Abstract
This study was designed to investigate a genetic moderation effect of dopamine receptor-4 gene (DRD4) alleles that have 7 or more repeats (“long” alleles, l) on an intervention to deter drug use among rural African American adolescents in high-risk families. Adolescents (N = 291, M age = 17) were assigned randomly to the Adults in the Making (AIM) program or to a control condition and were followed for 27.5 months. Adolescents provided data on drug use and vulnerability cognitions 3 times after pretest. Pretest assessments of caregiver depressive symptoms, disruption in the home, and support toward the adolescent were used to construct a family risk index. Adolescents living in high-risk families who carried at least one l allele and were assigned to the control condition evinced greater escalations in drug use than did (a) adolescents who lived in high-risk families, carried the l allele, and were assigned to AIM or (b) adolescents assigned to either condition who carried no l alleles. AIM-induced reductions in vulnerability cognitions were responsible for the family risk × AIM × DRD4 status drug use prevention effects. These findings support differential susceptibility predictions and imply that prevention effects on genetically susceptible individuals may be underestimated.
Keywords: adolescence, DRD4, drug use, family environment, prevention
Background
To date, etiological models of drug use disorder, psychopathology, and the studies they have sponsored have focused primarily on social (e.g., family, peer, and community-level processes) and psychological (e.g., temperament and self-regulation) determinants. Such models, however, are incomplete. Vulnerabilities to tobacco, alcohol, and marijuana dependence are likely to be influenced by a combination of environmental and genetic factors, mediated in part through psychological processes (Kreek, Nielsen, Butelman, & LaForge, 2005; Rutter, Moffitt, & Caspi, 2006). These contributions include genetic main effects, gene × environment interactions (G×E) and gene-environment correlations (rGE), collectively called gene-environment (G-E) interplay (Rutter et al., 2006). G×E can be seen either as the moderation of a genetic effect by environmental influence or the moderation of environmental influence by genotype. For example, human children and primates whose genotypes predispose them to high impulsivity may develop poorly in adverse environments but normally in nurturing environments (Barr et al., 2004; Pauli-Pott, Friedl, Hinney, & Hebebrand, 2009). Gene-environment correlations occur (a) when genetic factors contribute to individual differences in exposure to positive or negative life events (such as when genetically influenced characteristics such as sociability or irritability evoke positive or negative responses from others), or (b) when genetically influenced behavior (such as risk-taking propensities) affects the individual’s choice of environmental experiences.
Most existing findings on G-E interplay in humans as it relates to drug use have come from genetic epidemiology, a branch of science that seems ideal for demonstrating that G×E and rGE exist in nature and affect etiology. Prevention science has less often been considered as an important source of basic information about the impact of genetic variation on drug use and psychological adjustment outcomes. This oversight is unfortunate, because prevention science has considerable potential to refine G-E hypotheses and to investigate causal mechanisms that are difficult to explicate in traditional genetic epidemiological designs. We propose that, through manipulated environments in randomized prevention trials, preventive interventions permit a more facile disentangling of environments from genetic influences and therefore greater flexibility in characterizing the nature of G-E interplay. Thus, the use of randomized intervention designs brings the power of experimental manipulation to the study of G-E interplay, advancing understanding of drug use and abuse, and thereby increasing the power of future prevention efforts (Howe, Reiss, & Yuh, 2002).
Through the implementation of prevention trials, a causal relationship between an environmental manipulation and the alteration of a targeted outcome can be identified (Rutter, 2005). Randomized prevention trials rule out rGE as rival explanations. Experimental random assignment of participants to a prevention or control condition eliminates biases that reflect rGE. For example, youths with certain genotypes may select deviant peers (active rGE), or parents with specific genotypes that their children share produce particular kinds of family environments (passive rGE). Accordingly, random assignment has the advantage of ruling out these potential rGE confounds that, in epidemiological designs, may be mistaken for pure environmental effects. In addition, the testing of G×E hypotheses using randomized prevention trials may enhance statistical power as much as five-fold over epidemiological genetic approaches (Bakermans-Kranenburg & van IJzendoorn, in press; Van IJzendoorn, 2013); consequently, fewer participants may be needed to detect a G×E interaction in a randomized trial. Most importantly, testing G×E hypotheses in the context of prevention trials broadens the conceptual models guiding such trials, contributing to progress within the Institute of Medicine prevention development cycle (O’Connell, Boat, & Warner, 2009).
First- and Second-Generation Gene × Intervention Research
Existing prevention trials can serve as experimental contexts into which genetic assessments can be integrated. We term this first-generation gene × intervention (G×I) research. Several first-generation studies have provided provocative initial evidence of the utility of randomized controlled trials in circumventing the issues inherent in epidemiological G×E studies. Evidence has shown intervention efficacy to be genetically moderated by the 7-repeat version of the dopamine receptor-4 gene (DRD4). Specifically, toddlers who carried this allele showed a greater reduction in disruptive behavior after parenting skill intervention than did children who did not carry this allele (Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, & Juffer, 2008). In another experiment, kindergarten students with this genotype were affected more positively than were those without it when randomly assigned to play computer games designed to enhance their phoneme awareness skills (Kegel, Bus, & van IJzendoorn, 2011). Beach, Brody, Lei, and Philibert (2010) demonstrated that preadolescents who carried the 7-repeat version of DRD4 and were assigned to take part in the Strong African American Families intervention program (Brody et al., 2004) evinced considerably less drug use across 2 years than did youths with the same genotype who were assigned to the control group. Brody, Chen, et al. (2013) found that African American adolescents carrying the 7-repeat allele benefitted most from the family-centered Strong African American Families–Teen intervention program (Brody, Chen, Kogan, et al., 2012) designed to prevent the use of alcohol and other drugs. These studies also indicate that, for genetic reasons, individuals differ in the extent to which they are affected by exposure to environmental influences. Bakermans-Kranenburg and van IJzendoorn (2011) executed a meta-analysis supporting the proposition that dopamine-related genes fostered differential susceptibility to environmental influences, including constructed environments such as prevention programs. Prevention scientists only recently have begun to examine the processes that account for or mediate first-generation G×I findings. Research designed to lead to an understanding of the locus of G×I effects can be termed second-generation G×I research. For example, in the context of a family-centered substance use prevention trial, Brody, Chen, et al. (2013) demonstrated that G×I effects on increases in protective parenting accounted for G×I effects on adolescent drug use. The purpose of the present study was to test both first- and second-generation G×I hypotheses with a sample of rural African American youths during their transitions to emerging adulthood.
