocal activation cascade involving both kinases. This mechanism may prove to be relevant to oocytes of externally fertilizing species where rapid activation of SFKs and the FAK family kinase PYK2 (PTK2) occurs in response to fertilization [121]. In the zebrafish oocyte, PYK2 can be activated by IP3-mediated calcium release and it is possible that activated PYK2 could induce or accelerate SFK activation in the oocyte cortex. However, at present, there is no convincing data supporting any of the above mechanisms of SFK activation in fertilized oocytes.
Summary
The Src-family PTKs perform multiple functions in oocytes as they do in most highly specialized cell types. Oocytes express a characteristic subset of this protein kinase family including FYN, YES, and FGR with the other SFKs being under-represented at the protein level. This array of kinases provides unique signaling capabilities that meet the requirements of this germ cell during meiotic maturation and fertilization. Oocytes from all species examined appear to require SFKs for establishment or maintenance of the cortical actin cytoskeleton structure as well as spindle organization and events at the G2/M transition. Oocytes that are fertilized externally also exhibit a requirement for SFKs during the fertilization-induced calcium transient which plays such an important role in rapidly establishing a permanent block to polyspermy. Since the ovulated oocyte must make the proper response to fertilization and carry out a pre-programmed egg activation process until the zygotic genome can direct operations, it must be prepared during maturation by pre-assembly of the signal transduction components needed to complete fertilization and egg activation. The SFKs must be properly expressed, undergo important post-translational modifications and protein-protein interactions, and be appropriately localized within the oocyte in order to prepare the oocyte for the responses to fertilization which occur fairly rapidly even in mammalian oocytes. This state of readiness must then be maintained for some period of time until fertilization occurs. The development of Assisted Reproduction Techniques and their application to human health have presented us with a compelling need to understand how the oocyte signaling pathways can be maintained at a proper state of readiness in the culture environment, and the biochemistry of SFK regulation in oocytes is an important aspect of this question.
Acknowledgments
The field of PTK signaling during fertilization benefitted significantly from the contributions of Dr. David L. Garbers who provided important inspiration to this author as well as many others.
Grant support: RO1 HD062860
Reference List
- 1.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Conti M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann Endocrinol (Paris) 2010;71:189–190. doi: 10.1016/j.ando.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Conti M, Hsieh M, Musa ZA, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol. 2005;15:1458–1468. doi: 10.1016/j.cub.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Maller JL. Pioneering the Xenopus oocyte and egg extract system. J Biol Chem. 2012;287:21640–21653. doi: 10.1074/jbc.X112.371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun QY. Regulating the orderly progression of oocyte meiotic maturation events in mammals. Reprod Fertil Dev. 2013;25:iii–iiv. doi: 10.1071/RDv25n3_FO. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Tokmakov AA, Fukami Y. Fertilization signalling and protein-tyrosine kinases. Comp Biochem Physiol [B] 2000;126:129–148. doi: 10.1016/s0305-0491(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 8.Kinsey WH. Tyrosine kinase signalling at fertilization. Biochem-Biophys-Res-Commun. 1997;240:519–522. doi: 10.1006/bbrc.1997.7586. [DOI] [PubMed] [Google Scholar]
