Abstract
The connective tissue of any organ in the body is generally referred to as stroma. This complex network is commonly composed of leukocytes, extracellular matrix components, mesenchymal cells and a collection of nerves, blood and lymphoid vessels. Once viewed primarily as a structural entity, stromal cells of mesenchymal origin are now being intensely examined for their ability to directly regulate various components of immune cell function. There is particular interest in the ability of stromal cells to influence the homeostasis, activation and proliferation of T lymphocytes. One example of this regulation occurs in the lymph node (LN) where fibroblastic reticular cells (FRCs) support the maintenance of naïve T cells, induce antigen-specific tolerance and restrict the expansion of newly activated T cells. In an effort to highlight the varied immunoregulatory properties of FRCs, we have reviewed the most recent advances in this field and provide some insights into potential future directions.
Introduction
The life cycle of T lymphocytes begins in the thymus as immature precursor T cells undergo positive and negative selection to mature into CD4+ and CD8+ single-positive cells (1). Following migration from the thymus, T cells recirculate from the blood through lymph nodes (LN) into lymphatics and back into the blood, searching for the presence of their target antigen (2). When a naïve T cell becomes activated in the LN by a professional antigen-presenting cell (APC) presenting its cognate antigen, the T cell will either mount an effector response or will become tolerant to avoid autoimmunity. In the presence of appropriate co-stimulation, activated T cells undergo rapid clonal expansion in the LN, acquire effector functions and gain the ability to migrate to their antigen source in peripheral tissues. The vast majority of effector T cells will die during the contraction phase of an immune response but a small fraction will remain as circulating long-lived effector or central memory cells, poised to mount a robust recall response in non-lymphoid and lymphoid tissues (3). As such, the LN serves as a central site for every stage of the T cell life cycle by: recruiting naïve T cells from the blood, promoting naïve T cell survival, providing an environment for T cell differentiation or tolerance and by influencing the homing properties of memory T cells.
In addition to hematopoietic cells, the LN contains specialized stromal cells including: blood endothelial cells (BECs), lymphatic endothelial cells (LECs), follicular dendritic cells (FDCs), marginal reticular cells (MRCs), integrin α7+ pericytes (IAPs) and fibroblastic reticular cells (FRCs) (4–6). LN-resident stromal cells were long viewed simply as structural determinants, uninvolved in immune cell homeostasis or ongoing immune responses. A series of publications over the past decade, however, have uncovered several fascinating immunoregulatory properties of LN stromal cells. In particular, FRCs are concentrated in the paracortical region (T cell zone) of the LN and are endowed with several functions that regulate the activity of T lymphocytes.
FRCs are thought to originate from mesenchymal preadipocyte precursors in the microenvironment of the LN anlagen during ontogeny (7). Engagement of the lymphotoxin-β receptor on these precursors drives their differentiation into lymphoid-tissue organizing cells, which ultimately leads to the development of myofibroblastic precursors that give rise to mature FRCs in the postnatal LN (7–11). The T cell zone of the adult LN is especially enriched with the presence of mature FRCs characterized by the expression of podoplanin (gp38) and extracellular matrix proteins such as ERTR-7 and collagens (6). We now know that naïve T cell recruitment to and survival within LNs are maintained by FRC-derived chemokines and cytokines (12, 13). FRCs also directly induce deletional T cell tolerance and can restrict the expansion of newly activated T cells (14–19). In the following, we will review the immunoregulatory characteristics of LN FRCs with particular emphasis on how these cells organize and regulate several phases of the T lymphocyte life cycle.