The Current Study
For young African American adults living in the rural Southern United States, transitions after secondary school are unstructured and left largely to individual initiative. When they leave school, many have no jobs. Eventually, they find part-time or full-time employment performing simple functions in retail service-sector jobs that offer little training and no opportunity for advancement. Job turnover rates are high during this period, as the combined effects of poor preparation for employment and disadvantageous hiring practices make the transition to the workforce a protracted and demoralizing process (Gore & Aseltine, 2003). Some who see no pathway to adequate subsistence, much less the attainment of life goals, cope by increasing drug use (Brody, Chen, & Kogan, 2010). These circumstances and the need for prevention programs for rural African American young adults led to the development of the Adults in the Making (AIM) program (Brody, Chen, Kogan, Smith, & Brown, 2010).
AIM is a universal, family-centered preventive intervention that was designed to enhance protective family and self-regulatory processes, promote resilience, and deter drug use. A cluster of protective parenting processes was identified from longitudinal, epidemiological research with rural African Americans. This cluster, targeted in AIM, includes the provision of developmentally appropriate emotional and instrumental support, occupational and educational mentoring, and racial socialization that includes strategies for dealing with discrimination. AIM training experiences for adolescents included enhancement of developmentally appropriate, planful self-control skills and problem-focused coping in response to racial discrimination; development and pursuit of educational or occupational plans; and formation of strategies for accessing support from community resources. Recent studies have shown AIM’s efficacy in deterring drug use, particularly for youths experiencing high levels of life stress (Brody, Chen, Kogan, et al., 2010) and contextual risk (Brody, Yu, Chen, Kogan, & Smith, 2012). As part of these evaluations, saliva samples were obtained from which the dopamine receptor-4 (DRD4) was genotyped.
A basic premise of the study is that living in a high-risk family context sponsors increases in drug use. The risky family model offers a psychosocial account of the impact that family stress exerts on drug use (Repetti, Taylor, & Seeman, 2002). It posits that some families confer risk for later drug use by producing emotionally cold, disorganized, and nonsupportive environments in which primary caregivers evince elevated levels of depression. Cross-sectional and prospective surveys with adolescents have found initiation and escalation of drug use to be positively associated with family environments characterized by low levels of positive affect along with a lack of parental instrumental and emotional support (Stone, Becker, Huber, & Catalano, 2012). We term such family environments high-risk. Baumeister and Scher (1988) advanced a parsimonious interpretation of this link. People desire the quickest possible escape from life stress and the negative affect that accompanies it; this increases the attraction of activities that provide rapid, though short-term, relief. Thus, the “quick fix” that drug use offers becomes appealing regardless of possible long-term costs to health and well-being.
The primary purpose of this study was to test multilevel predictors regarding a genetic moderation effect on the efficacy of AIM in deterring drug use for young adults living in high-risk family contexts. We did not expect genetic variation to have a direct linear association with drug use escalation; instead, we expected genetic status to predict variation in young adults’ responses to AIM participation, particularly among those living in high-risk family environments. This perspective is consistent with differential susceptibility theory, in which genetic variation is hypothesized to render individuals more or less vulnerable to both positive and negative aspects of the environment (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pluess, 2009, 2013; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011).
Who: DRD4
One such genetic factor is a variation at the dopamine receptor D4 (DRD4) gene. We tested a hypothesis involving the presence of an allele with 7 or more repeats. The 7-repeat allele of DRD4 is associated with reduced gene expression (Schoots & Van Tol, 2003) and altered functioning (Asghari et al., 1995; Asghari et al., 1994). This gene is also associated with behavioral self-control problems, such as alcoholism (Laucht, Becker, Blomeyer, & Schmidt, 2007), use of alcohol and other drugs (Brody, Chen, Yu, et al., 2012), and impulsivity (Eisenberg et al., 2007). Relevant to the current study’s purposes, dopamine may be important in the development of cognitions that orient youths toward drug use. Studies have shown that the pairing of drug-related cues—such as peer talk about drugs, peer use, or media depictions of drug use—with subsequent dopamine release in the limbic system and prefrontal cortex increase drug-seeking behaviors (Hyman, Malenka, & Nestler, 2006). One explanation for the link between dopamine and drug-related cues is the incentive sensitization theory (Franken, Stam, Hendriks, & van den Brink, 2003). When youths are presented with a drug-related cue, they may experience an increase in dopaminergic activity in brain regions related to selective attention (i.e., amygdala, anterior cingulate cortex, regions of the prefrontal cortex). Subsequently, the youth becomes focused on drug use, leading to drug-seeking behavior. In this study, we propose that carriers of the DRD4 allele with 7 or more repeats will be likely to develop drug use vulnerability cognitions, particularly if they live in a high-risk family context and are in the control condition of the AIM trial.
As mentioned previously, evidence is accumulating to indicate that carriers of at least one allele of DRD4 with 7 or more repeats experience heightened susceptibility to environmental influences, including exposure to prevention and intervention programs (Bakermans-Kranenburg & van IJzendoorn, 2011; Brody, Beach, et al., 2013). Most studies characterize DRD4 alleles as either “short” (s) or “long” (l), with the short allele defined as having 6 or fewer repeats and the long allele as having 7 or more repeats (McGeary, 2009). Accordingly, this convention is followed in the present study, in which individuals with two s alleles were contrasted with those carrying one or two l alleles. We proposed that young adults who carried at least one l allele of DRD4, who were assigned to the control condition and lived in a high-risk family context would evince more drug use over time than would (a) carriers of at least one l allele who were assigned to the AIM condition and lived in a high-risk family context, (b) young adults assigned to either condition who carried two s alleles, or (c) young adults who did not live in a high-risk family context.
How: Vulnerability Cognitions
Even with its complexity, the aforementioned hypotheses provide an example of first generation gene × environment (G×E) interaction research. As mentioned previously, this is an important and necessary step in understanding the etiology of drug use and abuse, it does not further understanding of the reasons why or the processes through which the G×E interaction operates to influence a phenotype such as drug use trajectories. To address the need for second-generation G×E research, in the present study we also hypothesized a G×E interaction in which young adults who live in a high-risk family context, are assigned to the control condition, and carry one or two l alleles of DRD4 would evince increases in vulnerability cognitions for drug use, a proximal risk factor for escalation. We further proposed that this interaction would account for the family risk × AIM participation × DRD4 interaction. We discuss this mediated moderation effect next.
