To the Editor:
Primary immunodeficiency diseases (PIDDs) are congenital disorders caused by inherent defects in the immune system that typically present with recurrent or severe infections but can also involve autoimmune disease, lymphoproliferation, or allergy.1 Allergic diseases are an important expression of misdirected immunity, and certain PIDDs are frequently associated with atopy. Selective IgA deficiency (SIgAD) has been associated with asthma, allergic rhinitis, atopic dermatitis (AD), and food allergy (FA).2-4 AD/eczema with an increased IgE level is often associated with hyper-IgE syndrome (HIES), Omenn syndrome, immune dysregulation–polyendrocrinopathy–enteropathy–X-linked syndrome, and Comel-Netherton syndrome, whereas 80% to 100% of patients with Wiskott-Aldrich syndrome (WAS) present with AD-like skin problems4,5 and many have evidence of food reactivity. However, there are no definitive data in the United States on the overall prevalence of food allergies as a specific expression of clinically relevant atopy in patients with PIDDs. We present the first broad consideration of the prevalence of FA in patients with PIDDs using the US Immunodeficiency Network (USIDNET).
The USIDNET is a research consortium established to advance scientific research in PIDDs.6 A query of 2923 patients with PIDDs registered in the USIDNET was performed to determine the responses to fields in the core registry form relevant to “allergic reactions to food” and “AD/eczema,” which were then cross-referenced to diagnosis. AD/eczema was included in the query as another manifestation of atopy.
Of 2923 patients registered, 2263 provided responses to the relevant fields (allergic reactions to food and AD/eczema). There were 14 diagnoses associated with FA, AD, or both (Table I). Surprisingly, in aggregate, the prevalence of both FA and AD in patients with PIDDs (FA, 1.8%; AD, 6%) was lower than that in the general population (FA, 2.5%; AD, 10.7%; Fig 1).7,8 This stands in contrast to experience in other countries, such as Kuwait, where 19% of patients with PIDDs have FA.4 Currently, there are no other prevalence data available for FA and AD in the United States, and the USIDNET experience potentially distinguishes American patients from those in other countries or reflects the experience of those centers and clinics in the United States contributing data to the USIDNET.
TABLE I.
Prevalence of FA and AD among patients with PIDDs
| PIDDs (n = 2263) | Age (y), mean/median (range) | FA (n = 40) | AD (n = 136) |
|---|---|---|---|
| Agammaglobulinemia (n = 339) | 37.5/42 (18-42) | 0.6% (2) | 1.5% (5) |
| X-linked (n = 332) | 0.3% (1) | 1.5% (5) | |
| Unknown genetic cause (n = 7) | 14.29% (1) | NR | |
| CD40 ligand deficiency (n = 13) | NR | 7.7% (1) | NR |
| Chronic granulomatous disease, X-linked (n = 283) | 16.5/16.5 (13-20) | NR | 0.7% (2) |
| CID (n = 3) | 15 | 33.3% (1) | 33.3% (1) |
| CSR defects and HIGM syndromes, unknown genetic cause (n = 112) | 32 | NR | 0.9% (1) |
| CVID (n = 773) | 36.6/33 (10-82) | 3.1% (24) | 4.4% (34) |
| DiGeorge syndrome (n = 362) | 6.7/7 (6-7) | 0.3% (1) | 0.6% (2) |
| Chromosome 22q11.2 deletion (n = 314) | NR | 0.3% (1) | |
| Unknown genetic cause (n = 48) | 2.1% (1) | 2.1% (1) | |
| HIES (n = 16) | 22/18.5 (14-37) | 6.3% (1) | 25% (4) |
| STAT3 (n = 5) | 20% (1) | 60% (3) | |
| Unknown genetic cause (n = 11) | NR | 9.1% (1) | |
| Selective IgA deficiency (n = 4) | 8 | 25% (1) | NR |
| Other hypogammaglobulinemias (n = 28) | 52/63.5 (10-71) | 7.1% (2) | 7.1% (2) |
| NEMO deficiency (n = 8) | 11.2/10 (7-18) | NR | 62.5% (5) |
| SCID, undefined (n = 131) | 17 | NR | 0.8% (1) |
| Selective IgM deficiency (n = 3) | 53 | NR | 33.3% (1) |
| WAS (n = 188) | 31.7/30.5 (6-62) | 3.7% (7) | 41.5% (78) |
| Mutations in WASP (n = 14) | 21.4% (3) | 7.1% (1) | |
| Unknown genetic cause (n = 173) | 1.7% (3) | 44.5% (77) | |
| X-linked thrombocytopenia with mutations in WASP (n = 1) | 100% (1) | NR | |
| Total | 1.77% | 6.01% |
CSR, Class-switch recombination; HIGM, hyper-IgM; NEMO, nuclear factor κB essential modulator; NR, not reported; SCID, severe combined immunodeficiency; STAT3, Signal transducer and activator of transcription 3; WASP, Wiskott-Aldrich syndrome protein.