FRCs facilitate lymphocyte arrival and organization in the lymph node
The random joining of T cell receptor (TCR) regions during T cell development produces a naïve T cell repertoire with only a few cells with high affinity for any individual peptide-major histocompatibility complex (MHC) (20, 21). To trigger an effective immune response, this rare population of T cells must initially engage an APC presenting its cognate antigen. To increase the likelihood of encountering its target antigen, naïve T cells continuously circulate between lymphoid organs, such as the Peyer’s patches (PP), spleen and LN (22). Circulating T lymphocytes enter the LN through specialized blood vessels named high endothelial venules (HEVs) (2, 23). FRCs surround HEVs and interact with extravasated platelets in the perivenular space to maintain HEV integrity during lymphocyte trafficking (24). This regulation requires the ligation of FRC-bound gp38 to the C-type lectin receptor CLEC-2 on platelets. Activated platelets then release sphingosine-1-phosphate in the perivenular space, which maintains adherens junctions between HEVs (24). Loss of FRC-bound gp38 or CLEC-2 expression on platelets compromises LN vascular integrity at steady state and during immune responses (24). The absence of CLEC-2 on platelets also leads to a defect in T and B cell recirculation through the LNs after repeated immunizations (25).
Lymphocyte chemoattractants CCL19, CCL21, and CXCL12 are all expressed by LN FRCs and function to support naïve T cell trafficking across HEVs and retains T cells in the LN paracortex through their ligation to CCR7 and CXCR4 (6, 12, 26–28) (Fig. 1A). After exiting the HEVs, naïve T cells use the FRC network as a defined structural path to migrate within the paracortex according to the chemokine gradients. The survival and homeostasis of naïve T cells that reach the T cell zone is also supported by FRC-derived interleukin-7 (IL-7) (13) (Fig. 1B). Accordingly, disruption of the FRC network during human immunodeficiency virus (HIV)-associated LN fibrosis significantly correlates with a reduced number of naïve CD4+ T cells in the LN (29). Indeed, LN fibrosis in models of simian immunodeficiency virus (SIV) in rhesus macaques restricts T cell access to FRC-derived IL-7, which drives apoptosis in both naïve CD4+ and CD8+ T cell populations (30). Treatment of SIV-infected macaques with agents that reduce LN fibrosis preserves the FRC network and is associated with larger CD4+ T cell populations in the LNs compared to untreated controls (31, 32). Genetic ablation of LN FRCs in mouse models alters T cell localization in the paracortex, decreases T cell survival and impairs antigen-specific T cell priming (33). By maintaining HEV integrity and secreting soluble mediators to facilitate migration and survival, FRCs place T cells in position to locate their cognate antigen.
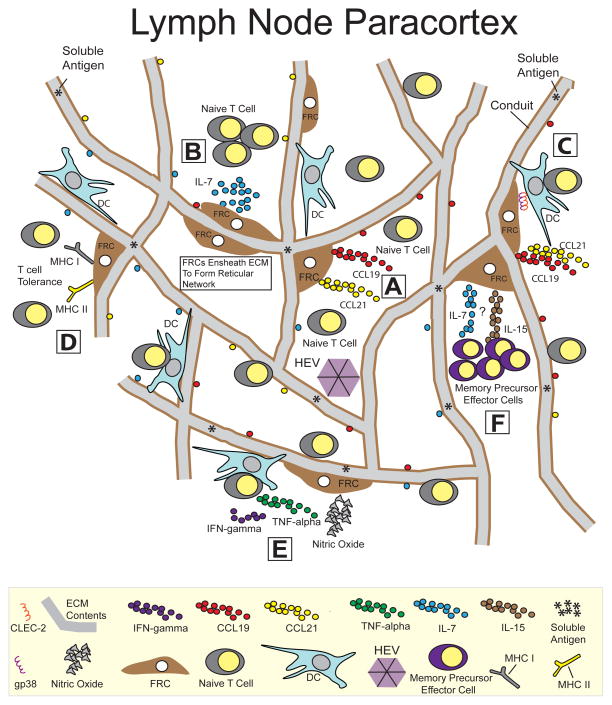
Figure 1.
