Abstract
Objective
To evaluate factors affecting sentinel lymph node (SLN) identification after neoadjuvant chemotherapy (NAC) in patients with initial node-positive breast cancer.
Summary Background Data
SLN surgery is increasingly used for nodal staging after NAC and optimal technique for SLN identification is important.
Methods
The American College of Surgeons Oncology Group Z1071 prospective trial enrolled clinical T0-4,N1-2,M0 breast cancer patients. Following NAC, SLN surgery and axillary lymph node dissection (ALND) were planned. Multivariate logistic regression modeling assessing factors influencing SLN identification was performed.
Results
Of 756 patients enrolled, 34 women withdrew, 21 were ineligible, 12 underwent ALND only, and 689 had SLN surgery attempted. At least one SLN was identified in 639 patients (92.7%: 95%CI: 90.5–94.6%). Among factors evaluated, mapping technique was the only factor found to impact SLN identification; with use of blue dye alone increasing the likelihood of failure to identify the SLN relative to using radiolabelled colloid +/− blue dye (p=0.006; OR=3.82 95%CI: 1.47-9.92). The SLN identification rate was 78.6% with blue dye alone; 91.4% with radiolabelled colloid and 93.8% with dual mapping agents. Patient factors (age, BMI), tumor factors (clinical T or N stage), pathologic nodal response to chemotherapy, site of tracer injection and length of chemotherapy treatment did not significantly affect the SLN identification rate.
Conclusions
The SLN identification rate after NAC was higher when mapping was performed using radiolabelled colloid alone or with blue dye compared to blue dye alone. Optimal tracer use is important to ensure successful identification of SLN(s) after NAC.
Keywords: sentinel node, neoadjuvant chemotherapy, breast cancer, identification rate
Introduction
The status of the axillary lymph nodes is an important prognostic factor in breast cancer. It has been used to guide local-regional and systemic treatment decisions and surgical removal of the axillary nodes facilitates staging and provides regional control in those with axillary metastases. In the past, axillary staging was accomplished through axillary lymph node dissection (ALND). However, in women with clinically node-negative (cN0) disease, sentinel lymph node (SLN) surgery has replaced ALND as the initial approach. SLN identification rates in primary breast surgery have increased from 64% in the early 1990s(1) to 81% in the late 1990s(2,3) and 91 to 100% in the 2000s.(4,5) Randomized trials have demonstrated that SLN surgery is technically feasible in women presenting with cN0 disease, with identification rates exceeding 97% and false-negative rates of less than 10%.(6) For patients with a negative SLN, regional control, disease-free and overall survival are equivalent between SLN surgery compared with those undergoing ALND. This allows patients with negative SLNs to avoid ALND and its associated morbidities which can include paresthesias, lymphedema and decreased range of motion.(7–9)
While SLN surgery for axillary staging has been firmly established in the management of patients with cN0 disease undergoing primary surgery, the question of when to perform SLN surgery in patients receiving neoadjuvant chemotherapy (NAC) is debated. Some prefer to perform SLN surgery prior to NAC primarily for staging the axilla. However, as the importance of determining response to chemotherapy has emerged and chemotherapy has been shown to reduce the incidence of node positive disease after chemotherapy, SLN surgery after NAC has become more widely utilized. Initial reports from single institutions showed variability in SLN identification rates after NAC from 72 to 100% and false-negative rates of 0-33%,(10–24) with pooled data reporting a SLN identification rate of 90% and false-negative rate of 12%.(25)
Some of the initial studies of SLN surgery after NAC included patients with clinically node-positive disease at presentation. Overall, the data for SLN surgery after NAC in women who present with node-positive disease is sparse with identification rates ranging from 78% to 98% and false-negative rates as high as 40%.(26,27,28,29,30) For these reasons, ALND has remained the standard practice in this patient population.(27)
With current systemic therapy regimens, approximately 40% of patients with node-positive disease convert to node-negative after NAC. Therefore there has been significant enthusiasm for exploring the use of SLN surgery for nodal staging after NAC as a less invasive alternative compared with ALND. This was the focus of the American College of Surgeons Oncology Group (ACOSOG) Z1071 trial which assessed feasibility and accuracy of SLN surgery after NAC in patients presenting with node-positive disease documented by needle biopsy at diagnosis. In this report, we evaluate the factors affecting the SLN identification rate in this multi-institutional clinical trial.