FIG. 1.
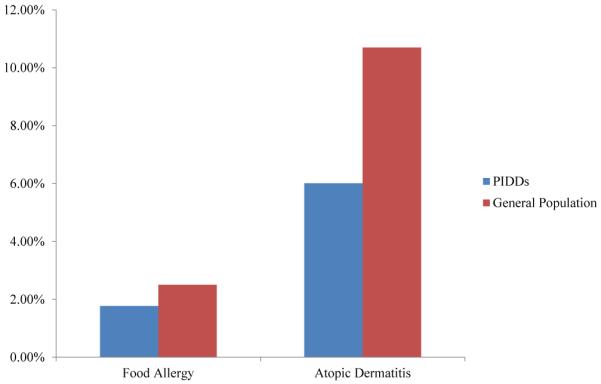
Prevalence of FA and AD among patients with PIDDs compared with the general population.
Despite there not being an overall increased prevalence, FA was more commonly reported in patients with specific PIDDs when compared with what would be expected in the general population. This included patients with CD40 ligand deficiency (7.7%), primary hypogammaglobulinemia (7.1%), and HIES (6.3%). There was also an increased prevalence in patients with combined immunodeficiency (CID; 33.3%) and SIgAD (25%), but there were very few of these patients in the registry. Reported reactions include anaphylaxis, angioedema, asthma, bronchospasm, dermatitis, unspecified gastrointestinal reactions, and urticaria, with the most frequent being anaphylaxis (20%). Seventeen of the 40 patients with FA did not have a specified type of allergic reaction. Meanwhile, AD was most commonly reported in patients with nuclear factor κB essential modulator deficiency (62.5%), WAS (41.5%), CID (33.3%), selective IgM deficiency (33.3%), and HIES (25%). PIDDs that were associated with both FA and AD included common variable immunodeficiency (CVID), CID, and HIES.
Despite the low numbers of patients, FA was found in 25% of those with SIgAD, which is similar to the findings (30%) of Aghamohammadi et al9 in a prospective cohort of Iranian patients with SIgAD and self-reported FA. In another prospective study children with SIgAD had an increased risk of parentally reported food hypersensitivity at 4 years of age.2 Because the majority of patients with SIgAD have substitution of secretory IgA with secretory IgM, it is not known whether they have adequate mucosal protection. This might allow food antigens to pass more efficiently through the gastrointestinal mucosa and predispose patients with SIgAD to FA. On the other hand, AD was not present in patients with SIgAD at the time of our query. It is possible that patients with SIgAD have a higher incidence of pure IgE-mediated rather than mixed or non–IgE-mediated manifestations of disease.
The reports on AD in patients around the world with SIgAD are conflicting. In the Iranian cohort AD was present in 52% of patients with SIgAD,9 whereas it was found in only 2.3% of Brazilian patients with SIgAD.2 This discrepancy might be due to differing physician algorithms for AD diagnosis. In another study from Sweden by Janzi et al,2 parentally reported eczema was not associated with SIgAD.
Other diagnoses associated with FA-related disease were more prevalent than in the general presentation, such as CD40 ligand deficiency, hypogammaglobulinemia, HIES, WAS, and CVID. HIES and WAS are associated with increased IgE levels and increased sensitization to specific food antigens. However, a limitation in this query is that genetic diagnosis of HIES was not further characterized, thus allowing for inclusion of patients with increased IgE levels for reasons other than signal transducer and activator of transcription 3, dedicator of cytokinesis 8, or tyrosine kinase 2, some of which might be purely atopic in nature. These molecularly defined PIDDs, except for primary hypogammaglobulinemia, are definitively associated with the substantially altered or defective T-cell immunity necessary for oral tolerance. This deficient immune regulation can lead to a skewed response, resulting in atopic disease. The high prevalence of FA in patients with primary hypogammaglobulinemia might suggest a concomitant T-cell dysfunction in these cases.