- 9.Tomashov-Matar R, Levi M, Shalgi R. The involvement of Src family kinases (SFKs) in the events leading to resumption of meiosis. Mol Cell Endocrinol. 2008;282:56–62. doi: 10.1016/j.mce.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis L, Kinsey WH, Albertini DF. The functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol Reprod Dev. 2011;78:831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DC, Jia Z. Emerging structural insights into bacterial tyrosine kinases. Trends Biochem Sci. 2009;34:351–357. doi: 10.1016/j.tibs.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Manning G, Young SL, Miller WT, Zhai Y. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. Proc Natl Acad Sci U S A. 2008;105:9674–9679. doi: 10.1073/pnas.0801314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Fuente van Bentem, Hirt H. Protein tyrosine phosphorylation in plants: More abundant than expected? Trends Plant Sci. 2009;14:71–76. doi: 10.1016/j.tplants.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008;451:783–788. doi: 10.1038/nature06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim WA, Pawson T. Phosphotyrosine signaling: evolving a new cellular communication system. Cell. 2010;142:661–667. doi: 10.1016/j.cell.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu BA, Nash PD. Evolution of SH2 domains and phosphotyrosine signalling networks. Philos Trans R Soc Lond B Biol Sci. 2012;367:2556–2573. doi: 10.1098/rstb.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suga H, Dacre M, de MA, Shalchian-Tabrizi K, Manning G, Ruiz-Trillo I. Genomic survey of premetazoans shows deep conservation of cytoplasmic tyrosine kinases and multiple radiations of receptor tyrosine kinases. Sci Signal. 2012;5:ra35. doi: 10.1126/scisignal.2002733. [DOI] [PubMed] [Google Scholar]
- 19.Li W, Young SL, King N, Miller WT. Signaling properties of a non-metazoan Src kinase and the evolutionary history of Src negative regulation. J Biol Chem. 2008;283:15491–15501. doi: 10.1074/jbc.M800002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang XQ, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- 21.Perez Y, Gairi M, Pons M, Bernado P. Structural characterization of the natively unfolded N-terminal domain of human c-Src kinase: insights into the role of phosphorylation of the unique domain. J Mol Biol. 2009;391:136–148. doi: 10.1016/j.jmb.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Liang XQ, Lu Y, Wilkes M, Neubert TA, Resh MD. The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation - Role in membrane targeting, cell adhesion, and spreading. J Biol Chem. 2004;279:8133–8139. doi: 10.1074/jbc.M311180200. [DOI] [PubMed] [Google Scholar]
- 23.Xu D, Kishi H, Kawamichi H, Kajiya K, Takada Y, Kobayashi S. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. 2007;581:5227–5233. doi: 10.1016/j.febslet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Pleiman CM, Clark MR, Gauen LK, Winitz S, Coggeshall KM, Johnson GL, Shaw AS, Cambier JC. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, p56lyn which interact with the effector molecues phospholipase Cgamma, MAP kinase, GTPase activating protein, and phosphatidylinositol 3 kinase. Mol Cell Biol. 1993;13:5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16:3236–3246. doi: 10.1091/mbc.E05-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad K, Janssen O, Kapeller RRM, Cantley LC, Rudd C. SH3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3 kinase PI-3 kinase in T-cells. Proc-Natl-Acad-Sci-U-S-A. 1993;90:7366–7370. doi: 10.1073/pnas.90.15.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng Z, Thomas SM, Rickles RJ, Taylor JA, Brauer AW, Seidel-Dugan C, Michael WM, Dreyfuss G, Brugge JS. Identification of Src, Fyn, and Lyn SH3-binding proteins: implications for a function of SH3 domains. Mol Cell Biol. 1994;14:4509–4521. doi: 10.1128/mcb.14.7.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato M, Sawahata R, Takenouchi T, Kitani H. Identification of Fyn as the binding partner for the WASP N-terminal domain in T cells. Int Immunol. 2011;23:493–502. doi: 10.1093/intimm/dxr042. [DOI] [PubMed] [Google Scholar]