Methods
ACOSOG Z1071 was a prospective clinical trial enrolling women with clinical T0-4, N1-2, M0 breast cancer who received NAC. Details of the study have been previously described.(31) A total of 756 women were enrolled from July 2009 to July 2011. All patients had cytologically or histologically proven axillary lymph node metastases by percutaneous fine needle aspiration (FNA) or core needle biopsy of at least one axillary lymph node prior to initiation of NAC. Axillary surgery prior to NAC was not allowed. All patients were treated with NAC with the type and length of chemotherapy left to the discretion of the treating medical oncologist. After completion of NAC at the time of definitive breast surgery, all patients were to undergo SLN surgery with concomitant ALND.
The protocol language encouraged the use of dual tracer SLN mapping with both radiolabeled colloid and a blue dye (isosulfan or methylene blue) however this was not mandatory. The site and timing of mapping agent administration was at the physician’s discretion. All radioactive and/or blue lymph nodes and palpable nodes were to be excised and submitted as SLNs. After SLN surgery had been completed, ALND was performed. Data was collected prospectively and operative reports and pathology reports were submitted for central review.
The study was designed to assess whether the false-negative rate of SLN surgery after NAC in women with cN1 who had at least two SLNs identified was greater than 10%. It was anticipated that a number of women would not have a SLN identified. As such, a secondary aim was to examine the factors impacting failure to identify a SLN. Patient and disease characteristics, as well as SLN mapping techniques, were examined for their impact on the failure to identify a SLN. Chi-square test and logistic regression modeling with likelihood ratio tests were used to assess whether the likelihood of identifying a SLN differed with respect to patient, tumor, or surgical treatment related factors. All statistical analyses were performed by Alliance statisticians using SAS 9.2. A p-value ≤ 0.05 was considered significant.
All patients provided informed consent and all enrolling institutions were required to have IRB approval. The trial was registered on clinicaltrials.gov with trial identifier NCT00881361.
Results
Of the 756 patients enrolled on ACOSOG Z1071, 34 women withdrew prior to surgery, 21 were ineligible and 12 underwent ALND only (Figure 1). The 689 women who had attempted SLN surgery make up the cohort for this analysis, two of these women did not undergo ALND. Table 1 shows the demographic, disease and surgical treatment characteristics of the 689 women who underwent SLN surgery after NAC.
Figure 1.
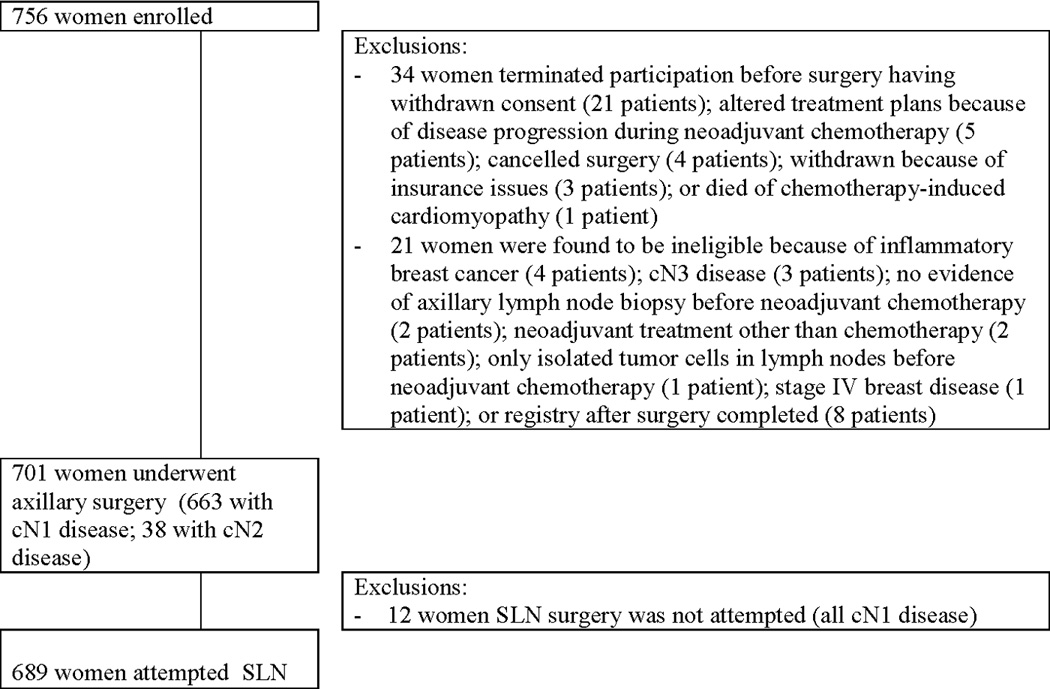
CONSORT diagram
Table 1.