Importantly, the number of patients with FA, AD, or both registered in the USIDNET at the time of the query was small, and this might underrepresent PIDDs associated with atopy. The overall lower prevalence of FA in patients with PIDDs might also be underestimated because 55% of patients had severe combined immunodeficiency, X-linked agammaglobulinemia, or CVID. A follow-up study should be undertaken in the future. Another limitation of the study is that it is based on a physician report questionnaire form; hence FA and AD rates could be overestimated. Moreover, there was a limitation in the core registry form for further description of reactions to food and AD/eczema.
Manifestations of atopy, such as FA and AD, occurring early in life might be a presenting feature before diagnosis of a PIDD4,5 and, if associated with recurrent infections, should warrant clinicians to pursue an immunologic evaluation. Early diagnosis of PIDDs might have a potential benefit of better outcome. Although the prevalence of both FA and AD in patients with PIDDs is lower than in the general population, there should be a lower threshold for suspecting FA in patients with certain PIDDs.
Acknowledgments
We thank the staff of the USIDNET and contributing sites for enabling this query. Per USIDNET policy, any site contributing more than 10% of the patients included in this analysis was to be offered authorship; however, no single site contributing to the registry contributed more than 10% of patients with PIDDs and FA or AD.
Footnotes
Disclosure of potential conflict of interest: K. S. Tuano is employed by Baylor College of Medicine. J. S. Orange has consultant arrangements with Baxter Healthcare, CSL Behring, ASD Healthcare, and Atlantic Research; has received research support from CSL Behring; and has received royalties from UpToDate, Unimed, and Springer. K. Sullivan is a board member for and has consultant arrangements with the Immune Deficiency Foundation, has received research support from Baxter, has received royalties from UpToDate, and has received payment for development of educational presentations from Yeshiva University. F. A. Bonilla has consultant arrangements with Baxter, The Cowen Group, CSL Behring, Gerson-Lehrman Group, Grand Rounds Health, Green Cross/American Research Group, the Immune Deficiency Foundation, and Octapharma; has received payment for lectures from Albany Medical College; has received royalties from UpToDate in Medicine; and has received travel expenses from Sheikh Khalifa Medical City. C. M. Davis is employed by Baylor College of Medicine; has provided expert testimony on behalf of Lapin and Landa, LLP; and has received research support from the Penland Foundation, the Food Allergy Initiative, and the American Academy of Allergy, Asthma & Immunology. C. Cunningham-Rundles declares no relevant conflicts of interest.
REFERENCES
- 1.Notarangelo LD. Primary immunodeficiencies. J Allergy Clin Immunol. 2010;125(suppl):S182–94. doi: 10.1016/j.jaci.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 2.Janzi M, Kull I, Sjoberg R, Wan J, Melen E, Bayat N, et al. Selective IgA deficiency in early life: Association to infections and allergic diseases during childhood. Clin Immunol. 2009;133:78–85. doi: 10.1016/j.clim.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Jacob CMA, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC. Autoimmunity in IgA deficiency: revisiting the role of IgA as a silent housekeeper. J Clin Immunol. 2008;28(suppl 1):S56–61. doi: 10.1007/s10875-007-9163-2. [DOI] [PubMed] [Google Scholar]
- 4.Sillevis Smitt JH, Kuijpers TW. Cutaneous manifestations of primary immunodeficiency. Curr Opin Pediatr. 2013;25:492–7. doi: 10.1097/MOP.0b013e3283623b9f. [DOI] [PubMed] [Google Scholar]
- 5.Al-Herz W, Nanda A. Skin manifestations in primary immunodeficient children. Pediatr Dermatol. 2011;28:494–501. doi: 10.1111/j.1525-1470.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 6.USIDNET.org [Accessed January 14, 2014]; Available at: http://www.usidnet.org/
- 7.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126:798–806. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanifin JM, Reed ML, Eczema Prevalence and Impact Working Group A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91. doi: 10.2310/6620.2007.06034. [DOI] [PubMed] [Google Scholar]
- 9.Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, et al. IgA deficiency: correlation between clinical and immuno-logical phenotypes. J Clin Immunol. 2009;29:130–6. doi: 10.1007/s10875-008-9229-9. [DOI] [PubMed] [Google Scholar]