We conjectured that African American emerging adults who experience high levels of family stress may come to believe that they have little to lose by abandoning planful, conventional orientations in favor of a present orientation that promotes “living in the moment.” These young adults are at heightened risk of developing cognitions that increase their likelihood of drug use, such as intentions to use drugs, willingness to use, and positive prototypes or images of drug-using peers. These cognitions start to develop at an early age and continue to develop during young adulthood, serving as proximal risk mechanisms in longitudinal, etiological research forecasting drug use escalation (Chassin, Tetzloff, & Hershey, 1985; Cleveland, Gibbons, Gerrard, Pomery, & Brody, 2005). Behavioral willingness is defined as an openness to using drugs given an opportunity to do so—that which a young adult might do under certain circumstances such as the presence of drug-using friends (Cleveland et al., 2005). Intentions to use drugs predict actual use more strongly with increasing age, as drug use becomes more deliberate and, in some cases, habitual (Pomery, Gibbons, Reis-Bergan, & Gerrard, 2009). Prototypes are images of a particular type of person, for example, young adults who use drugs (Chassin, Presson, Sherman, Corty, & Olshavsky, 1984). Positive images of drug-using peers predict drug use and other health-risk behaviors on the part of those who hold the images (Cleveland et al., 2005). To maximize predictive power, we formed a construct labeled vulnerability cognitions that comprises willingness, intentions, and prototypes. We predicted that (a) African American young adults who live in a high-risk family context, were assigned to the AIM condition, and carried at least one l allele of DRD4 would evince decreases in vulnerability cognitions for drug use, whereas similar youths in the control condition would evince increases in vulnerability cognitions over time; and (b) these changes would serve as a mediator connecting the family risk × AIM participation × DRD4 genotype interaction with changes in drug use.
Method
Participants
Participants in the AIM trial included 367 African American youths who were in high school at the beginning of the study (M age = 17.0 years, SD = 0.75). Their families had an average of 2.4 children. Of the youths in the sample, 59.1% were female, and 63.6% lived in single-mother-headed households. Primary caregivers, whose mean age was 44.0 years (SD = 8.50), were the target youths’ biological mothers. A majority of the youths’ caregivers (78.7%) had completed high school or earned a GED. The median family income of $2,012 per month was representative of the sampled population (Boatright, 2005). The genetic data were collected when the youths were 17 years of age. Of the 367 youths who participated in the AIM trial, 291 agreed to provide DNA (79.3% of the original sample). These 291 participants constituted the sample in this study. Of this subsample, 142 were assigned to the intervention condition and 149 were assigned to the control condition. Participants provided data at pretest (2.5 months pre-intervention), at posttest (Wave 2, 6.4 months after pretest), and at two long-term follow up assessments (Wave 3, 16.6 months after pretest; Wave 4, 27.5 months after pretest). Wave 4 data were collected from 255 participants (87.6%). The equivalence of the baseline assessments of the study variables for participants who provided or did not provide DNA data by prevention group assignment were evaluated via two-factor multivariate analyses of variance (MANOVAs). No significant main effects or interaction effects emerged for any study or confounder variables. Additional analyses, using ANOVAs and chi-square, of the study variables’ baseline equivalence among participants in the AIM and control groups who provided DNA data did not detect any differences (see Table 1).
Table 1.
Pretest Equivalence of Prevention Condition by Genotype Status
| Variables at Pretest | Control
|
AIM
|
F | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
DRD4 s alleles (n = 94)
|
DRD4 l alleles (n = 55)
|
DRD4 s alleles (n = 84)
|
DRD4 l alleles (n = 58)
|
|||||||
| M | SD | M | SD | M | SD | M | SD | |||
| Gender, male | 0.43 | 0.50 | 0.47 | 0.50 | 0.37 | 0.48 | 0.29 | 0.46 | 1.51 | .21 |
| SES risk index | 2.05 | 1.48 | 1.93 | 1.45 | 1.99 | 1.33 | 2.26 | 1.24 | 0.64 | .59 |
| Family risk | 0.02 | 2.94 | −0.12 | 2.80 | −0.08 | 2.96 | 0.20 | 3.13 | 0.14 | .94 |
| Vulnerability cognitions | 0.10 | 2.41 | 0.25 | 2.24 | −0.18 | 1.61 | −0.11 | 1.91 | 0.61 | .61 |
|
| ||||||||||
| n | % | n | % | n | % | n | % | χ2 | p | |
|
| ||||||||||
| Drug use | 3.91 | .69 | ||||||||
| No use | 71 | 75.5 | 41 | 74.5 | 63 | 75.0 | 44 | 75.9 | ||
| Occasional use | 11 | 11.7 | 8 | 14.5 | 16 | 19.0 | 9 | 15.5 | ||
| Regular use | 12 | 12.8 | 6 | 10.9 | 5 | 6.0 | 5 | 8.6 | ||
Procedure
To enhance rapport and cultural understanding, African American students and community members served as field researchers to collect data. Prior to data collection, the researchers received 12 hours of training in administering the protocol. The instruments and procedures were developed and refined with the help of a focus group of 40 African American community members who were representative of the population from which the sample was drawn. The focus group process has been described in detail elsewhere (Brody, Murry, Kim, & Brown, 2002).
At each wave of data collection, one home visit lasting 2 hours was made to each family. Both the field researchers and the project staff who arranged the visits were unaware of the families’ assignments to the intervention or control group. Primary caregivers consented to their own participation and the participation of youths under age 18; youths assented or, if 18 or older, consented to their own participation. At the home visit, self-report questionnaires were administered to caregivers and youths in an interview format. Each interview was conducted privately, with no other family members present or able to overhear the conversation. Each family was paid $100 at each assessment. All procedures were approved by the University of Georgia Institutional Review Board.
Intervention Implementation, Attendance, and Fidelity
The AIM prevention program, modeled after an existing family-based skills-training intervention in a group format for rural African American preadolescents (see Brody et al., 2004), consists of six consecutive weekly group meetings held at community facilities. Each meeting includes separate, concurrent training sessions for parents and youths, followed by a joint parent–youth session during which the families practice the skills they learned in their separate sessions. Concurrent and family sessions each last 1 hour. Thus, both parents and youths receive 12 hours of prevention training.