Patient and tumor characteristics of 689 women enrolled in Z1071 who underwent SLN surgery after neoadjuvant chemotherapy
| N=689 | |
|---|---|
| Median age (range) | 49 (23-93) |
| N (%) | |
| Age | |
| Age < 50 years old | 345 (50.0) |
| Age ≥ 50 years old | 344 (50.0) |
| ECOG performance status | |
| 0 | 556 (80.7) |
| 1 | 133 (19.3) |
| Race | |
| White | 555 (80.6) |
| Black or African American | 98 (14.2) |
| Other/Not reported | 36 (5.2) |
| Type of axillary LN biopsy | |
| Core needle biopsy | 420 (61.0) |
| Fine needle aspiration | 269 (39.0) |
| Clinical N stage at diagnosis | |
| N1 | 651 (94.5) |
| N2 | 38 (5.5) |
| Clinical T stage at diagnosis | |
| T0/Tis | 6 (0.9) |
| T1 | 90 (13.1) |
| T2 | 379 (55.0) |
| T3 | 182 (26.4) |
| T4 | 32 (4.6) |
| Approximated subtype | |
| Hormone receptor positive/HER2 negative | 311 (45.3) |
| Triple negative | 166 (24.1) |
| HER2 positive | 209 (30.5) |
| Not available | 3 (0.4) |
| Neoadjuvant chemotherapy regimen | |
| Anthracycline and taxane | 515 (74.8) |
| Anthracycline-based chemotherapy | 44 (6.4) |
| Taxane based chemotherapy | 118 (17.1) |
| No anthracycline or taxane | 12 (1.7) |
| Anti-HER2 therapy (HER2 positive patients (n=209) | |
| Yes | 185 (88.5) |
| No | 24 (11.5) |
SLN technique and surgical findings
SLN mapping was performed using blue dye only in 28 (4.1%) cases; radiolabeled colloid only in 116 (16.8%) cases and dual tracer in 545 (79.1%) cases. The radiolabeled colloid was injected the day before surgery in 165 (25.0%) cases and on the morning of surgery in 496 (75.0%) cases.
At least one SLN was detected in 639 women, resulting in a SLN identification rate of 92.7% (95% CI: 90.5 – 94.6%). The SLN identification rate was 92.9% among the 651 patients with cN1 disease (95%CI: 90.7-94.8) and 89.5% among the 38 women with cN2 disease (95%CI: 75.2-97.1) at presentation (p=0.42).
The median number of SLNs resected by the surgeon was 2. The median number of SLNs identified by the pathologist at histologic examination was 3 (range 0 to 13). At surgery there were no SLNs identified in 50 (7.3%) patients, one in 86 (12.5%) patients, two in 165 (23.9%), three in 154 (22.4%), four in 95 (13.8%) and five or more in 139 (20.2%). Figure 2 provides the distribution of the number of SLNs examined histologically by the pathologists.
Figure 2.