LN FRCs regulate several aspects of the T cell life cycle. |A| FRCs facilitate lymphocyte arrival and organization in the LN. FRC-derived chemokines CCL19 and CCL21 support naïve T cell trafficking across HEVs and retains T cells in the LN paracortex through their ligation to CCR7. |B| T cell survival is maintained by FRCs. FRC-derived IL-7 supports the survival and homeostasis of naïve T cells that reach the T cell zone. |C| FRCs facilitate the interactions between antigen-presenting dendritic cells and T cells. The trafficking of migratory DCs to the LN is induced by the ligation between the CCR7 receptor on DCs and FRC-derived chemokines CCL19 and CCL21. Upon arrival in the LN, DC migration along the FRC network requires the engagement of DC-expressed CLEC-2 to gp38 on FRCs. Disruption of this signaling axis ultimately leads to reduced T cell priming. |D| FRCs induce T cell tolerance via the expression of peripheral tissue-restricted antigens. Upon antigen presentation, FRCs induce deletional tolerance of MHC class I-restricted CD8+ T cells and hyporesponsiveness of MHC II-restricted CD4+ T cells. |E| FRCs restrict the expansion of newly activated T cells. Activated T cells release IFN-γ and TNF-α, which act synergistically to endow FRCs with suppressive capabilities, mediated through the activity of nitric oxide. This suppression occurs in an antigen-independent fashion, which may ultimately be required to protect the organ from excessive swelling and damage during ongoing immune responses. |F| FRCs influence the maintenance of memory precursor effector cells. Ablation of FRCs during the late phase of an immune response leads to a modest reduction in the percentage of memory precursor effector cells. The mechanism controlling this reduction has not been determined although FRC-derived IL-7 and IL-15 are hypothesized to be involved.
FRCs support the interactions between antigen-presenting dendritic cells and T cells
After arrival in the LN via HEVs, naïve T cells spend ~8–12 hours exploring the LN parenchyma for their cognate antigen (34). FRCs directly facilitate antigen availability to T cells by: 1) creating a conduit system that extends deep into the LN parenchyma; and by 2) supporting migratory dendritic cell (DC) entry, maturation and trafficking into the LN from peripheral tissues. FRCs secrete and ensheathe extracellular matrix (ECM) components to form a reticular conduit network within the T cell zone of the LN and spleen (6, 35–38). This system functions as a molecular sieve, allowing expedited delivery of chemokines and small soluble antigens from upstream tissues into the parenchyma of draining LNs (37, 39) (Fig. 1). Small lymph-borne antigens from the conduit are sampled by LN-resident DCs and subsequently presented to T cells (37, 40). Under inflammatory conditions, a second flux of antigen-loaded migratory DCs arrive in the LN and present antigen to primed T cells (40). This trafficking and upregulation of co-stimulatory molecules is induced by the ligation between the CCR7 receptor on DCs and FRC-derived chemokines CCL19 and CCL21 (41, 42) (Fig. 1C). Upon arrival in the LN, migratory DCs cross the floor of the subcapsular sinus and infiltrate the parenchyma. Similar to naïve T cells, DCs also use the reticular FRC network as a scaffold to navigate within the T cell zone (26), which increases potential interactions between antigen-bearing DCs and naïve T cells. In addition to chemokine gradients (41), DC migration along FRC networks also depend on signaling between CLEC-2 and its ligand gp38 (43). Engagement of DC-expressed CLEC-2 to gp38 on FRCs promotes actin polymerization in DCs, which facilitates spreading, protrusion extension and migration along FRC networks (43). Disruption of this signaling pathway causes impaired DC trafficking to and within the LN, ultimately leading to reduced T cell priming (43). In addition to modulating DC motility, the CLEC-2-gp38 axis also influences the contractility of FRCs (44, 45). At steady state, gp38 maintains FRCs in a highly tense and contracted state within the LN reticular network. CLEC-2 ligation inhibits gp38 signaling which causes FRCs to stretch and expand due to the relaxation of their actomyosin cytoskeleton (44, 45). Effectively, the same antigen-bearing DCs that initiate T cell priming during an immune response also causes relaxation of the FRC network which allows space for T cell influx and clonal expansion (44, 45).