Parents in the prevention condition were taught how (a) to provide developmentally appropriate emotional and instrumental support, (b) to provide ongoing racial socialization that includes strategies for dealing with discrimination, (c) to provide occupational and educational mentoring, (d) to promote autonomy and adult responsibility, and (e) to encourage responsible decisions about risk behaviors. Program content was delivered by narrators on videotapes that also depicted family interactions illustrating targeted behaviors. African American group leaders presented the prevention curriculum, organized role-playing activities, guided discussions among parents, and answered parents’ questions. Youths were taught how to develop a future orientation, to plan for meeting goals, to identify people in their communities who could help them attain goals, to cope with barriers and racial discrimination, and to formulate self-care strategies. Videotapes were also used in the youth sessions, along with structured activities, role playing, and group discussions.
Of the pretested families, 67% took part in four or more sessions, with 35% attending all six of them. Families took part in an average of four sessions. Each team of group leaders was videotaped while conducting program sessions. For each group, two parent and two youth sessions were selected randomly and scored for adherence to and coverage of the prevention curriculum. Coverage of the curriculum components exceeded 80% for both the parent and the youth sessions.
Measures
Socioeconomic risk index
Six dichotomous variables formed a socioeconomic risk index administered at pretest that was used as a control in the data analyses. A score of 1 was assigned to each of the following characteristics: family poverty based on federal guidelines, primary caregiver unemployment, receipt of Temporary Assistance for Needy Families, primary caregiver single parenthood, primary caregiver education level less than high school graduation, and caregiver-reported inadequacy of family income. The scores were summed to form an index (M = 2.39, SD = 1.48). Because this index is composed of count data, internal consistency was not computed.
Intervention status and gender
Intervention status and gender were dummy coded. AIM participants were coded 1 and control participants were coded 0; male participants were coded 1 and female participants were coded 0.
Family risk
The family risk construct, evaluated at pretest, was composed of four variables: parental depression, parent-child conflict, chaos in the home, and parental involvement and support. Parental depressive symptoms were measured via self-report on the Center for Epidemiologic Studies Depression scale (CES–D; Radloff, 1977), which is widely used with community samples. Primary caregivers rated each of 20 symptoms on a scale of 0 (rarely or none of the time), 1 (some or little of the time), 2 (occasionally or a moderate amount of time), or 3 (most or all of the time), α = .85. Parent-child conflict was assessed using a seven-item version of the Ineffective Arguing Inventory (IAI; Kurdek, 1994) adapted for use with parents and children. Parents rated statements about the conflicts they had with their children on a scale ranging from 1 (disagree strongly) to 5 (agree strongly), α = .79. Examples include, “You and your child’s arguments are left hanging and unsettled” and “You and your child go for days being mad at each other.” Chaotic home environment was assessed by the 15-item Confusion, Hubbub, and Order Scale (CHAOS; Matheny, Wachs, Ludwig, & Phillips, 1995). Parents were asked to endorse as true (1) or false (0) several statements about their homes, α = .76. Examples include “there is often a fuss going on at our home”; “no matter what our family plans, it usually doesn’t seem to work out”; and “I often get drawn into other people’s arguments at home.” Support, involvement, and communication in the parent-child relationship were assessed using the 20-item Interaction Behavior Questionnaire (IBQ; Prinz, Foster, Kent, & O’Leary, 1979). Parents rated 20 statements as true (1) or false (0), α = .88. Examples include “your child usually listens to what you tell him/her” and “you and your child reach an agreement during arguments.” Parental depression, parent-child conflict, chaos in the home, and parental support scores were standardized, and parental support was subtracted from the summed scores of parental depression, parent-child conflict, and chaos in the home; high values indicated highly negative home environments. Prior to combining them, the individual measures were determined to be intercorrelated (rs = −.22 to .58, ps < .001).
Vulnerability cognitions
The measures used to assess vulnerability cognitions were administered at each wave. This construct was composed of behavioral willingness and intentions to use alcohol and other drugs and included prototypes of peers who use them. Youths’ willingness to use drugs was measured with three items, worded as in previous studies (Brody et al., 2004). A scenario was presented: “Suppose you were with a group of friends and there were some drugs there that you could have if you wanted. How willing would you be to do the following things: (a) take some and use them; (b) use enough to get high; and (c) take some with you to use later?” Responses ranged from 1 (not at all) to 3 (very); Cronbach’s alphas ranged from .73 to .89 across the study. The vulnerability cognition measure also included an eight-item scale composed of two items measuring intentions to engage in each of four drug-use behaviors: smoking cigarettes, smoking marijuana, drinking alcohol, and drinking alcohol heavily. The items were, “Do you plan to use [drug] in the next year?” and “How likely is it that you will use [drug] in the next year?” (Warshaw & Davis, 1985). Cronbach’s alphas ranged from .77 to .82 across the study. The measure of prototypical images of drinkers (Brody et al., 2004) was introduced with the lead-in statement, “Take a moment to think about the type of person your age who frequently drinks alcohol. We are not talking about anyone in particular, just your image of people your age who frequently drink alcohol.” Using a response set ranging from 1 (not at all) to 4 (very), youths indicated how “popular,” “smart,” “cool,” “attractive (good looking),” and “dull (boring)” they considered such peers to be. Youths also indicated, on the same response set, how similar they considered themselves to be to alcohol-drinking peers. The six items were summed to create a variable measuring youths’ images of drinkers; Cronbach’s alphas ranged from .73 to .82 across the study. The intentions, willingness, and prototypical images scores were then standardized and summed to form an indicator of vulnerability cognitions for each wave. Prior to combining them, the individual measures were determined to be intercorrelated within each wave (rs = .27 to .61, ps < .001 for Wave 1; rs = .31 to .62, ps < .001 for Wave 2; rs = .28 to .69, ps < .001 for Wave 3; rs = .35 to .66, ps < .001 for Wave 4).
Drug use
Four items widely used in the public health literature (DiClemente, et al., 2001) and our previous research with rural African American youths (Wills, Gibbons, Gerrard, & Brody, 2000; Wills, Gibbons, Gerrard, Murry, and Brody, 2003) were used to assess past-month drug use (Johnston, O’Malley, & Bachman, 2000). Youths were asked how often during the past month they had engaged in each of the forms of drug use included in the study. A 7-point response set ranging from not at all to about two packs a day was used for cigarette smoking; a 6-point scale ranging from none to 20 or more indexed the other forms of use. These items have commonly been summed to characterize drug use in evaluations of prevention programs (Brody et al., 2006; Brody et al., 2012). The data were re-coded into three developmentally appropriate drug use categories, defined as follows: never, no use of any drugs; occasional use, less than one cigarette a day or no more than three occasions of smoking marijuana, drinking alcohol, or drinking heavily; and regular use, at least one cigarette per day, engaging in one of the other three forms of use on four or more occasions, or a combination of two or more forms of drug use on at least three occasions.