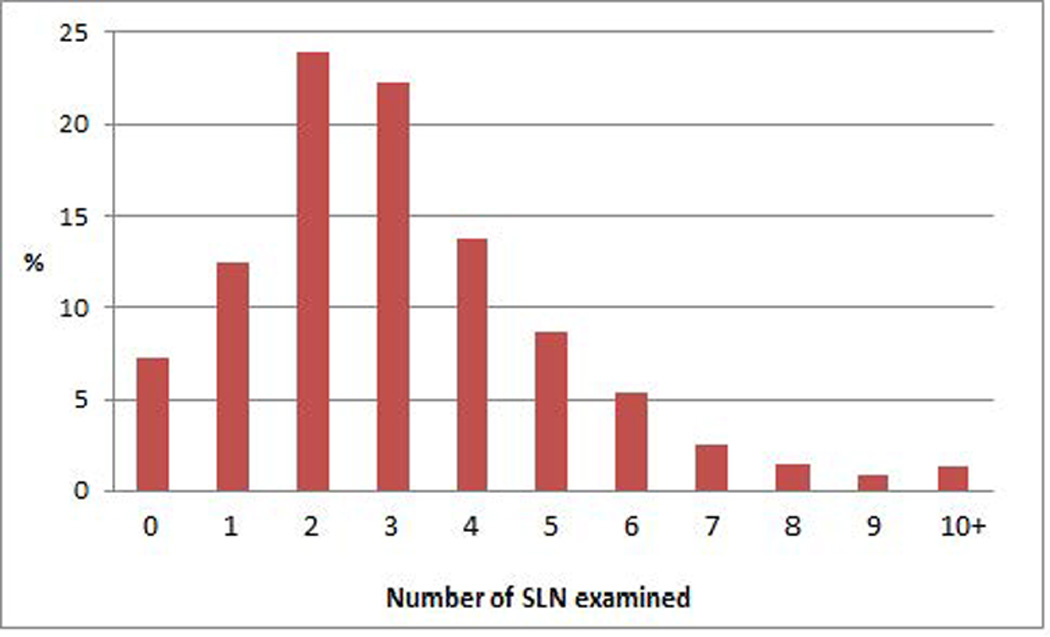
Number of sentinel lymph nodes examined histologically
Factors impacting SLN identification
On univariate analysis, SLN identification rates did not differ by age (50 or older); being overweight or obese; clinical T stage (T0-1 vs. T2 vs. T3-4); clinical N stage (N1 vs. N2); histologic subtype; duration of chemotherapy (≤ 6 months vs. > 6 months); presence of palpable adenopathy after NAC; or site of injection of mapping agents (Table 2). Tumor subtype did not impact SLN identification with similar identification rates between hormone receptor positive/HER2-negative disease (92.3%), HER2-positive disease (91.8%) (FISH amplified and/or IHC 3+ expression) and triple receptor negative disease (95.2%). In addition, type of breast surgery (lumpectomy vs. mastectomy) did not impact the likelihood of SLN identification (93.7% vs. 92.3%).
Table 2.
Factors associated with failure to identify a SLN
| Variable | SLN ID rate (%) |
Failure to identify a SLN (%) |
Odds Ratio** (95% CI) |
p-value |
|---|---|---|---|---|
| Age | ||||
| < 50 years old | 324/345 (93.9) | 21/345 (6.1) | 0.70 (0.39-1.26) | 0.238 |
| ≥ 50 years old | 315/344 (91.6) | 29/344 (8.4) | 1 | |
| BMI | ||||
| < 25.0 (underweight/normal) | 185/195 (94.9) | 10/195 (5.1) | 1 | 0.176 |
| ≥ 25.0 (overweight/obese) | 452/492 (91.9) | 40/492 (8.1) | 1.64 (0.80-3.31) | |
| Unknown | 2/2 (100) | 0/2 (0) | -- | |
| Clinical T stage at presentation | ||||
| Tis/T0/T1 | 88/95 (92.6) | 7/95 (7.4) | 1 | |
| T2 | 356/379 (93.9) | 23/379 (6.1) | 0.81 (0.34-1.95) | 0.642 |
| T3/4 | 194/214 (90.7) | 20/214 (9.3) | 1.30 (0.53-3.18) | 0.571 |
| Unknown | 1/1 (100) | 0/1 (0) | ||
| Clinical N stage at presentation | ||||
| N1 | 605/651 (92.9) | 46/651 (7.1) | 1 | 0.428 |
| N2 | 34/38 (89.5) | 4/38 (10.5) | 1.55 (0.53-4.55) | |
| Approximated subtype | ||||
| HR positive/HER2 negative | 287/311 (92.3) | 24/311 (7.7) | 1 | |
| HER2 positive | 192/209 (91.9) | 17/209 (8.2) | 1.06 (0.55-2.02) | 0.863 |
| Triple negative | 158/166 (95.2) | 8/166 (4.8) | 0.61 (0.27-1.38) | 0.232 |
| Unable to define | 2/3 (66.0) | 1/3 (33.0) | ||
| Duration of NAC | ||||
| < 135 days | 390/415 (94.0) | 25/415 (6.0) | 1 | 0.127 |
| ≥ 135 days | 249/274 (90.9) | 25/274 (9.1) | 1.57 (0.88-2.79) | |
| Palpable adenopathy after NAC | ||||
| Yes | 85/88 (96.6) | 3/88 (3.4) | 0.41 (0.13-1.36) | 0.145 |
| No | 526/571 (92.1) | 45/571 (7.9) | 1 | |
| Not stated | 28/30 (93.3) | 2/30 (6.7) | ||
| Anti-HER2 therapy (among the 209 HER2 positive cases) | ||||
| Yes | 168/185 (90.8) | 17/185 (9.2) | 0.229 | |
| No | 24/24 (100) | 0/27 (0) | ||
| Site of tracer injection | ||||
| Multiple sites | 138/149 (92.6) | 11/149 (7.4) | 1 | |
| Subareolar | 409/435 (94.0) | 26/435 (6.0) | 0.80 (0.38-1.66) | 0.631 |
| Peri-tumoral | 49/57 (86.0) | 8/57 (14.0) | 2.05 (0.78-5.39) | 0.146 |