- 29.Lang ML, Chen YW, Shen L, Gao H, Lang GA, Wade TK, Wade WF. IgA Fc receptor (FcalphaR) cross-linking recruits tyrosine kinases, phosphoinositide kinases and serine/threonine kinases to glycolipid rafts. Biochem J. 2002;364:517–525. doi: 10.1042/BJ20011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beacham D, Ahn M, Catterall WA, Scheuer T. Sites and molecular mechanisms of modulation of Na(v)1. 2 channels by Fyn tyrosine kinase. J Neurosci. 2007;27:11543–11551. doi: 10.1523/JNEUROSCI.1743-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao R, Xi XD, Chen Z, Chen SJ, Meng G. Structural framework of c-Src activation by integrin beta3. Blood. 2013;121:700–706. doi: 10.1182/blood-2012-07-440644. [DOI] [PubMed] [Google Scholar]
- 32.Liu BA, Engelmann BW, Nash PD. The language of SH2 domain interactions defines phosphotyrosine-mediated signal transduction. FEBS Lett. 2012;586:2597–2605. doi: 10.1016/j.febslet.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 33.Zheng XM, Resnick RJ, Shalloway D. Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation. J Biol Chem. 2002;277:21922–21929. doi: 10.1074/jbc.M201394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng XM, Resnick RJ, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPa. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem. 2004;279:18887–18894. doi: 10.1074/jbc.M311274200. [DOI] [PubMed] [Google Scholar]
- 36.Twamley-Stein GM, Pepperkok R, Ansorge W, Courtneidge SA. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993;90:7696–7700. doi: 10.1073/pnas.90.16.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, Kuster B. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics. 2012;75:3465–3477. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Basu N, Bhandari R, Natarajan VT, Visweswariah SS. Cross talk between receptor guanylyl cyclase C and c-src tyrosine kinase regulates colon cancer cell cytostasis. Mol Cell Biol. 2009;29:5277–5289. doi: 10.1128/MCB.00001-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindfors HE, Drijfhout JW, Ubbink M. The Src SH2 domain interacts dynamically with the focal adhesion kinase binding site as demonstrated by paramagnetic NMR spectroscopy. IUBMB Life. 2012;64:538–544. doi: 10.1002/iub.1038. [DOI] [PubMed] [Google Scholar]
- 40.Leischner H, Albers C, Grundler R, Razumovskaya E, Spiekermann K, Bohlander S, Ronnstrand L, Gotze K, Peschel C, Duyster J. SRC is a signaling mediator in FLT3-ITD- but not in FLT3-TKD-positive AML. Blood. 2102;119:4026–4033. doi: 10.1182/blood-2011-07-365726. [DOI] [PubMed] [Google Scholar]
- 41.Evans JV, Ammer AG, Jett JE, Bolcato CA, Breaux JC, Martin KH, Culp MV, Gannett PM, Weed SA. Src binds cortactin through an SH2 domain cystine-mediated linkage. J Cell Sci. 2012;125:6185–6197. doi: 10.1242/jcs.121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banavali NK, Roux B. Flexibility and charge asymmetry in the activation loop of Src tyrosine kinases. Proteins. 2009;74:378–389. doi: 10.1002/prot.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang S, Roux B. Src kinase conformational activation: thermodynamics, pathways, and mechanisms. PLoS Comput Biol. 2008;4:e1000047. doi: 10.1371/journal.pcbi.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 45.Yadav SS, Yeh BJ, Craddock BP, Lim WA, Miller WT. Reengineering the signaling properties of a Src family kinase. Biochemistry. 2009;48:10956–10962. doi: 10.1021/bi900978f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandari V, Lim KL, Pallen CJ. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J Biol Chem. 1998;273:8691–8698. doi: 10.1074/jbc.273.15.8691. [DOI] [PubMed] [Google Scholar]
- 47.Djagaeva I, Doronkin S, Beckendorf SK. Src64 is involved in fusome development and karyosome formation during Drosophila oogenesis. Dev Biol. 2005;284:143–156. doi: 10.1016/j.ydbio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 48.O’Neill FJ, Gillett J, Foltz KR. Distinct roles for multiple Src family kinases at fertilization. J Cell Sci. 2004;117:6227–6238. doi: 10.1242/jcs.01547. [DOI] [PubMed] [Google Scholar]