Antigen presentation by FRCs induces peripheral tolerance
The priming instructions received by naïve T cells in the LN will either trigger effector T cell differentiation or produce functionally inert clones that remain in a hyporesponsive state or eventually become deleted. The immune system has evolved such that this priming fate depends primarily on whether naïve T cells harbor a TCR specific for a potentially dangerous foreign antigen or contain receptors that recognize self-peptides. Despite the immune system’s design to eliminate self-reactive clones in the thymus during negative selection, a relatively high frequency of T cells with auto-reactive potential will escape into the periphery (46). Therefore, mechanisms to enforce peripheral tolerance are critical for the prevention of auto-inflammation and tissue destruction. Until recently, steady state trafficking of antigen-loaded immature DCs was widely accepted as the primary mechanism of peripheral deletional tolerance (47, 48). In 2007 however, a report from Lee et al (14) identified an important role for LN stromal cells in inducing CD8+ T cell tolerance (Fig. 1D). A transgenic model system was used for this study whereby a truncated form of ovalbumin (tOVA) was expressed as a self-antigen under the control of the intestinal fatty acid-binding protein (iFABP) promoter (49). As expected, adoptive transfer of OVA-specific CD8+ T cells (OTI) led to proliferation in gut draining tissues such as the mesenteric LN and Peyer’s patches. Surprisingly, however, transferred cells also proliferated in the non-draining LNs. This proliferation occurred even under conditions when DCs and other bone marrow-derived APCs were prevented from presenting antigen (14). These data led to the discovery that CD45− gp38+ non-hematopoietic stromal cells were responsible for the presentation of tOVA and subsequent activation of OTI cells (14). Most notably, after some initial proliferation, the adoptively transferred T cells were subsequently lost from the T cell pool, highlighting the tolerogenic capacity of LN stroma (14).
Advances in purification techniques for LN stromal cells (50) led to the identification of FRCs as the specific stromal cell population responsible for the ectopic expression of tOVA in the iFABP-tOVA mouse (15). These techniques also allowed Malhotra et al (6) to conduct a comprehensive transcriptomic analysis of different LN stroma cell subsets. Pairwise analyses of ligands and cognate receptors across stroma and hematopoietic cells suggested a number of potentially interesting interactions. Notably, upregulation of MHC class II (MHC II) by FRCs in response to inflammatory stimuli suggest that FRCs might also tolerize class II-restricted CD4+ T cells (6). Dubrot et al (16) substantiated this notion with a recent study which showed that FRCs express low levels of endogenous MHC II through the PIV promoter region of CIITA, a known master regulator of class II expression. The study also provided evidence that MHC class II on FRCs can also be acquired from DCs via a contact-dependent mechanism involving the transfer of DC-derived MHC II+ exosomes (16). Accordingly, DCs pulsed with FITC-labeled OVA were also able to transfer peptide-loaded MHC II (pMHC II) complexes to FRCs (16). To investigate the influence of this pMHC II transfer on CD4+ T cells in vivo, the authors used the CD11cDOG mouse model in which OVA protein is exclusively expressed by DCs (51). OVA-specific CD4+ T cells (OTII) pre-cultured with FRCs from these mice exhibited a delayed proliferative response upon restimulation with anti-CD3/CD28 antibodies (16). Based on these findings, it was proposed that FRCs acquired OVA-MHC II complexes from DCs in vivo which endowed them with the ability to induce antigen-specific CD4+ T cell hyporesponsiveness (16) (Fig. 1D). Altogether, these studies have elucidated a role for FRCs in antigen presentation and T cell anergy.
FRCs restrict the expansion of newly activated T cells
In addition to tolerizing T cells through direct antigen presentation, FRCs can also limit T cell responses by curtailing the expansion of newly activated T cell pools. This proliferative restriction applies to CD4+ and CD8+ T cells and surprisingly occurs independently of antigen presentation (17–19). According to several reports, T cells activated by either anti-CD3/CD28 antibodies or peptide-pulsed DCs experienced delayed division kinetics when co-cultured with FRCs (17–19). Additional FRC:T cell co-culture experiments from mice deficient for candidate mediators revealed a molecular crosstalk whereby T cell-derived IFN-γ and TNF-α act synergistically to endow FRCs with suppressive capabilities that are mediated through the activity of inducible nitric oxide synthase (iNOS) (17–19) (Fig. 1E). In transwell assays, the suppressive affects of FRCs were significantly diminished, indicating contact-dependency or a requirement for close cellular proximity (17–19). The existence of FRC-mediated T cell suppression was corroborated in vivo. When compared to WT iFABP-tOVA mice, Lukacs-Kornek et al (17) showed enhanced proliferation of OTI T cells transferred into iFABP-tOVA mice lacking iNOS expression. Similar proliferation trends were observed using iNOS−/− mice infected with VSV-OVA (19) or immunized with OVA-loaded bone marrow DCs (18).