| Intradermal | 17/19 (89.5) | 2/19 (10.5) | 1.48 (0.30-7.23) | 0.544 |
| Unknown | 26/29 (89.7) | 3/29 (10.3) | -- | -- |
| Tracers used | ||||
| Blue dye alone | 22/28 (78.6) | 6/28 (21.4) | 4.10 (1.56-10.78) | 0.004 |
| Radiocolloid alone | 106/116 (91.4) | 10/116 (8.6) | 1.42 (0.68-2.96) | 0.352 |
| Dual tracers | 511/545 (93.8) | 34/545 (6.2) | 1 | |
| Mapping approach | ||||
| Blue dye alone | 22/28 (78.6) | 6/28 (21.4) | 3.82 (1.47-9.92) | 0.006 |
| Radiocolloid +/− Blue dye | 617/661(93.3) | 44/661 (6.7) | 1 | |
| Number of tracers used | ||||
| Single tracer | 128/144 (88.9) | 16/144 (11.1) | 1 | 0.048 |
| Dual tracer | 511/545 (93.8) | 34/545 (6.2) | 0.53 (0.29-0.99) | |
| Breast Surgery* | ||||
| Lumpectomy | 255/272 (93.7) | 17/272 (6.3) | 0.79 (0.43-1.46) | 0.458 |
| Mastectomy | 381/413 (92.3) | 32/413 (7.6) | 1 | |
| Year of Surgery | ||||
| 2009-2010 | 339/368 (92.1) | 29/368 (7.9) | 1.22 (0.68-2.190 | 0.500 |
| 2011-2012 | 300/321 (93.5) | 21/300 (6.5) | 1 | |
4 patients did not have breast surgery
estimates from logistic regression modeling
The only factor associated with the failure to identify a SLN was the type of mapping agent used (blue dye vs. radiocolloid vs. both; Chi-square test p=0.0086). The rate of failure to identify a SLN was 21.4% (6/28; 95%CI: 8.3-41.0%) when blue dye alone was used; 8.6% (10/116; 95%CI: 4.2-15.3%) with use of radiolabeled colloid alone and 6.2% (34/545; 95%CI: 4.4-8.6%) with use of dual mapping agents. In terms of single agent (blue dye or radiocolloid) versus dual agent mapping, the SLN identification rate was significantly higher with use of dual agent compared to single agent mapping (93.8% vs. 88.9%, Chi-square test p=0.048).
Multivariate logistic regression analysis did not reveal any other factor significantly associated with likelihood of failure to identify a SLN, once number of mapping agents was accounted for in the model. The likelihood of failing to identify a SLN was 3.8 times higher (95%CI: 1.47-9.92) in those who underwent SLN surgery with blue dye alone compared with those who underwent SLN with radiocolloid with or without blue dye.
SLN identification in those with and without residual nodal disease after NAC
Among the 687 women who underwent both SLN and ALND, the SLN identification rate was 93.6% (382/408) for those with residual nodal disease after chemotherapy and 91.4% (255/279) for those with no residual nodal disease after chemotherapy indicating that nodal response to NAC did not impact SLN identification rates (Chi square test p=0.269) (Table 3). Nodal positivity was not significantly different based on ability to identify a SLN. Residual nodal disease was found in 382 (60%) of the 637 patients with a SLN identified and 26 (52%) of the 50 patients where there was a failed attempt to identify a SLN (p=0.30).
Table 3.
