Abstract
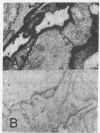
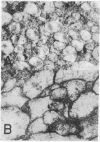
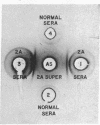
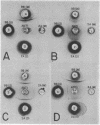
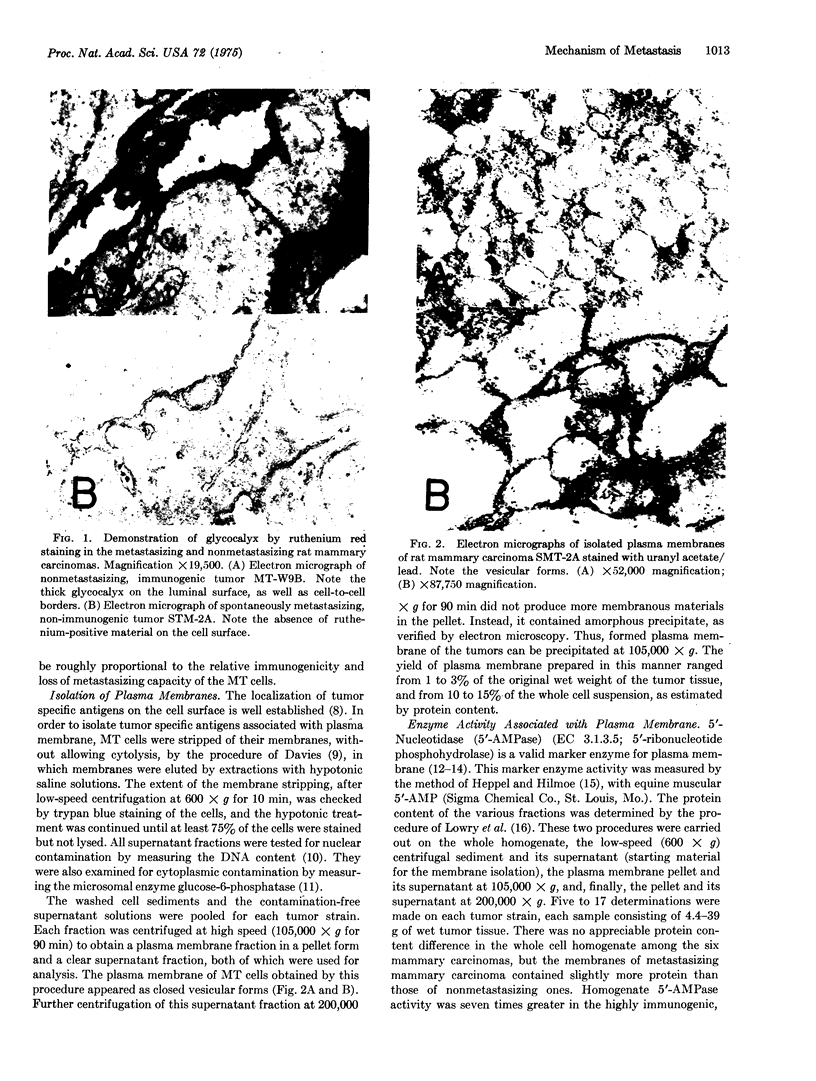
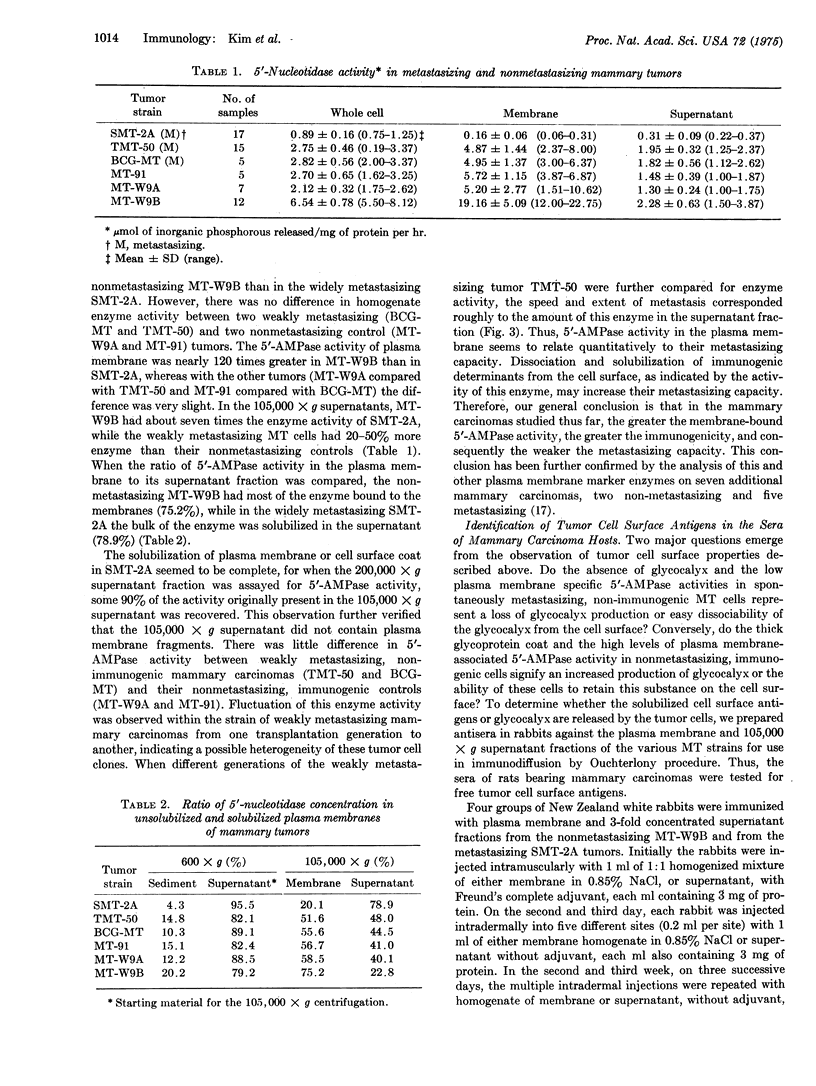
Immunological and biochemical studies of spontaneously metastasizing and nonmetastasizing rat mammary carcinomas and their plasma membranes indicated that: (i) all spontaneously metastasizing tumors have little or no demonstrable glycocalyx, while all nonmetastasizing tumors have a thick glycocalyx; (ii) there is a direct relationship between the glycocalyx and immunogenicity, and an inverse relationship with the metastasizing capacity of tumor cells, properties which can be quantitated by levels of the plasma membrane marker enzyme 5'-nucleotidase (EC3.1.3.5;5'-ribonucleotide phosphohydrolase) activity; (iii) the absence of glycocalyx from the metastasizing tumor cell surface seems to result from its dissociation from plasma membranes, for solubilized cell surface antigen is readily found in the blood of metastasizing tumor bearing rats, while there was no detectable tumor cell surface antigen in the blood of the nonmetastasizing tumor hosts tested; (iv) both metastasizing and nonmetastasizing mammary tumors appear to have a common soluble cell surface antigen; (v) in addition to this common antigen, there is another membrane-bound antigen in the nonmetastasizing, immunogenic tumor cell surface which presumably is the tumor specific transplantation antigen; and (vi) this antigen is immunobiologically unique, but seems to be immunochemically related to the common soluble antigen. It is postulated that the lack of an immunogenic coat and/or the presence of solubilized tumor cell surface antigen in the blood may provide an immune escape mechanism for tumor cells by interfering with cell-mediated immune response of tumor hosts, leading to their dissemination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose K. R., Anderson N. G., Coggin J. H., Jr Cytostatic antibody and SV40 tumour immunity in hamsters. Nature. 1971 Oct 1;233(5318):321–324. doi: 10.1038/233321a0. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Bowen J. G., Price M. R. Detection of circulating hepatoma D23 antigen and immune complexes in tumour bearer serum. Br J Cancer. 1973 Jul;28(1):16–24. doi: 10.1038/bjc.1973.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Embleton M. J., Robins R. A. Cellular and humoral immunity to rat hepatoma-specific antigens correlated with tumour status. Int J Cancer. 1973 Jan 15;11(1):1–10. doi: 10.1002/ijc.2910110102. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Price M. R., Robins R. A. Inhibition of hepatoma-immune lymph-node cell cytotoxicity by tumour-bearer serum, and solubilized hepatoma antigen. Int J Cancer. 1973 May;11(3):527–535. doi: 10.1002/ijc.2910110304. [DOI] [PubMed] [Google Scholar]