Genotyping
Youths’ DNA was obtained using Oragene™ DNA kits (DNA Genotek, Kanata, Ontario, Canada). Youths rinsed their mouths with tap water, then deposited 4 ml of saliva in the Oragene sample vial. The vial was sealed, inverted, and shipped via courier to a central laboratory in Iowa City, where samples were prepared according to the manufacturer’s specifications. Genotype at DRD4 was determined for each youth as Bradley, Dodelzon, Sandhu, and Philibert, 2005 described using the primers F-GGCGTTGCCGCTCTGAATGC and R-GAGGGACTGAGCTGGACAACCAC, standard Taq polymerase and buffer, standard dNTPs with the addition of 100 μM 7-deaza GTP, and 10% DMSO. The resulting PCR products were electrophoresed on a 6% non-denaturing polyacrylamide gel and visualized using silver staining. Genotype was then called by two individuals blind to the study hypotheses and other information about the participants. For tests of the G×E×I hypotheses, DRD4 status was dummy coded; participants with at least one l allele were assigned a code of 1 (38.8% of the sample), and participants who were homozygous for the s allele were assigned a code of 0 (61.2% of the sample). None of the alleles deviated from Hardy-Weinberg equilibrium (p = .87, ns).
Data Analysis
To test the study hypotheses, we conducted latent growth modeling (LGM) using Mplus 7.1 (B. O. Muthén & Muthén, 1998–2012). Missing data were handled using full information maximum likelihood (FIML) estimation, which yields unbiased parameter estimates and appropriate standard errors when data are missing at random (B. O. Muthén & Muthén, 1998–2012). Because of the relatively low rates of drug use among rural African American adolescents (Brody & Ge, 2001; Cleveland et al., 2005), the outcome was modeled as three-level ordinal variables: the absence of any drug use was coded as 0, occasional drug use was coded as 1, and regular drug use was coded as 2. Latent growth curve models with ordinal outcomes were used for this study (B. O. Muthén, 2001). We tested a mediated-moderation model (Little, Bovaird, & Card, 2007; Preacher, Rucker, & Hayes, 2007) to examine the hypothesis that family risk × AIM participation× DRD4 status interaction effects on drug use are mediated by their effects on vulnerability cognitions.
Participant age within the AIM and control groups varied at all waves of data collection. These variations were managed in the growth models by specifying growth as a function of age rather than a function of data collection wave; the random t-score option in Mplus was used. The models included two individual growth parameters: (a) an intercept parameter with time centered at age 17, and (b) a linear slope parameter representing the average linear change in drug use and vulnerability cognitions over time.
Results
Preliminary Analyses
Drug use rates from Wave 1 to Wave 4 are presented in Table 2. As expected, the proportion of participants indicating no use decreased and the proportions indicating occasional use and regular use increased in a linear fashion. Overall, 75.3% of participants reported no drug use, 15.1% indicated occasional use, and 9.6% reported regular use at pretest. At Wave 4, 56.1% of participants reported no drug use, 18.8% reported occasional use, and 25.1% reported regular use.
Table 2.
Numbers and Proportions of Drug Use for Each Time Point
| Time Point | Drug Use
|
||
|---|---|---|---|
| No Use | Occasional Use | Regular Use | |
| Pretest | 219 (75.3%) | 44 (15.1%) | 28 (9.6%) |
| Wave 2 | 198 (71.0%) | 42 (15.1%) | 39 (14.0%) |
| Wave 3 | 169 (61.7%) | 41 (15.0%) | 64 (23.4%) |
| Wave 4 | 143 (56.1%) | 48 (18.8%) | 64 (25.1%) |
p< .05.
Associations of Family Risk, AIM participation, and DRD4 Status with Drug Use Trajectories
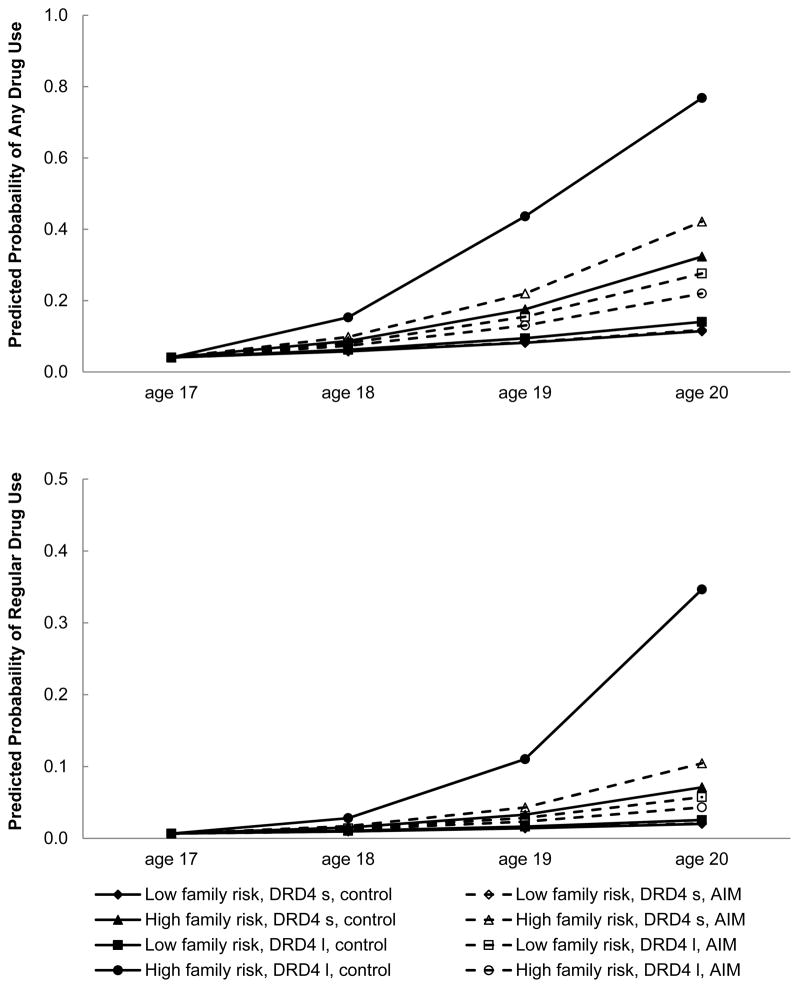
Participant gender and the SES risk index were regressed on the intercept of drug use; gender, SES risk index, family risk, AIM participation, and DRD4 status, as well as the two-way interactions and the three-way interaction among these variables, were regressed on the slope of drug use. As shown in Table 3 (left column), gender and family risk were positively associated with the slope of drug use, indicating that male participants and those living in high-risk families were likely to report an increase in drug use over time. A significant family risk × AIM participation × DRD4 status three-way interaction predicted the slope of drug use. To interpret this interaction, we calculated the effects of prevention status (simple slopes) on the slope of drug use at low (1 standard deviation below to mean; −1 SD) and high (1 standard deviation above the mean; +1 SD) levels of family risk for each genotype status (see Table 4, left column). These simple slopes served as the estimation of effect size for prevention assignment. Comparisons among the family risk × genotype × prevention assignment groups revealed that, for participants living in high-risk families who carried at least one l allele of DRD4, assignment to the prevention group significantly reduced the increase of drug use over time (coefficient of intervention effect = −0.82, SE = 0.32, t(280) = −2.56, p = .011). Prevention assignment was not associated with changes in drug use over time for youths who lived in low-risk families or carried two s alleles of DRD4. This three-way interaction, also depicted in Figure 1, suggests that participants in the control group who lived in high-risk families and carried at least one l allele of DRD4 were more likely than similar AIM participants to increase their drug use over time. No significant differences between the control and AIM groups emerged for drug use when youths lived in low-risk families or carried two s alleles of DRD4.