SLN identification rates according to clinical and pathologic stage
| Patients | N | SLN identified |
SLN identification rate (%) |
CI | P value |
|---|---|---|---|---|---|
| All patients | 689 | 639 | 92.7 | 90.5 - 94.6 | - |
| Clinical N stage at presentation | |||||
| cN1 | 651 | 605 | 92.9 | 90.7 - 94.8 | 0.424 |
| cN2 | 38 | 34 | 89.5 | 75.2 - 97.1 | |
| Final Pathologic Nodal Status* | |||||
| Node-negative | 279 | 255 | 91.4% | 87.5 - 94.4 | 0.269 |
| Node-positive | 408 | 382 | 93.6% | 90.8 - 95.8 | |
| Final Pathologic Tumor Stage** | |||||
| ypTX, Tis, T0 | 232 | 216 | 93.1% | 89.0 - 96.0 | 0.696 |
| ypT1/T2 | 392 | 365 | 93.1% | 90.1 - 95.4 | |
| ypT3/T4 | 61 | 55 | 90.2% | 79.8 - 96.3 | |
2 pts did not undergo ALND
4 pts had nodal surgery only
Discussion
ACOSOG Z1071 is the first study specifically evaluating SLN identification rates after NAC in patients with known nodal disease at presentation. The SLN identification rate in this multicenter prospective study in women with confirmed node-positive breast cancer at presentation treated with neoadjuvant chemotherapy was 92.7%. The use of dual agent mapping resulted in a significantly higher SLN identification rate (93.8%) compared with single agent mapping (88.9%). This highlights the importance of technical factors in the performance of SLN surgery in these patients.
The use of NAC in women presenting with operable breast cancer is increasing with decisions based on tumor biology along with clinical tumor stage and evidence of nodal involvement at presentation. More clinicians are utilizing axillary ultrasound at presentation in women with invasive breast cancer and percutaneous biopsy of axillary nodes can document the presence of metastasis using FNA or core biopsy. To date, use of ALND after completion of chemotherapy has been considered standard practice in these cases based on low SLN identification rates and high false-negative rates in previous retrospective reports.(27,28,29,30) Based on data from prospective trials including ACOSOG Z1071(31) and the SENTINA study (32), which detail information regarding surgical and pathologic considerations, surgeons are considering use of SLN surgery for women who present with node-positive disease and receive NAC. To optimize the technique of performing SLN surgery in these patients, it is critical to understand factors impacting the success of SLN identification.
Initial studies reported lower SLN identification rates after chemotherapy. In the NSABP B-27 trial, SLN surgery followed by ALND was performed after NAC in 428 patients. SLN surgery was not a planned component of the B-27 trial and there were no specifications for the performance of the procedure. The SLN identification rate in B-27 was 84.8%.(24) Over time, the SLN identification rate has improved. In the French prospective multicenter study (26) of locally advanced breast cancer, SLN surgery was the primary endpoint of the trial. This study included both clinically node-positive and node-negative patients but did not require histologic confirmation of nodal involvement at presentation. The overall SLN identification rate was 90.1%. Axillary status was determined based on clinical examination alone and the investigators found that the SLN identification rate was significantly higher in patients with a clinically negative axilla at initial presentation (94.6%) compared to patients with palpable lymph nodes at presentation (81.5%). In ACOSOG Z1071, palpable adenopathy was not significantly associated with failure to identify a SLN, however all of the patients had confirmation of nodal metastasis with needle biopsy.
In ACOSOG Z1071, the SLN identification rate was 92.7%, higher than what has been reported in earlier studies. It is higher than the 80.1% SLN identification rate seen in similar patient population in the SENTINA study. (32) The identification rate in Z1071 is similar to the 93% SLN identification rate reported by Krag et al in the 1998 publication of a multicenter validation trial of SLN surgery without NAC.(33) It is also in line with the single institution experience from MD Anderson Cancer Center of SLN after NAC in 150 patients with node-positive disease who were not enrolled on Z1071 where SLN was identified in 93% of cases.(27) This SLN identification rate is slightly lower than their previous report of SLN after NAC in patients with a clinically node negative axilla treated with NAC where the SLN identification rate was 97.4%.(34) This suggests that nodal disease detected at presentation along with chemotherapy effect on the axilla impacts lymphatic mapping to decrease the SLN identification rate, however not to the extent that it should prohibit SLN surgery.