- 49.Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- 50.Townley IK, Schuyler E, Parker-Gur M, Foltz KR. Expression of multiple Src family kinases in sea urchin eggs and their function in Ca2+ release at fertilization. Dev Biol. 2009;327:465–477. doi: 10.1016/j.ydbio.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 51.Stricker SA, Carroll DJ, Tsui WL. Roles of Src family kinase signaling during fertilization and the first cell cycle in the marine protostome worm Cerebratulus. Int J Dev Biol. 2010;54:787–793. doi: 10.1387/ijdb.092918ss. [DOI] [PubMed] [Google Scholar]
- 52.Steele RE, Deng JC, Ghosn CR, Fero JB. Structure and expression of Fyn genes in Xenopus Laevis. Oncogene. 1990;5:369–376-376. [PubMed] [Google Scholar]
- 53.Steele RE, Irwin M, Knudsen CL, Collett JW, Fero JB. The yes oncogene is present in amphibians and contributes to the maternal pool of rna in the oocyte. Oncogene-Res. 1989;4:223–233. [PubMed] [Google Scholar]
- 54.Kamel C, Veno PA, Kinsey WH. Quantitation of a src-like tyrosine protein kinase during fertilization of the sea urchin egg. Biochem-Biophys-Res-Commun. 1986;138:349–355. doi: 10.1016/0006-291x(86)90287-1. [DOI] [PubMed] [Google Scholar]
- 55.Kinsey WH. Biphasic activation of Fyn kinase upon fertilization of the sea urchin egg. Devel Biol. 1996;174:281–287. doi: 10.1006/dbio.1996.0073. [DOI] [PubMed] [Google Scholar]
- 56.Steele RE, Unger TF, MArdis MJ, Fero JB. The two xenopus laevis SRC genes are coexpressed and each produces functional pp60src. J Biol Chem. 1989;264:10649–10653. [PubMed] [Google Scholar]
- 57.Sato K, Aoto M, Mori K, Akasofu S, Tokmakov AA, Sahara S, Fukami Y. Purification and characterization of a Src-related p57 protein-tyrosine kinase from Xenopus oocytes. Isolation of an inactive form of the enzyme and its activation and translocation upon fertilization. J Biol Chem. 1996;271:13250–13257. doi: 10.1074/jbc.271.22.13250. [DOI] [PubMed] [Google Scholar]
- 58.Sato K, Iwao Y, Fujimura T, Tamaki I, Ogawa K, Iwasaki T, Tokmakov AA, Hatano O, Fukami Y. Evidence for the involvement of a Src-related tyrosine kinase in Xenopus egg activation. Dev Biol. 1999;209:308–320. doi: 10.1006/dbio.1999.9255. [DOI] [PubMed] [Google Scholar]
- 59.Iwasaki T, Sato K, Yoshino K, Itakura S, Kosuge K, Tokmakov AA, Owada K, Yonezawa K, Fukami Y. Phylogeny of vertebrate Src tyrosine kinases revealed by the epitope region of mAb327. J Biochem. 2006;139:347–354. doi: 10.1093/jb/mvj059. [DOI] [PubMed] [Google Scholar]
- 60.Rongish BJ, Kinsey WH. Transient nuclear localization of Fyn kinase during development in zebrafish. Anat Rec. 2000;260:115–123. doi: 10.1002/1097-0185(20001001)260:2<115::AID-AR10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, Kinsey W. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int J Devel Biol. 2000;44:837–841. [PubMed] [Google Scholar]
- 62.Talmor A, Kinsey WH, Shalgi R. Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- 63.Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev-Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- 64.Meng L, Luo J, Li C, Kinsey WH. Role of SH2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- 65.Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction. 2004;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- 66.Tsai WB, Zhang X, Sharma D, Wu W, Kinsey WH. Role of Yes kinase during early zebrafish development. Dev-Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto Y, Maruyama T, Sakai N, Sakurai R, Shimizu A, Hamatani T, Masuda H, Uchida H, Sabe H, Yoshimura Y. Expression and subcellular distribution of the active form of c-Src tyrosine kinase in differentiating human endometrial stromal cells. Mol Hum Reprod. 2002;8:1117–1124. doi: 10.1093/molehr/8.12.1117. [DOI] [PubMed] [Google Scholar]
- 68.Peaucellier G, Veno PA, Kinsey WH. Protein tyrosine phosphorylation in response to fertilization. J-Biol-Chem. 1988;263:13806–13811. [PubMed] [Google Scholar]
- 69.Jiang WP, Veno PA, Wood RW, Peaucellier G, Kinsey WH. pH regulation of an egg cortex tyrosine kinase. Dev-Biol. 1991 Jul;@146:81–88. doi: 10.1016/0012-1606(91)90448-c. [DOI] [PubMed] [Google Scholar]
- 70.Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levi M, Maro B, Shalgi R. Fyn kinase is involved in cleavage furrow ingression during meiosis and mitosis. Reproduction. 2010;140:827–834. doi: 10.1530/REP-10-0312. [DOI] [PubMed] [Google Scholar]