Table 3.
Associations among Intervention Status, DRD4 Status, Family Risk, and Growth in Drug Use and Vulnerability Cognitions (N = 291)
| Predictors | Drug Use
|
Vulnerability Cognitions
|
||
|---|---|---|---|---|
| Intercept | Slope | Intercept | Slope | |
| 1. Male gender | −.588 (.696) | .537* (.225) | .258 (.326) | .101 (.095) |
| 2. SES risk index | .251 (.223) | −.083 (.077) | .089 (.121) | −.054 (.036) |
| 3. AIM | .076 (.153) | −.052 (.068) | ||
| 4. DRD4 status | .362 (.200) | .092 (.102) | ||
| 5. Family risk | .074* (.036) | .009 (.016) | ||
| 6. DRD4 × Family risk | .096 (.066) | .077* (.032) | ||
| 7. DRD4 × AIM | −.345 (.265) | −.044 (.131) | ||
| 8. Family risk × AIM | .022 (.046) | .016 (.021) | ||
| 9. DRD4 × Family risk × AIM | −.209* (.085) | −.097* (.037) | ||
Table 4.
Intervention Effects on the Slopes of Drug Use and Vulnerability Cognitions by Family Risk and DRD4 Status
| Subgroups | Slope of Drug Use
|
Slope of Vulnerability Cognitions
|
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficients | SE | t | p | Coefficients | SE | t | p | |
| Low family risk, DRD4 s alleles | 0.011 | 0.218 | 0.051 | .959 | −0.099 | 0.084 | −1.174 | .242 |
| High family risk, DRD4 s alleles | 0.141 | 0.191 | 0.739 | .461 | −0.005 | 0.099 | −0.048 | .961 |
| Low family risk, DRD4 l alleles | 0.283 | 0.281 | 1.004 | .316 | 0.143 | 0.114 | 1.257 | .210 |
| High family risk, DRD4 l alleles | −0.821 | 0.321 | −2.559 | .011 | −0.335 | 0.168 | −1.991 | .047 |
Note. Low family risk: 1 SD below the mean; high family risk: 1 SD above the mean.
Figure 1.
Growth in probability of past-month drug use by family risk, AIM assignment, and DRD4 status. Low family risk: 1 SD below the mean; high family risk: 1 SD above the mean.
Associations of Family Risk, AIM Participation, and DRD4 Status with Trajectories for Vulnerability Cognitions
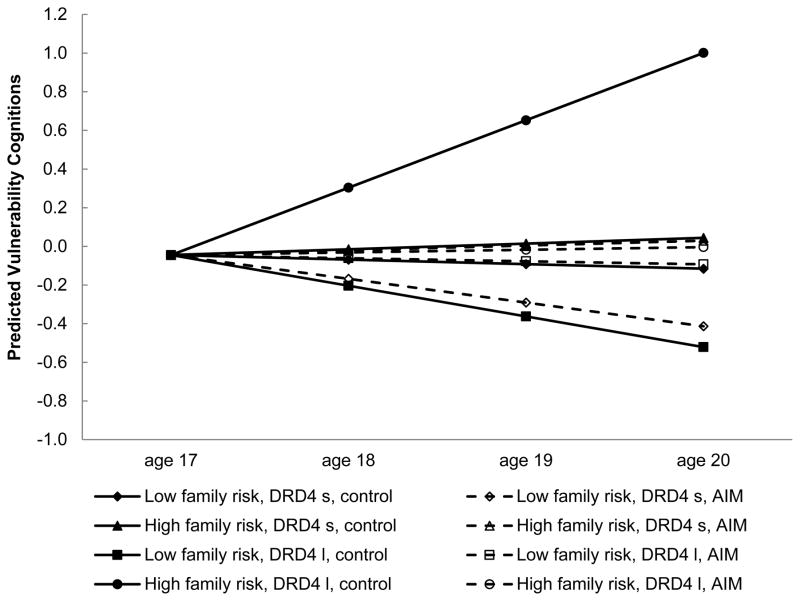
The LGM was estimated for the hypothesized mediator, vulnerability cognitions, which we conjectured would account for AIM’s prevention effects among participants who lived in high-risk families and carried an l allele of DRD4. Participant gender and SES risk index were regressed on the intercept of vulnerability cognitions; gender, SES risk index, family risk, AIM participation, and DRD4 status, as well as the two-way interactions and the three-way interaction among the latter three variables, were regressed on the slope of vulnerability cognitions. As shown in Table 3 (right column), a significant family risk × AIM participation × DRD4 status three-way interaction predicted the slope of vulnerability cognitions. We also calculated the effects of prevention status (simple slopes) on the slope of vulnerability cognitions at low (−1 SD) and high (+1 SD) levels of family risk for each genotype status (see Table 4, right column). Comparisons among the family risk × genotype × prevention status groups on the intervention effect revealed that, for participants who lived in high-risk families and carried at least one l allele of DRD4, assignment to the prevention group significantly reduced the increase of vulnerability cognitions over time (coefficient of prevention effect = −0.34, SE = 0.17, t(280) = −1.99, p = .047). Prevention assignment was not associated with changes in vulnerability cognitions over time for youths living in low-risk families or those who carried two s alleles of DRD4. This three-way interaction, also depicted in Figure 2, suggests a pattern similar to that for drug use. Youths in the control group who lived in high-risk families and carried an l allele of DRD4 were more likely than similar AIM participants to increase in vulnerability cognitions over time. Again, no significant differences between the control and AIM groups emerged for vulnerability cognitions when youths lived in low-risk families and carried two s alleles of DRD4.