Technical factors have been shown to impact SLN identification rates in multiple studies. Dual agent mapping was shown to significantly improve SLN identification rates in Z1071, with an identification rate of 93.8% with use of dual agents and only 88.9% when a single agent was used. Use of blue dye alone was the technique with the lowest SLN identification rate (78.6%). These findings are in keeping with previous reports from Mamounas et al from the NSABP B-27 trial which showed that the SLN rate after chemotherapy was significantly higher with use of radiocolloid with or without blue dye (88-89%) compared with blue dye alone (78%).(24) Similarly, Hunt et al showed in the MD Anderson experience that dual agent mapping had a higher technical success for SLN identification (99%) compared with single agent mapping (97.5%, p<0.0001).(34) The SENTINA study also found that combined radiocolloid and blue dye had better SLN detection rate than radiocolloid alone. (32)
Other factors that have been suggested in previous studies to impact SLN identification rates were not confirmed in this large contemporary study of patients undergoing SLN after chemotherapy for node-positive disease at presentation. Tumor location has previously been reported to influence ability to identify SLN,(33,35) however in more recent series, this has not been substantiated. Similarly, BMI and older age had been reported to impact SLN identification rate, however this was not seen in the current study.
As the SLN identification rate in this patient population remains lower than the 98-99% rates currently reported in patients undergoing surgery first, patients should be appropriately counseled regarding the SLN procedure. Discussion should include the possibility of failure to identify a SLN and the surgical plan for axillary staging in the event that this occurs, ALND remains the standard recommended in this setting.
In conclusion, in the ACOSOG Z1071 trial, we have shown a high rate of SLN identification following completion of NAC. SLN identification rates are higher with use of radiolabeled colloid or dual tracer technique. Optimal technique is important to ensure success in performing SLN surgery after chemotherapy.
ACKNOWLEDGMENTS
The authors thank the ACOSOG staff, in particular the leadership of Heidi Nelson, MD (Mayo Clinic, Rochester, MN) and David Ota, MD (Duke University, Durham, NC). Both of these individuals contributed to study design and did not receive compensation for their contributions. They also thank the patients with breast cancer who participated in the study and their caregivers; the investigators and their site research teams; Sue Paxton and Amy Oeltjen for their work with data quality; Karla Ballman for initial statistical planning; and Susan Budinger for protocol development.
Funding/Support: This work was supported by National Cancer Institute grant U10 CA76001 to the American College of Surgeons Oncology Group (ACOSOG). The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Role of the Sponsor: The National Cancer Institute (NCI) approved the study design and had a representative on the DSMC of this cooperative group study. It had no role in the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Additional Contributions: We thank the ACOSOG staff, in particular the leadership of Heidi Nelson, MD (Mayo Clinic, Rochester, Minnesota) and David Ota, MD (Duke University, Durham, North Carolina). Both of these individuals contributed to study design, manuscript review, or both; none received compensation for their contributions.
We also thank the patients with breast cancer who participated in the study and their caregivers, and we thank the investigators and their site research teams. We thank Sue Paxton and Amy Oeltjen for their work with data quality, Karla Ballman for initial statistical planning, and Susan Budinger for protocol development.
Trial Registration clinicaltrials.gov; trial identifier NCT00881361.
References
- 1.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. doi: 10.1097/00000658-199409000-00015. discussion 398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nwariaku FE, Euhus DM, Beitsch PD, et al. Sentinel lymph node biopsy, an alternative to elective axillary dissection for breast cancer. Am J Surg. 1998;176(6):529–531. doi: 10.1016/s0002-9610(98)00276-1. [DOI] [PubMed] [Google Scholar]
- 3.Schlag PM, Bembenek A. Specification of potential indications and contraindications of sentinel lymph node biopsy in breast cancer. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 2000;157:228–236. doi: 10.1007/978-3-642-57151-0_20. [DOI] [PubMed] [Google Scholar]
- 4.Chagpar AB, Martin RC, Scoggins CR, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery. 2005;138(1):56–63. doi: 10.1016/j.surg.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17(7):1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 7.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 10.Tafra L, Verbanac KM, Lannin DR. Preoperative chemotherapy and sentinel lymphadenectomy for breast cancer. Am J Surg. 2001;182(4):312–315. doi: 10.1016/s0002-9610(01)00718-8. [DOI] [PubMed] [Google Scholar]