- 72.Sharma D, Kinsey WH. Regionalized calcium signaling in zebrafish fertilization. Int J Dev Biol. 2008;52:561–570. doi: 10.1387/ijdb.072523ds. [DOI] [PubMed] [Google Scholar]
- 73.Levi M, Maro B, Shalgi R. The involvement of Fyn kinase in resumption of the first meiotic division in mouse oocytes (NOTE:not free access) Cell Cycle. 2010:9. doi: 10.4161/cc.9.8.11299. [DOI] [PubMed] [Google Scholar]
- 74.Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- 75.Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 2002;21:5386–5395. doi: 10.1093/emboj/cdf553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng KG, Meng XQ, Yang Y, Yu YS, Liu DC, Li YL. Requirements of Src family kinase during meiotic maturation in mouse oocyte. Mol Reprod Dev. 2007;74:125–130. doi: 10.1002/mrd.20613. [DOI] [PubMed] [Google Scholar]
- 77.Ley SC, Verbi W, Pappin D, Davies A, Crumpton M. Tyrosine phosphorylation of alpha tubulin in human T lymphocytes. Eur J Immunol. 1994;24:99–106. doi: 10.1002/eji.1830240116. [DOI] [PubMed] [Google Scholar]
- 78.Campbell KS, Cooper S, Dessing M, Yates S, Buder A. Interaction of p59fyn kinase with the dynein light chain, Tctex-1, and colocalization during cytokinesis. J Immunol. 1998;161:1728–1737. [PubMed] [Google Scholar]
- 79.Wu Y, Ozaki Y, Inoue K, Satoh K, Ohmori T, Yatomi Y, Owada K. Differential activation and redistribution of c-Src and Fyn in platelets, assessed by MoAb specific for C-terminal tyrosine-dephosphorylated c-Src and Fyn. Biochim Biophys Acta Mol Cell Res. 2000;1497:27–36. doi: 10.1016/s0167-4889(00)00043-4. [DOI] [PubMed] [Google Scholar]
- 80.Yamada T, Aoyama Y, Owada MK, Kawakatsu H, Kitajima Y. Scraped-wounding causes activation and association of C-Src tyrosine kinase with microtubules in cultured keratinocytes. Cell Struct Funct. 2000;25:351–359. doi: 10.1247/csf.25.351. [DOI] [PubMed] [Google Scholar]
- 81.McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peaucellier G, Andersen AC, Kinsey WH. Protein tyrosine phosphorylation during meiotic divisions of starfish oocytes. Dev-Biol. 1990;138:391–399. doi: 10.1016/0012-1606(90)90205-w. [DOI] [PubMed] [Google Scholar]
- 83.Spivack JG, Erikson RL, Maller JL. Microinjection of pp60v-src into Xenopus oocytes increases phosphorylation of ribosomal protein S6 and accelerates the rate of progesterone-induced meiotic maturation. Mol Cell Biol. 1984;4:1631–1634. doi: 10.1128/mcb.4.8.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tokmakov A, Iwasaki T, Itakura S, Sato K, Shirouzu M, Fukami Y, Yokoyama S. Regulation of Src kinase activity during Xenopus oocyte maturation. Dev Biol. 2005;278:289–300. doi: 10.1016/j.ydbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 86.Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, Boghaert E, Arndt K, Ye F, Boschelli DH, Li F, Titsch C, Huselton C, Chaudhary I, Boschelli F. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005;65:5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- 87.Hanke JH, Gardner JP, Dow RLCPSBWH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck and Fyn-dependent T cell activation. J-Biol-Chem. 2002;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 88.Blake RA, Broome MA, Liu XD, Wu JM, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo J, McGinnis LK, Kinsey WH. Role of Fyn kinase in oocyte developmental potential. Reprod Fertil Dev. 2010;22:966–976. doi: 10.1071/RD09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Unger TF, Steele RE. Biochemical and cytological changes associated with expression of deregulated pp60src in Xenopus oocytes. Mol Cell Biol. 1992;12:5485–5498. doi: 10.1128/mcb.12.12.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thorn JM, Armstrong NA, Cantrell LA, Kay BK. Identification and characterisation of Xenopus moesin, a Src substrate in Xenopus laevis oocytes. Zygote. 1999;7:113–122. doi: 10.1017/s0967199499000465. [DOI] [PubMed] [Google Scholar]