Given the current data, we can only speculate on the reasons for this suppressive relationship. One possible clue may come from the in vivo expression profile of iNOS in FRCs. As reported by immunostaining of skin-draining LNs (SLN) and mesenteric LNs (MLNs), OTI T cells transferred into iFABP-tOVA mice induced iNOS expression in only a proportion of FRCs in vivo (17). This result, coupled with the fact that FRCs in vitro attenuated T cell proliferation without complete abrogation (17–19), suggests that FRCs may exist as a heterogeneous population in regards to their suppressive capacity. Indeed, functional heterogeneity of FRCs has been recently described for their B cell homeostatic potential (33). Therefore, it is possible that FRCs reside in discrete microdomains that promote T cell proliferation in a spatially and temporally restricted fashion, while limiting uncontrolled T cell expansion. Ultimately, this mechanism may be required to protect lymphoid organs from excessive swelling and damage during ongoing immune responses. Although we know that FRCs proliferate and expand to accommodate LN enlargement during immune responses (52, 53), whether and how these cells regulate changes in LN structure during inflammation remains completely unknown. More in vivo studies are needed to test the validity of this hypothesis.
Can FRCs influence the differentiation of T cells?
Differentiation of naïve T cells into regulatory cells; specific helper subsets or long-lived memory cells represent the final stages of the T lymphocyte life cycle. The involvement of LN stroma in this final stage can be investigated using a variety of techniques including LN transplantation models. Upon LN transplantation, graft-derived LN stroma are largely retained in the transplant, whereas all hematopoietic cells migrate out of the LN and are replaced by host-derived hematopoietic cells (54, 55). As such, this system has provided a useful tool for investigating location-specific properties that are intrinsic to stroma. The capacity of LN stroma to induce the generation of de novo regulatory T cells (Tregs) was explored in a recent study (56) in which liver draining celiac LNs and gut-draining mesenteric LNs were transplanted into the popliteal fossa after excision of the endogenous popliteal LN. When compared to transplanted popliteal LN controls, celiac and mesenteric LNs represented a significantly superior environment for de novo Treg differentiation from adoptively transferred naïve Foxp3− CD4+ OTII T cells (56). Interestingly, the ability to induce Tregs was not recapitulated when LNs were transplanted from germ-free mice or mice with vitamin A deficiency (56). The phenomenon also required the presence of DCs as Treg generation was abolished when DCs were depleted (56). Overall, these data suggest a model whereby the intestinal microenvironment imprints LN stroma with Treg inducing properties that are ultimately fulfilled via a synergistic relationship with DCs. Given their interactions with DCs (43) and their density within the T cell zone (4), FRCs may be the main LN stromal population responsible for the Treg induction in this model. It is also possible that FRC-derived nitric oxide might act directly on newly activated T cells to induce Tregs. In line with this hypothesis, work by Liew and colleagues has shown that nitric oxide can promote the generation of CD4+ CD25+ Foxp3− regulatory T cells with both in vitro and in vivo suppressive functions (57, 58).
