Abstract
We used data from a prospective study of 300 women attending a sexually transmitted infection clinic in Kingston, Jamaica, to compare participant self-report of recent semen exposure to actual semen exposure measured by prostate-specific antigen in vaginal swabs. Underreporting of semen exposure was significantly more frequent at follow-up than baseline, suggesting the accuracy of reports of sexual behavior may vary over time.
Study participants may misreport sexual behaviors (intentionally or not). Efforts to quantify misreporting have ranged widely from measuring self-reported virginal status among pregnant women1 or sexually transmitted infections (STIs) among young adults reporting sexual abstinence2 to detecting biological markers such as semen among women reporting no recent sexual exposure3 and drug levels in hair as evidence of pre-exposure prophylaxis use.4 Despite the increasing recognition of the need for improved measures, HIV prevention studies continue to depend on participant reports of sensitive behaviors. This practice is problematic. For example, the association detected in observational studies between injectable contraception and HIV acquisition5 could be explained by differential condom use between study arms. Hormonal contraception users might have more unprotected sex with HIV-infected partners than nonusers, yet participant reports of sexual activity and condom use may be inadequate for controlling for this difference in risk.6
One argument supporting continued use of self-reported measures could be that any misreporting is assumed to be nondifferential between arms in comparison trials or in a population over time, which would tend to conservatively bias results toward the null.7 To examine this question, we assessed the consistency of misreporting over time by comparing self-reports to a semen biomarker at baseline and follow-up among females participating in a trial on the effectiveness of counseling messages conducted during 2010–2011.8
Participants consisted of nonpregnant, HIV-negative women ≥18 years of age attending a public STI clinic in Kingston, Jamaica who were prescribed syndromic treatment for cervicitis or vaginal discharge according to standard care.8 Women were randomized to receive a counseling message promoting either abstinence alone or abstinence backed up by promotion and provision of condoms for the treatment period of ≥7 days. At enrollment and the 6-day follow-up visit, a study clinician collected vaginal swabs to test for prostate-specific antigen (PSA), and study staff administered a questionnaire. Women had to give written consent for screening, enrollment and PSA testing to be part of this analysis. Ethical review committees at the Jamaican Ministry of Health and the US Centers for Disease Control and Prevention approved the research.
PSA detected in vaginal fluid is a marker of semen exposure occurring within the past 48 hours.9 On-site laboratory staff used previously described methods10 to test for PSA with ABAcard p30 (Abacus Diagnostics, West Hills, CA), which produces results that can be interpreted semi-quantitatively (negative, low positive, or high positive). We dichotomized the results (negative versus low or high positive). We used a chi-squared test to evaluate differences between enrollment and follow-up visits in the proportion of women who self-reported no recent semen exposure (i.e., either no sex without a condom or no sex in the past 2 days) among those with PSA positivity.
Of 300 women enrolled in the trial, 7 failed to return for follow up, 7 declined to consent to PSA testing, and 1 had missing PSA data at enrollment. Thus, the current analysis is based on 286 women (95% of randomized women) who completed 571 visits (285 enrollment and 286 follow-up visits). Altogether, 16.8% of participants (48/286) had PSA detected at ≥1 study visit. Similar proportions of women had PSA detected at enrollment (8.4%) and follow up (10.1%) (Figure). Fifteen percent of women reported having sex without a condom in the past 2 days, with similar percentages at enrollment (15.3%) and follow up (14.7%). Among visits where PSA was detected, however, in nearly two-thirds (64%) of visits, the participant denied having unprotected sex in the preceding 2 days. Notably, the proportion of women with biological evidence of semen exposure who reported no unprotected sex increased significantly between enrollment (50%) and follow up (75.9%) (P=0.05).
Figure.
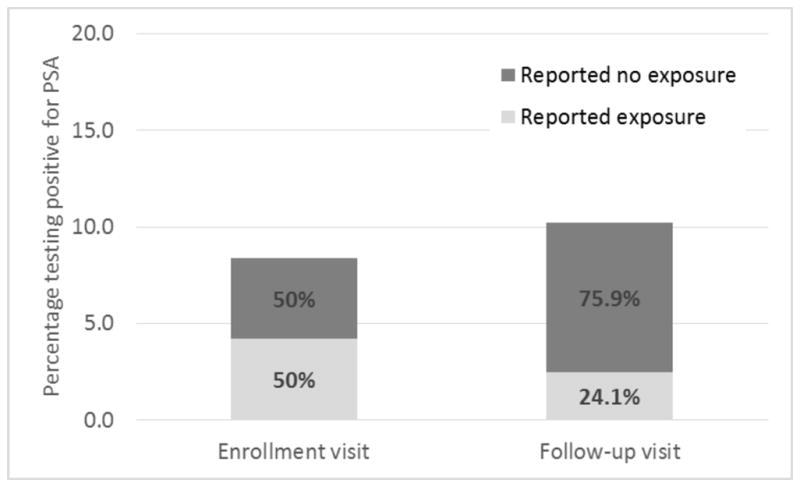
Reported versus actual semen exposurea among women attending a sexually transmitted infection clinic (n=300), Kingston, Jamaica, 2010–2011
aAs determined by vaginal fluid swabs testing positive for prostate-specific antigen, a marker of semen exposure.
Women may have perceived more pressure not to disclose unprotected sex at follow up because of the counseling at enrollment, which instructed them to be abstinent while on syndromic treatment. As previously revealed in a qualitative study conducted among a subset of the participants informed about their PSA positivity (following rapid testing at follow up), this social desirability bias may have prevented women from reporting failure to adhere to clinic staff’s instructions.11 In contrast, at enrollment, women were asked to report on their behaviors, including unprotected sex, before any counseling messages were provided. Whether misreporting would have regressed to baseline levels (from participants becoming desensitized to counseling messages) or would have increased (from effect of cumulative messages) had our trial been longer is unknown. A previous study of female sex workers in Kenya, which compared participant reports and PSA outcomes at both enrollment and 12-month follow-up visits, did not find differences in discordancy between the two study visits.12 Collectively, these findings suggest that the changes in accuracy could differ by study population, research question, or trial procedures.