- 94.McGinnis LK, Kinsey WH, Albertini DF. Functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wellbrock C, Schartl M. Activation of phosphatidylinositol 3-kinase by a complex of p59fyn and the receptor tyrosine kinase Xmrk is involved in malignant transformation of pigment cells. Eur J Biochem. 2000;267:3513–3522. doi: 10.1046/j.1432-1327.2000.01378.x. [DOI] [PubMed] [Google Scholar]
- 96.Saksena S, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl-/OH- exchange activity in human intestinal epithelial cells. Biochem J. 2008;416:99–108. doi: 10.1042/BJ20070960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roche S, Koegl M, Barone MV, Roussel MF, Courtneidge SA. DNA synthesis induced by some but not all growth factors requires Src family protein tyrosine kinases. Mol Cell Biol. 1995;15:1102–1109. doi: 10.1128/mcb.15.2.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taylor SJ, Shalloway D. Src and the control of cell division. Bioessays. 1996;18:9–11. doi: 10.1002/bies.950180105. [DOI] [PubMed] [Google Scholar]
- 99.Messina S, Onofri F, Bongiorno-Borbone L, Giovedi S, Valtorta F, Girault JA, Benfenati F. Specific interactions of neuronal focal adhesion kinase isoforms with Src kinases and amphiphysin. J Neurochem. 2003;84:253–265. doi: 10.1046/j.1471-4159.2003.01519.x. [DOI] [PubMed] [Google Scholar]
- 100.Samayawardhena LA, Kapur R, Craig AW. Involvement of Fyn kinase in Kit and integrin-mediated Rac activation, cytoskeletal reorganization, and chemotaxis of mast cells. Blood. 2007;109:3679–3686. doi: 10.1182/blood-2006-11-057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tournaviti S, Hannemann S, Terjung S, Kitzing TM, Stegmayer C, Ritzerfeld J, Walther P, Grosse R, Nickel W, Fackler OT. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility. J Cell Sci. 2007;120:3820–3829. doi: 10.1242/jcs.011130. [DOI] [PubMed] [Google Scholar]
- 102.Ciapa B, Borg B, Whitaker M. Polyphosphoinositide metabolism during the fertilization wave in sea urchin eggs. Development. 1992;115:187–195. doi: 10.1242/dev.115.1.187. [DOI] [PubMed] [Google Scholar]
- 103.De-Nadai C, Cailliau K, Epel D, Ciapa B. Detection of phospholipase Cgamma in sea urchin eggs. Dev-Growth-Differ. 1998;40:669–676. doi: 10.1046/j.1440-169x.1998.t01-5-00011.x. [DOI] [PubMed] [Google Scholar]
- 104.Shearer J, De Nadai C, Emily-Fenouil F, Gache C, Whitaker M, Ciapa B. Role of phospholipase Cgamma at fertilization and during mitosis in sea urchin eggs and embryos. Development. 1999;126:2273–2284. doi: 10.1242/dev.126.10.2273. [DOI] [PubMed] [Google Scholar]
- 105.Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem. 1999;274:29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- 106.Shen SS, Kinsey WH, Lee SJ. Protein tyrosine kinase-dependent release of intracellular calcium in the sea urchin egg. Dev Growth Diff. 1999;41:345–355. doi: 10.1046/j.1440-169x.1999.413436.x. [DOI] [PubMed] [Google Scholar]
- 107.Kinsey WH, Shen SS. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Devel Biol. 2000;225:253–264. doi: 10.1006/dbio.2000.9830. [DOI] [PubMed] [Google Scholar]
- 108.Runft LL, Jaffe LA. Sperm extract injection into ascidian eggs signals Ca2+ release by the same pathway as fertilization. Development. 2000;127:3227–3236. doi: 10.1242/dev.127.15.3227. [DOI] [PubMed] [Google Scholar]
- 109.Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: Where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- 110.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Genazzani AA, Mezna M, Dickey DM, Michelangeli F, Walseth TF, Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br J Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parrington J, Davis LC, Galione A, Wessel G. Flipping the switch: how a sperm activates the egg at fertilization. Dev Dyn. 2007;236:2027–2038. doi: 10.1002/dvdy.21255. [DOI] [PubMed] [Google Scholar]
- 113.Churamani D, Boulware MJ, Ramakrishnan L, Geach TJ, Martin AC, Vacquier VD, Marchant JS, Dale L, Patel S. Molecular characterization of a novel cell surface ADP-ribosyl cyclase from the sea urchin. Cell Signal. 2008;20:2347–2355. doi: 10.1016/j.cellsig.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Glahn D, Mark SD, Behr RK, Nuccitelli R. Tyrosine kinase inhibitors block sperm-induced egg activation in Xenopus laevis. Dev-Biol. 1999;205:171–180. doi: 10.1006/dbio.1998.9042. [DOI] [PubMed] [Google Scholar]