FRCs may also regulate T cell differentiation by supporting the maintenance of memory precursor effector cells (MPECs) and long-live memory populations. A recent report by Denton et al (59) provided some validation to this hypothesis by showing a modest reduction in MPEC percentages following FRC depletion during the late phase of an ongoing influenza virus infection. Interestingly, FRC ablation reduced the percentage of MPECs without negatively impacting the abundance of short lived effector cells (59). Selective reduction of MPECs likely occurred in response to decreased FRC-derived IL-7, which is known to support MPEC formation (60) (Fig. 1F). IL-7 along with IL-15 are pro-survival factors for both CD4+ and CD8+ LN-homing central memory T cells (TCM) (61–65). Therefore, it is not surprising to find that resting LN CD8+ TCM closely mirror the microanatomical distribution of naïve CD8+ T cells (66), whose survival is also supported by FRC-derived IL-7 (13). Although CD4+ memory T cells are already known to associate with IL-7-expressing VCAM-1+ stroma cells in the bone marrow (67), additional studies are needed to formally test if CD4+ memory T cells preferentially reside on IL-7-producing LN FRCs. Additionally, FRCs were recently shown to express IL-15 in vivo (68), adding to their potential role as supporters of memory T cell maintenance (Fig. 1F).
Conclusions
Over the past decade, understanding of the immunological relevance of the stroma has grown significantly. Based on recent and emerging evidence, the role of stromal cells within the LN is now appreciated to be more complex than their previous categorization as a mere structural entity. We now know that FRCs govern lymphocyte recruitment and organization in the LN and support encounters between antigen-presenting DCs and T cells. FRCs also induce deletional tolerance via antigen presentation and are expected to participate in the generation of inducible Tregs within gut-draining LNs. During clonal expansion, FRCs restrict proliferation within the expanding T cell pool and are involved in the maintenance of memory precursor effector cells. By participating in the homeostasis, activation and differentiation of T lymphocytes, FRCs are emerging as essential regulators of T cell immunity.
Given their diverse immunoregulatory properties, FRCs are now being explored for their therapeutic potential in settings with immunological dysregulation. In a recent report from Fletcher et al (69), FRCs were used as a novel anti-inflammatory therapy for the treatment of high-mortality murine sepsis. In this system, FRCs offer enhanced survival in mice with either lipopolysaccharide endotoxemia or cecal ligation and puncture sepsis by dampening the expression of proinflammatory cytokines in the peritoneum and blood via a iNOS-dependent mechanism (69). To fully uncover the therapeutic potential of FRCs, additional research will be needed to identify the underlying molecular mechanisms that induce these cells to become immuno-stimulatory or immuno-suppressive. Identifying the soluble mediators and signaling pathways that control the function of FRCs should allow researchers to better predict how these cells might behave in a particular therapeutic setting. In the coming years, we anticipate additional discoveries that will expand our understanding of the immune functions of FRCs, and inform the development of therapies to treat infections, cancer and autoimmune disorders.
Acknowledgments
The authors thank Viviana Cremasco, W Nicholas Haining and Austyn Ellese Mayfield for critical reading of the manuscript.
Footnotes
This work was supported by a Howard Hughes Medical Institute Gilliam Fellowship for Advanced Study (to F.D.B)
Disclosures
The authors have no competing financial interest or conflicts.