- Barclay M., Terebus-Kekish O. Enzyme activities and extrinsic proteins in plasma membranes from normal liver and Morris hepatoma 5123tc. J Natl Cancer Inst. 1973 Nov;51(5):1709–1710. doi: 10.1093/jnci/51.5.1709. [DOI] [PubMed] [Google Scholar]
- Barski G., Youn J. K. Evolution of cell-mediated immunity in mice bearing an antigenic tumor. Influence of tumor growth and surgical removal. J Natl Cancer Inst. 1969 Jul;43(1):111–121. [PubMed] [Google Scholar]
- Blomberg F., Perlmann P. Immunochemical studies of detergent-soluble nucleoside phosphatases in rat liver plasma membranes. Biochim Biophys Acta. 1971 Mar 9;233(1):53–60. doi: 10.1016/0005-2736(71)90357-9. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Alexander P. Spontaneous shedding of TSTA by viable sarcoma cells: its possible role in facilitating metastatic spread. Br J Cancer. 1974 Jan;29(1):72–75. doi: 10.1038/bjc.1974.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Effect of active immunization with irradiated tumour cells on specific serum inhibitors of cell-mediated immunity in patients with disseminated cancer. Br J Cancer. 1973 Jul;28(1):25–35. doi: 10.1038/bjc.1973.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. A. Mouse histocompatibility isoantigens derived from normal and from tumour cells. Immunology. 1966 Aug;11(2):115–125. [PMC free article] [PubMed] [Google Scholar]
- Dermer G. B., Kern W. H. Changes in the affinity of phosphotungstic acid and positively charged colloidal particles for the surfaces of malignant human transitional epithelium of the urinary bladder. Cancer Res. 1974 Aug;34(8):2011–2014. [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. V. On the lipid dependence of some phosphohydrolases of isolated rat-liver plasma membranes. Biochim Biophys Acta. 1968 Apr 29;150(3):341–353. doi: 10.1016/0005-2736(68)90133-8. [DOI] [PubMed] [Google Scholar]
- GASIC G., BERWICK L. HALE STAIN FOR SIALIC ACID-CONTAINING MUCINS. ADAPTATION TO ELECTRON MICROSCOPY. J Cell Biol. 1963 Oct;19:223–228. doi: 10.1083/jcb.19.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASIC G., LOEBEL F., BADINEZ O. Cementing substances in metastasizing and non-metastasizing transplantable tumours in mice. Nature. 1960 Mar 19;185:864–865. doi: 10.1038/185864a0. [DOI] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Kim U. Metastasizing mammary carcinomas in rats: induction and study of their immunogenicity. Science. 1970 Jan 2;167(3914):72–74. doi: 10.1126/science.167.3914.72. [DOI] [PubMed] [Google Scholar]