Figure 2.
Growth in vulnerability cognitions by family risk, AIM assignment, and DRD4 status. Low family risk: 1 SD below the mean; high family risk: 1 SD above the mean.
Vulnerability Cognitions Mediate the Relations among Family Risk, AIM Participation, DRD4 Status, and Substance Use Trajectories
Before investigating the mediation effect of vulnerability cognitions, we executed a cross-lagged model to determine whether the direction of causality among the variables conformed to the study hypotheses. The path coefficient from vulnerability cognitions at pretest to past-month drug use at Wave 4 was .27 (p < .01), and the path coefficient from drug use at pretest to vulnerability cognitions at Wave 4 was .12 (p < .01). A Wald test demonstrated a significant difference between the two path coefficients, χ2(1) = 4.45, p < .05, suggesting the direction of causality proceeded from vulnerability cognitions to past-month drug use rather than the reverse.
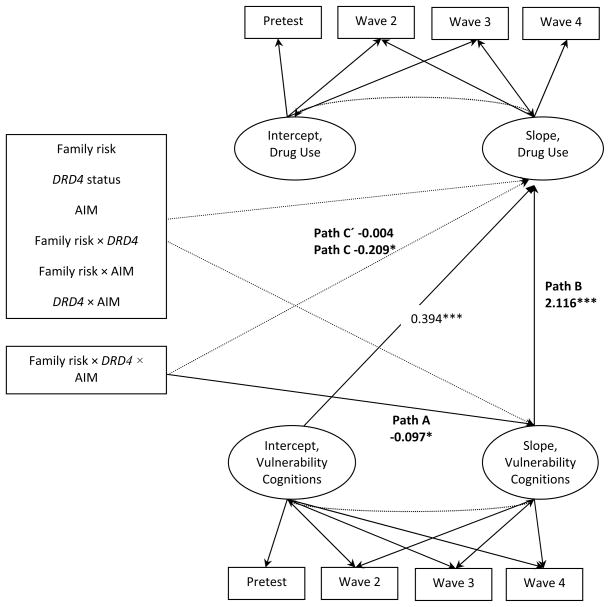
A parallel growth model (L. K. Muthén & Curran, 1997) was executed to test the mediated moderation hypothesis that AIM effects on increases in vulnerability cognitions for youths who lived in high-risk families and carried an l allele of DRD4 would account for its efficacy in reducing drug use among this vulnerable group. The models depicted in Figure 3 demonstrated that (a) the family risk × AIM participation × DRD4 status interaction effect on vulnerability cognitions found in previous analyses also emerged in the mediation model (path A); (b) the path from the slope for vulnerability cognitions to the slope for drug use (path B) was positive and significant; and (c) the path from the family risk × AIM participation × DRD4 status interaction to the slope for drug use became nonsignificant when the effect of the three-way interaction on vulnerability cognitions was included in the model (Path C: β = −0.209, p < .02 without the growth of vulnerability cognitions in the model; Path C′: β = −0.004, p = ns with the growth of vulnerability cognitions in the model). Thus, AIM’s efficacy in preventing increases in drug use among rural African American youths living in high-risk families and carrying an l allele of DRD4 occurred through its effect on deterring the development of cognitions that make the use of drugs attractive (indirect effect of Path A × Path B = −0.204, SE = 0.098, p = .037).
Figure 3.
Vulnerability cognitions as a mediator for the effect of AIM × DRD4 status × risky family on probability of past month drug use with gender and SES risk index controlled. Dashed lines indicate nonsignificant paths. *p < .05, ***p < .001.
All of these analyses were re-executed using other groupings of DRD4 alleles: 7 repeats (n = 96, 33.0% of the sample) versus all other variations (n = 195, 67.0% of the sample) and 7 repeats (n = 96, 33.0% of the sample) versus 4 repeats (n = 126, 43.3% of the sample). The results were identical to those of the previous analyses.
Discussion
Drug use and abuse among children and adolescents have well-documented environmental causes. First-and second-generation G×I research will help to expand scientific understanding of etiological mechanisms underlying these problems, and this progress will continue as more data become available during the next several years. Toward this end, the present study tested a G×E hypothesis about the genetic moderation of prevention effects on increases in young adult drug use was tested. The results indicated that adolescents who carried at least one l allele of DRD4, lived in a high-risk family context, and were assigned to the control condition evinced greater increases in drug use over time than did (a) similar youths assigned to AIM or (b) adolescents who carried two s alleles or lived in a low-risk family context. The results extend previous findings that carrying an l allele of DRD4 increases sensitivity to intervention or prevention programs among toddlers (Bakermans-Kranenburg et al., 2008), kindergarten children (Kegel et al., 2011), preadolescents (Beach et al., 2010), and adolescents (Brody, Chen, et al., 2013).
The results support Belsky and colleagues’ differential susceptibility hypothesis, in which variants of specific genes are proposed to render individuals more susceptible to the surrounding environment whether it is characterized by high positivity or high risk. The finding that, after exposure to the protective processes that AIM offered to adolescents living in high-risk families, carriers of at least one l allele of DRD4 evinced less drug use over time than did similar youths in the control condition supports differential susceptibility predictions. If supported on a broader basis, these results imply that general estimates of prevention-induced resilience effects on drug use both under- and overestimate protective effects. Resilience effects are underestimated for genetically susceptible individuals and overestimated for those without genetic susceptibility. Clearly, more genetically informed prevention/intervention research is needed to test this conjecture.