- 115.Sato KI, Tokmakov AA, Iwasaki T, Fukami Y. Tyrosine kinase-dependent activation of phospholipase Cgamma is required for calcium transient in Xenopus egg fertilization. Dev Biol. 2000;224:453–469. doi: 10.1006/dbio.2000.9782. [DOI] [PubMed] [Google Scholar]
- 116.Sato K, Tokmakov AA, He CL, Kurokawa M, Iwasaki T, Shirouzu M, Fissore RA, Yokoyama S, Fukami Y. Reconstitution of Src-dependent phospholipase Cgamma phosphorylation and transient calcium release by using membrane rafts and cell-free extracts from Xenopus eggs. J Biol Chem. 2003;278:38413–38420. doi: 10.1074/jbc.M302617200. [DOI] [PubMed] [Google Scholar]
- 117.Tokmakov AA, Sato KI, Iwasaki T, Fukami Y. Src kinase induces calcium release in Xenopus egg extracts via PLCgamma and IP3-dependent mechanism. Cell Calcium. 2002;32:11–20. doi: 10.1016/s0143-4160(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 118.Yamasaki-Mann M, Demuro A, Parker I. Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals. J Biol Chem. 2010;285:25053–25061. doi: 10.1074/jbc.M109.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Webb SE, Miller A. Calcium signalling during zebrafish embryonic development. BioEssays. 2000;22:113–123. doi: 10.1002/(SICI)1521-1878(200002)22:2<113::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 120.Webb SE, Miller AL. Ca2+ signaling and early embryonic patterning during the blastula and gastrula periods of zebrafish and Xenopus development. Biochim Biophys Acta. 2006;1763:1192–1208. doi: 10.1016/j.bbamcr.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Sharma D, Kinsey WH. PYK2: a calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Dev Biol. 2013;373:130–140. doi: 10.1016/j.ydbio.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McGinnis LK, Luo J, Kinsey WH. Protein tyrosine kinase signaling in the mouse oocyte cortex during sperm-egg interactions and anaphase resumption. Mol Reprod Dev. 2013 doi: 10.1002/mrd.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levi M, Maro B, Shalgi R. The conformation and activation of Fyn kinase in the oocyte determine its localisation to the spindle poles and cleavage furrow. Reprod Fertil Dev. 2011;23:846–857. doi: 10.1071/RD11033. [DOI] [PubMed] [Google Scholar]
- 124.Yasunaga M, Tagi T, Hanzawa N, Yasuda M, Yamanashi Y, Yamamoto T, Aizawa S, Miyauchi Y, Nishikawa S. Involvement of Fyn Tyrosine Kinase in Progression through Cytokinesis of B Lymphocyte Progenitor. J Cell Biol. 1996;132:91–99. doi: 10.1083/jcb.132.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil Cytoskeleton. 1995;30:1122–1135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- 126.Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakayama Y, Matsui Y, Takeda Y, Okamoto M, Abe K, Fukumoto Y, Yamaguchi N. c-Src but not Fyn promotes proper spindle orientation in early prometaphase. J Biol Chem. 2012;287:24905–24915. doi: 10.1074/jbc.M112.341578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Besterman B, Schultz RM. Regulation of mouse preimplantation development: inhibitory effect of genistein, an inhibitor of tyrosine protein phosphorylation, on cleavage of one-cell embryos. J Exp Zool. 1990;256:44–53. doi: 10.1002/jez.1402560107. [DOI] [PubMed] [Google Scholar]
- 129.Moore KL, Kinsey WH. Effects of protein tyrosine kinase inhibitors on egg activation and fertilization-dependent protein tyrosine kinase activity. Dev Biol. 1995;168:1–10. doi: 10.1006/dbio.1995.1056. [DOI] [PubMed] [Google Scholar]
- 130.Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev Cell. 2008;15:416–425. doi: 10.1016/j.devcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moos J, Visconti PE, Moore GD, Schultz RM, Kopf GS. Potential role of mitogen-activated protein kinase in pronuclear envelope assembly and disassembly following fertilization of mouse eggs. Biol Reprod. 1995;53:692–699. doi: 10.1095/biolreprod53.3.692. [DOI] [PubMed] [Google Scholar]