- 11.Breslin TM, Cohen L, Sahin A, et al. Sentinel lymph node biopsy is accurate after neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2000;18(20):3480–3486. doi: 10.1200/JCO.2000.18.20.3480. [DOI] [PubMed] [Google Scholar]
- 12.Julian TB, Patel N, Dusi D, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer. Am J Surg. 2001;182(4):407–410. doi: 10.1016/s0002-9610(01)00736-x. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez A, Cortes M, Benito E, et al. Gamma probe sentinel node localization and biopsy in breast cancer patients treated with a neoadjuvant chemotherapy scheme. Nucl Med Commun. 2001;22(4):361–366. doi: 10.1097/00006231-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Haid A, Tausch C, Lang A, et al. Is sentinel lymph node biopsy reliable and indicated after preoperative chemotherapy in patients with breast carcinoma? Cancer. 2001;92(5):1080–1084. [PubMed] [Google Scholar]
- 15.Stearns V, Ewing CA, Slack R, et al. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9(3):235–242. doi: 10.1007/BF02573060. [DOI] [PubMed] [Google Scholar]
- 16.Brady EW. Sentinel lymph node mapping following neoadjuvant chemotherapy for breast cancer. Breast J. 2002;8(2):97–100. doi: 10.1046/j.1524-4741.2002.08205.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller AR, Thomason VE, Yeh IT, et al. Analysis of sentinel lymph node mapping with immediate pathologic review in patients receiving preoperative chemotherapy for breast carcinoma. Ann Surg Oncol. 2002;9(3):243–247. doi: 10.1007/BF02573061. [DOI] [PubMed] [Google Scholar]
- 18.Balch GC, Mithani SK, Richards KR, et al. Lymphatic mapping and sentinel lymphadenectomy after preoperative therapy for stage II and III breast cancer. Ann Surg Oncol. 2003;10(6):616–621. doi: 10.1245/aso.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Piato JR, Barros AC, Pincerato KM, et al. Sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. A pilot study. Eur J Surg Oncol. 2003;29(2):118–120. doi: 10.1053/ejso.2002.1349. [DOI] [PubMed] [Google Scholar]
- 20.Reitsamer R, Peintinger F, Rettenbacher L, et al. Sentinel lymph node biopsy in breast cancer patients after neoadjuvant chemotherapy. J Surg Oncol. 2003;84(2):63–67. doi: 10.1002/jso.10294. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GF, Meltzer AJ. Accuracy of axillary sentinel lymph node biopsy following neoadjuvant (induction) chemotherapy for carcinoma of the breast. Breast J. 2003;9(5):374–379. doi: 10.1046/j.1524-4741.2003.09502.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel NA, Piper G, Patel JA, et al. Accurate axillary nodal staging can be achieved after neoadjuvant therapy for locally advanced breast cancer. Am Surg. 2004;70(8):696–699. discussion 699-700. [PubMed] [Google Scholar]
- 23.Lang JE, Esserman LJ, Ewing CA, et al. Accuracy of selective sentinel lymphadenectomy after neoadjuvant chemotherapy: effect of clinical node status at presentation. J Am Coll Surg. 2004;199(6):856–862. doi: 10.1016/j.jamcollsurg.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2005;23(12):2694–2702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 25.Xing Y, Foy M, Cox DD, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93(5):539–546. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 26.Classe JM, Bordes V, Campion L, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. Journal of Clinical Oncology. 2009;27(5):726–732. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]
- 27.Alvarado R, Yi M, Le-Petross H, et al. The Role for Sentinel Lymph Node Dissection after Neoadjuvant Chemotherapy in Patients who Present with Node-Positive Breast Cancer. Ann Surg Oncol. 2012;19(10):3177–3184. doi: 10.1245/s10434-012-2484-2. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Gilcrease MZ, Babiera GV, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007;109(7):1255–1263. doi: 10.1002/cncr.22540. [DOI] [PubMed] [Google Scholar]
- 29.Newman EA, Sabel MS, Nees AV, et al. Sentinel Lymph Node Biopsy Performed After Neoadjuvant Chemotherapy is Accurate in Patients with Documented Node-Positive Breast Cancer at Presentation. Ann Surg Oncol. 2007;14(10):2946–2952. doi: 10.1245/s10434-007-9403-y. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Kim EY, Kang SH, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat. 2007;102(3):283–288. doi: 10.1007/s10549-006-9330-9. [DOI] [PubMed] [Google Scholar]
- 31.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(4):1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 33.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer--a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 34.Hunt KK, Yi M, Mittendorf EA, et al. Sentinel Lymph Node Surgery After Neoadjuvant Chemotherapy is Accurate and Reduces the Need for Axillary Dissection in Breast Cancer Patients. Ann Surg. 2009;250:558–566. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 35.Ahrendt GM, Laud P, Tjoe J, et al. Does breast tumor location influence success of sentinel lymph node biopsy? J Am Coll Surg. 2002;194(3):278–284. doi: 10.1016/s1072-7515(01)01174-7. [DOI] [PubMed] [Google Scholar]