References
- 1.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 2.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Cui WG. Transcriptional control of effector and memory CD8(+) T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol. 2010;10:813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra D, Fletcher AL, Turley SJ. Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev. 2013;251:160–176. doi: 10.1111/imr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, Gonzalez SF, Elpek KG, Chang SK, Knoblich K, Hemler ME, Brenner MB, Carroll MC, Mooney DJ, Turley SJ, Project IG. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13:499–U107. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benezech C, Mader E, Desanti G, Khan M, Nakamura K, White A, Ware CF, Anderson G, Caamano JH. Lymphotoxin-beta receptor signaling through nf-kappa b2-relb pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37:721–734. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–U624. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 9.Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C, Cupovic J, Danuser R, Sparwasser T, Luther SA, Thiel V, Rulicke T, Stein JV, Hehlgans T, Ludewig B. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity. 2013;38:1013–1024. doi: 10.1016/j.immuni.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. In: Paul WE, Littman DR, Yokoyama WM, editors. Annual Review of Immunology. Vol. 29. 2011. pp. 23–43. [DOI] [PubMed] [Google Scholar]
- 11.Koning JJ, Mebius RE. Interdependence of stromal and immune cells for lymph node function. Trends Immunol. 2012;33:264–270. doi: 10.1016/j.it.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 14.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207:689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W, Swartz MA, Hugues S. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. 2014;211:1153–1166. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–U1105. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan O, Headley M, Gerard A, Wei W, Liu LM, Krummel MF. Regulation of T cell priming by lymphoid stroma. PloS ONE. 2011:6. doi: 10.1371/journal.pone.0026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, Nelson PJ, Heikenwalder M, Acha-Orbea H, Buckley CD, Marsland BJ, Zehn D, Luther SA. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PloS ONE. 2011:6. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for peptide-major histocompatibility complex ligands. In: Paul WE, Littman DR, Yokoyama WM, editors. Annual Review of Immunology. Vol. 28. 2010. pp. 275–294. [DOI] [PubMed] [Google Scholar]
- 22.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 23.Girard JP, Springer TA. High endothelial venules (HEVS) - specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 24.Herzog BH, Fu JX, Wilson SJ, Hess PR, Sen A, McDaniel JM, Pan YF, Sheng MJ, Yago T, Silasi-Mansat R, McGee S, May F, Nieswandt B, Morris AJ, Lupu F, Coughlin SR, McEver RP, Chen H, Kahn ML, Xia LJ. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502:105–+. doi: 10.1038/nature12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benezech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE, Marriott CL, Watson SP, Caamano JH, Buckley CD, Barone F. CLEC-2 is required for development and maintenance of lymph nodes. Blood. 2014;123:3200–3207. doi: 10.1182/blood-2013-03-489286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase independent manner. J Exp Med. 2007;204:1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, Skarda D, Larson M, Douek DC, Haase AT. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4(+) T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Estes JD, Reilly C, Trubey CM, Fletcher CV, Cory TJ, Piatak M, Russ S, Anderson J, Reimann TG, Star R, Smith A, Tracy RP, Berglund A, Schmidt T, Coalter V, Chertova E, Smedley J, Haase AT, Lifson JD, Schacker TW. Antifibrotic therapy in simian immunodeficiency virus infection preserves CD4+ t-cell populations and improves immune reconstitution with antiretroviral therapy. J Infect Dis. 2014 doi: 10.1093/infdis/jiu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabb B, Morcock DR, Trubey CM, Quinones OA, Hao XP, Smedley J, Macallister R, Piatak M, Harris LD, Paiardini M, Silvestri G, Brenchley JM, Alvord WG, Lifson JD, Estes JD. Reduced inflammation and lymphoid tissue immunopathology in rhesus macaques receiving anti-tumor necrosis factor treatment during primary simian immunodeficiency virus infection. J Infect Dis. 2013;207:880–892. doi: 10.1093/infdis/jis643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cremasco V, Woodruff MC, Onder L, Cupovic J, Nieves-Bonilla JM, Schildberg FA, Chang J, Cremasco F, Harvey CJ, Wucherpfennig K, Ludewig B, Carroll MC, Turley SJ. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol. 2014;15:973–981. doi: 10.1038/ni.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomura M, Yoshida N, Tanaka J, Karasawa S, Miwa Y, Miyawaki A, Kanagawa O. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc Natl Acad Sci U S A. 2008;105:10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: Critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 36.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20:1483–1487. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 37.Sixt M, Kanazawa N, Seig M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Katakai T, Hara T, Sugai M, Gonda H, Shimuzu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200:783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gretz JE, Norbury CC, Anderson AO, Proudfoot AEI, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1439. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 41.Schumann K, Laemmermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Foerster R, Lutz MB, Sorokin L, Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, Nakano H, Nembrini C, Saudan P, Kopf M, Bachmann MF. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the c-type lectin receptor CLEC-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acton SE, Farrugia AJ, Astarita JL, Mourao-Sa D, Jenkins RP, Nye E, Hooper S, van Blijswijk J, Rogers NC, Snelgrove KJ, Rosewell I, Moita LF, Stamp G, Turley SJ, Sahai E, Reis e Sousa C. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, Song K, Kondo Y, Woodruff MC, Gogineni A, Onder L, Ludewig B, Weimer RM, Carroll MC, Mooney DJ, Xia L, Turley SJ. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2014 doi: 10.1038/ni.3035. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohse AW, Dinkelmann M, Kimmig M, Herkel J, ZumBuschenfelde KHM. Estimation of the frequency of self-reactive T cells in health and inflammatory diseases by limiting dilution analysis and single cell cloning. J Autoimmun. 1996;9:667–675. doi: 10.1006/jaut.1996.0087. [DOI] [PubMed] [Google Scholar]
- 47.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. In: Chiorazzi N, Lahita RG, Capra JD, Ferrarini M, Zabriskie JB, editors. Immune Mechanisms and Disease. 2003. pp. 15–25. [DOI] [PubMed] [Google Scholar]
- 48.Carbone FR, Belz GT, Heath WR. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 2004;25:655–658. doi: 10.1016/j.it.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Vezys V, Olson S, Lefrancois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M, Turley SJ. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immunol. 2011:2. doi: 10.3389/fimmu.2011.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hochweller K, Striegler J, Hammerling GJ, Garbi N. A novel CD11c.DTR transgenic mouse for depletion of dendritic cells reveals their requirement for homeostatic proliferation of natural killer cells. Eur J Immunol. 2008;38:2776–2783. doi: 10.1002/eji.200838659. [DOI] [PubMed] [Google Scholar]
- 52.Yang CY, Vogt TK, Favre S, Scarpellino L, Huang HY, Tacchini-Cottier F, Luther SA. Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci U S A. 2014;111:E109–E118. doi: 10.1073/pnas.1312585111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chyou S, Benahmed F, Chen JF, Kumar V, Tian S, Lipp M, Lu TT. Coordinated regulation of lymph node vascular-stromal growth first by CD11c(+) cells and then by T and B cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Foerster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J Immunol. 2008;181:1898–1907. doi: 10.4049/jimmunol.181.3.1898. [DOI] [PubMed] [Google Scholar]
- 56.Cording S, Wahl B, Kulkarni D, Chopra H, Pezoldt J, Buettner M, Dummer A, Hadis U, Heimesaat M, Bereswill S, Falk C, Bode U, Hamann A, Fleissner D, Huehn J, Pabst O. The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol. 2014;7:359–368. doi: 10.1038/mi.2013.54. [DOI] [PubMed] [Google Scholar]
- 57.Niedbala W, Cai BL, Liu HY, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4(+)CD25(+) Foxp3(-) regulatory T cells from CD4(+)CD25(-) T cells via p53, IL-2, and OX40. Proc Natl Acad Sci U S A. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niedbala W, Besnard AG, Jiang HR, Alves JC, Fukada SY, Nascimento D, Mitani A, Pushparaj P, Alqahtani MH, Liew FY. Nitric oxide-induced regulatory T cells inhibit Th17 but not Th1 cell differentiation and function. J Immunol. 2013;191:164–170. doi: 10.4049/jimmunol.1202580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denton AE, Roberts EW, Linterman MA, Fearon DT. Fibroblastic reticular cells of the lymph node are required for retention of resting but not activated CD8(+) T cells. Proc Natl Acad Sci U S A. 2014;111:12139–12144. doi: 10.1073/pnas.1412910111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8(+) T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 62.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 63.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 65.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4(+) T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Cui GW, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, Ikuta K. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc Natl Acad Sci U S A. 2014;111:1915–1920. doi: 10.1073/pnas.1318281111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fletcher AL, Elman JS, Astarita J, Murray R, Saeidi N, D’Rozario J, Knoblich K, Brown FD, Schildberg FA, Nieves JM, Heng TSP, Boyd RL, Turley SJ, Parekkadan B. Lymph node fibroblastic reticular cell transplants show robust therapeutic efficacy in high-mortality murine sepsis. Sci Transl Med. 2014;6:249ra109. doi: 10.1126/scitranslmed.3009377. [DOI] [PMC free article] [PubMed] [Google Scholar]