A recent analysis found no evidence of differential misclassification of semen exposure between hormonal contraception users and nonusers.13 Previous studies, though, have found that having discordant biological and self-reported measures of semen exposure was associated with a range of characteristics and behaviors: study site, race/ethnicity, age, education, use of amphetamine-type stimulants, self-reported injection drug use, number of partners, self-perceived risk of HIV infection, or infection with HIV, human papillomavirus, bacterial vaginosis, or chlamydia.14–17 This further evidence that misclassification does not occur at random suggests that attempts to adjust for self-reported condom use could actually introduce bias, occurring in any direction.
Identifying those women at risk of HIV/STIs from engaging in unprotected sex could be useful in many settings. Public health interventions to prevent these diseases could improve their efficiency by targeting sexually-active people who are not consistent condom users. Also, research on interventions to prevent HIV/STIs could benefit from collection of objective biologic measures of semen exposure. The interpretation of data from HIV prevention trials involving only self-reports from participants is more complicated than previously suspected given the present results indicating that participant underreports of semen exposure may change over time.
Summary.
Participant underreporting of exposure to semen increased over time. Misclassification of participant reports of sensitive behaviors cannot be assumed to occur at random.
Acknowledgments
Source of Funding: The trial was funded by the Division of Reproductive Health, CDC through a cooperative agreement maintained by USAID with FHI 360 (Contraceptive and Reproductive Health Technology Research and Utilization Project (CRTU), Agreement no.GPO-A-00-05-00022). Laboratory activities for the study were supported in part by the Southeastern Sexually Transmitted Infections Cooperative Research Center of the NIAID (U19-AI031496). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Conflicts of Interest
The authors have no conflicts of interests.
References
- 1.Herring AH, Attard SM, Gordon-Larsen P, et al. Like a virgin (mother): analysis of data from a longitudinal, US population representative sample survey. BMJ. 2013;347:f7102. [Google Scholar]
- 2.DiClemente RJ, Sales JM, Danner F, et al. Association between sexually transmitted diseases and young adults’ self-reported abstinence. Pediatrics. 2011;127:208–213. doi: 10.1542/peds.2009-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo MF, Steiner MJ, Hobbs MM, et al. Biological markers of sexual activity: tools for improving measurement in HIV/STI prevention research. Sex Transm Dis. 2013;40:447–452. doi: 10.1097/OLQ.0b013e31828b2f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137:696–697. doi: 10.7326/0003-4819-137-8-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz SR, Pettifor A, Stuart GS, Cohen MS. Hormonal contraception and HIV: the methods have confused the message. AIDS. 2013;27:S45–53. doi: 10.1097/QAD.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 7.Rothman KJ, Greenland S, Lash TL. Validity in epidemiologic studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. pp. 128–147. [Google Scholar]
- 8.Anderson C, Gallo MF, Hylton-Kong T, et al. Randomized controlled trial on the effectiveness of counseling messages for avoiding unprotected sexual intercourse during STI and RTI treatment among female patients. Sex Transm Dis. 2013;40:105–110. doi: 10.1097/OLQ.0b013e31827938a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59:195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs MM, Steiner MJ, Rich KD, et al. Good performance of rapid prostate-specific antigen test for detection of semen exposure in women: implications for qualitative research. Sex Trans Dis. 2009;36:501–506. doi: 10.1097/OLQ.0b013e3181a2b4bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter MW, Bailey A, Snead MC, et al. Exploring discordance between biologic and self-reported measures of semen exposure: a qualitative study among female patients attending an STI clinic in Jamaica. AIDS Behav. 2013;17:728–736. doi: 10.1007/s10461-012-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo MF, Behets FM, Steiner MJ, et al. Validity of self-reported “safe sex” among female sex workers in Mombasa, Kenya – PSA analysis. Int J of STD & AIDS. 2007;18:33–38. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 13.McCoy SI, Ralph LJ, Padian NS, et al. Are hormonal contraceptive users more likely to misreport unprotected sex? Evidence from a biomarker validation study in Zimbabwe. AIDS Behav. 2014;18:2259–2264. doi: 10.1007/s10461-014-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo MF, Behets FM, Steiner MJ, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33:476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 15.Aho J, Koushik A, Diakite SL, et al. Biological validation of selfreported condom use among sex workers in Guinea. AIDS Behav. 2010;14:1287–1293. doi: 10.1007/s10461-009-9602-6. [DOI] [PubMed] [Google Scholar]
- 16.Gallo MF, Sobel JD, Rompalo AM, et al. Discordance between spermatozoa detection and self-reported semean exposure. Sex Transm Dis. 2011;38:909–912. doi: 10.1097/OLQ.0b013e318223be4b. [DOI] [PubMed] [Google Scholar]
- 17.Evans JL, Couture MC, Stein ES, et al. Biomarker validation of recent unprotected sexual intercourse in a prospective study of young women engaged in sex work in Phnom Penh, Cambodia. Sex Transm Dis. 2013;40:462–468. doi: 10.1097/OLQ.0b013e318286db8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mose F, Newman LP, Njunguna R, et al. Biomarker evaluation of self-reported condom use among women in HIV-discordant couples. Int J STD AIDS. 2013;24:537–540. doi: 10.1177/0956462412473892. [DOI] [PMC free article] [PubMed] [Google Scholar]