- Klein G. Tumor-specific transplantation antigens: G. H. A. Clowes memorial lecture. Cancer Res. 1968 Apr;28(4):625–635. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo Gerfo P., Krupey J., Hansen H. J. Demonstration of an antigen common to several varieties of neoplasia. N Engl J Med. 1971 Jul 15;285(3):138–141. doi: 10.1056/NEJM197107152850302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Ran M., Witz I. P. Tumor-associated immunoglobulins. The elution of IgG2 from mouse tumors. Int J Cancer. 1970 Nov 15;6(3):361–372. doi: 10.1002/ijc.2910060306. [DOI] [PubMed] [Google Scholar]
- Reynoso G., Chu T. M., Holyoke D., Cohen E., Nemoto T., Wang J. J., Chuang J., Guinan P., Murphy G. P. Carcinoembryonic antigen in patients with different cancers. JAMA. 1972 Apr 17;220(3):361–365. [PubMed] [Google Scholar]
- Roth J., Stiller D., Block F. Glycocalyx of tumor cells. Exp Pathol (Jena) 1972;6(1):35–46. [PubMed] [Google Scholar]
- SWANSON M. A. Phosphatases of liver. I. Glucose-6-phosphatase. J Biol Chem. 1950 Jun;184(2):647–659. [PubMed] [Google Scholar]
- Skarin A. T., Delwiche R., Zamcheck N., Lokich J. J., Frei E., 3rd Carcinoembryonic antigen: clinical correlation with chemotherapy for metastatic gastrointestinal cancer. Cancer. 1974 May;33(5):1239–1245. doi: 10.1002/1097-0142(197405)33:5<1239::aid-cncr2820330508>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Steward A. M., Nixon D., Zamcheck N., Aisenberg A. Carcinoembryonic antigen in breast cancer patients: serum levels and disease progress. Cancer. 1974 May;33(5):1246–1252. doi: 10.1002/1097-0142(197405)33:5<1246::aid-cncr2820330509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Thomson D. M., Alexander P. A cross-reacting embryonic antigen in the membrane of rat sarcoma cells which is immunogenic in the syngeneic host. Br J Cancer. 1973 Jan;27(1):35–47. doi: 10.1038/bjc.1973.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Eccles S., Alexander P. Antibodies and soluble tumour-specific antigens in blood and lymph of rats with chemically induced sarcomata. Br J Cancer. 1973 Jul;28(1):6–15. doi: 10.1038/bjc.1973.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Steele K., Alexander P. The presence of tumour-specific membrane antigen in the serum of rats with chemically induced sarcomata. Br J Cancer. 1973 Jan;27(1):27–34. doi: 10.1038/bjc.1973.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vánky F., Stjernswärd J., Klein G., Steiner L., Lindberg L. Tumor-associated specificity of serum-mediated inhibition of lymphocyte stimulation by autochthonous human tumors. J Natl Cancer Inst. 1973 Jul;51(1):25–32. doi: 10.1093/jnci/51.1.25. [DOI] [PubMed] [Google Scholar]