- 132.Sun QY, Wu GM, Lai L, Bonk A, Cabot R, Park KW, Day BN, Prather RS, Schatten H. Regulation of mitogen-activated protein kinase phosphorylation, microtubule organization, chromatin behavior, and cell cycle progression by protein phosphatases during pig oocyte maturation and fertilization in vitro. Biol Reprod. 2002;66:580–588. doi: 10.1095/biolreprod66.3.580. [DOI] [PubMed] [Google Scholar]
- 133.Jacquet P, Saint-Georges L, Barrio S, Baugnet-Mahieu L. Morphological effects of caffeine, okadaic acid and genistein in one-cell mouse embryos blocked in G2 by X-irradiation. Int J Radiat Biol. 1995;67:347–358. doi: 10.1080/09553009514550401. [DOI] [PubMed] [Google Scholar]
- 134.Fumagalli S, Totty NF, Hsuan JJ, Courtneidge SA. A target for Src in mitosis. Nature. 1994;368:871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- 135.Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Mol Hum Reprod. 2000;6:510–516. doi: 10.1093/molehr/6.6.510. [DOI] [PubMed] [Google Scholar]
- 136.Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127:431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- 137.Gardner AJ, Williams CJ, Evans JP. Establishment of the mammalian membrane block to polyspermy: evidence for calcium-dependent and -independent regulation. Reproduction. 2007;133:383–393. doi: 10.1530/REP-06-0304. [DOI] [PubMed] [Google Scholar]
- 138.Qian YW, Erikson E, Taieb FE, Maller JL. The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol Biol Cell. 2001;12:1791–1799. doi: 10.1091/mbc.12.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ashwell JD, D’Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20:412–416. doi: 10.1016/s0167-5699(99)01505-4. [DOI] [PubMed] [Google Scholar]
- 140.Tsujikawa K, Ichijo T, Moriyama K, Tadotsu N, Sakamoto K, Sakane N, Fukada S, Furukawa T, Saito H, Yamamoto H. Regulation of Lck and Fyn tyrosine kinase activities by transmembrane protein tyrosine phosphatase leukocyte common antigen-related molecule. Mol Cancer Res. 2002;1:155–163. [PubMed] [Google Scholar]
- 141.Pallen CJ. Protein tyrosine phosphatase alpha (PTPalpha): a Src family kinase activator and mediator of multiple biological effects. Curr Top Med Chem. 2003;3:821–835. doi: 10.2174/1568026033452320. [DOI] [PubMed] [Google Scholar]
- 142.Granot-Attas S, Elson A. Protein tyrosine phosphatase epsilon activates Yes and Fyn in Neu-induced mammary tumor cells. Exp Cell Res. 2004;294:236–243. doi: 10.1016/j.yexcr.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 143.Hebert CE, Dupuy JW, Letellier T, Dachary-Prigent J. Functional impact of PTP1B-mediated Src regulation on oxidative phosphorylation in rat brain mitochondria. Cell Mol Life Sci. 2011;68:2603–2613. doi: 10.1007/s00018-010-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu W, Kinsey WH. Role of PTPase(s) in regulating Fyn kinase at fertilization of the zebrafish egg. Dev Biol. 2002;247:286–294. doi: 10.1006/dbio.2002.0697. [DOI] [PubMed] [Google Scholar]
- 145.Maruyama R, Velarde NV, Klancer R, Gordon S, Kadandale P, Parry JM, Hang JS, Rubin J, Stewart-Michaelis A, Schweinsberg P, Grant BD, Piano F, Sugimoto A, Singson A. EGG-3 regulates cell-surface and cortex rearrangements during egg activation in Caenorhabditis elegans. Curr Biol. 2007;17:1555–1560. doi: 10.1016/j.cub.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 146.Parry JM, Velarde NV, Lefkovith AJ, Zegarek MH, Hang JS, Ohm J, Klancer R, Maruyama R, Druzhinina MK, Grant BD, Piano F, Singson A. EGG-4 and EGG-5 Link Events of the Oocyte-to-Embryo Transition with Meiotic Progression in C. elegans. Curr Biol. 2009;19:1752–1757. doi: 10.1016/j.cub.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parry JM, Singson A. EGG molecules couple the oocyte-to-embryo transition with cell cycle progression. Results Probl Cell Differ. 2011;53:135–151. doi: 10.1007/978-3-642-19065-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: A mechanism to regulate activity and subcellular localization of Src kinases. Mol Biol Cell. 1999;10:3489–3505. doi: 10.1091/mbc.10.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 150.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]