The present study was also designed to address questions about the mechanisms through which G×E prevention effects operate. The study demonstrated that the intermediate phenotype of vulnerability cognitions accounted for G×E effects on drug use across late adolescence. Carriers of at least one l allele of DRD4 who lived in high-risk family environments and were assigned randomly to the control condition evinced large increases in vulnerability cognitions. Similar adolescents in the AIM condition, as well as carriers of two s alleles of DRD4 in either the AIM or control condition who lived in high-risk family environments, did not evince increases in vulnerability cognitions or drug use. Although identification of the precise mechanism responsible for these results requires additional research, we can speculate on the ways in which AIM deterred the development of vulnerability cognitions for carriers of the l allele. We believe that living in a stressful, high-risk family environment and carrying an l allele of DRD4 is a toxic combination that increases sensitivity and attention to drug cues (see McGeary, 2009). It is possible that youths in the AIM condition were equipped with protective processes, such as familial and extrafamilial support and self-regulation strategies, that led them to direct less attention to drug-related cues and drug-seeking behavior. A potential application of this hypothesis is to determine whether AIM and other preventive interventions reduce attention biases to drug-related cues, particularly for youths who carry an l allele of DRD4. Presumably, heightened levels of support and diminished levels of stress would reduce sensitivity to drug cues and subsequent cognitions associated with cravings and use. This explanation, although plausible, should be empirically evaluated in future research.
Generalizability of the present research is limited because AIM was designed to meet a need in rural Southern communities for efficacious prevention programming for African American adolescents. The findings’ applicability with ethnically and socioeconomically diverse participants residing in urban and rural locations must be established empirically. Even though the distribution of DRD4 exon III VNTR alleles has been shown not to vary between persons of African and European descent (Chang, Kidd, Livak, Pakstis, & Kidd, 1996; Chen, Burton, Greenberger, & Dmitrieva, 1999), in attempting future replications, researchers should note whether their study populations differ in the distribution of these alleles. Such studies are important to an understanding of the etiology of substance use and abuse. These issues aside, the present study demonstrates the utility of using randomized prevention trials to test differential susceptibility and G×E hypotheses; it also furthers understanding of drug use etiology.
Acknowledgments
This research was supported by Awards Numbers R01 DA019230 and P30 DA027827 from the National Institute on Drug Abuse.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Asghari V, Schoots O, Van Kats S, Ohara K, Jovanovic V, Guan HC, et al. Dopamine D4 receptor repeat: Analysis of different native and mutant forms of the human and rat genes. Molecular Pharmacology. 1994;46:364–373. [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. The hidden efficacy of interventions: Gene × environment experiments from a differential susceptibility perspective. Annual Review of Psychology. doi: 10.1146/annurev-psych-010814-015407. in press. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Archives of General Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Scher SJ. Self-defeating behavior patterns among normal individuals: Review and analysis of common self-destructive tendencies. Psychological Bulletin. 1988;104:3–22. doi: 10.1037/0033-2909.104.1.3. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Lei MK, Philibert RA. Differential susceptibility to parenting among African American youths: Testing the DRD4 hypothesis. Journal of Family Psychology. 2010;24:513–521. doi: 10.1037/a0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Genetic moderation of early child-care effects on social functioning across childhood: A developmental analysis. Child Development. 2013;84:1209–1225. doi: 10.1111/cdev.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright SR. The Georgia county guide. 24. Athens: Center for Agribusiness and Economic Development; 2005. [Google Scholar]
- Bradley SL, Dodelzon K, Sandhu HK, Philibert RA. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136B:58–61. doi: 10.1002/ajmg.b.30185. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SRH, Hill KG, Howe GW, Prado G, Fullerton SM. Using genetically informed, randomized prevention trials to test etiological hypotheses about child and adolescent drug use and psychopathology. American Journal of Public Health. 2013;103(S1):S19–S24. doi: 10.2105/AJPH.2012.301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Beach SRH, Kogan SM, Yu T, DiClemente RJ, et al. Differential sensitivity to prevention programming: A dopaminergic polymorphism-enhanced prevention effect on protective parenting and adolescent substance use. Health Psychology. 2013 Feb 4; doi: 10.1037/a0031253. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Kogan SM. A cascade model connecting life stress to risk behavior among rural African American emerging adults. Development and Psychopathology. 2010;22:667–678. doi: 10.1017/S0954579410000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Kogan SM, Smith K, Brown AC. Buffering effects of a family-based intervention for African American emerging adults. Journal of Marriage and Family. 2010;72:1426–1435. doi: 10.1111/j.1741-3737.2010.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Kogan SM, Yu T, Molgaard VK, DiClemente RJ, et al. Family-centered program to prevent substance use, conduct problems, and depressive symptoms in Black adolescents. Pediatrics. 2012;129:108–115. doi: 10.1542/peds.2011-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y-f, Yu T, Beach SRH, Kogan SM, Simons RL, et al. Life stress, the dopamine receptor gene, and emerging adult drug use trajectories: A longitudinal, multilevel, mediated moderation analysis. Development and Psychopathology. 2012;24:941–951. doi: 10.1017/S0954579412000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Ge X. Linking parenting processes and self-regulation to psychological functioning and alcohol use during early adolescence. Journal of Family Psychology. 2001;15(1):82–94. doi: 10.1037//0893-3200.15.1.82. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, et al. The Strong African American Families program: Translating research into prevention programming. Child Development. 2004;75:900–917. doi: 10.1111/j.1467-8624.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Kim S, Brown AC. Longitudinal pathways to competence and psychological adjustment among African American children living in rural single-parent households. Child Development. 2002;73:1505–1516. doi: 10.1111/1467-8624.00486. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen Y-f, Kogan SM, Smith K. The Adults in the Making program: Long-term protective stabilizing effects on alcohol use and substance use problems for rural African American emerging adults. Journal of Consulting and Clinical Psychology. 2012;80:17–28. doi: 10.1037/a0026592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Chassin LA, Presson CC, Sherman SJ, Corty E, Olshavsky RW. Predicting the onset of cigarette smoking in adolescents: A longitudinal study. Journal of Applied Social Psychology. 1984;14:224–243. [Google Scholar]
- Chassin LA, Tetzloff C, Hershey M. Self-image and social-image factors in adolescent alcohol use. Journal of Studies on Alcohol. 1985;46:39–47. doi: 10.15288/jsa.1985.46.39. [DOI] [PubMed] [Google Scholar]
- Chen C, Burton M, Greenberger E, Dmitrieva J. Population migration and the variation of dopamine D4 receptor (DRD4) allele frequencies around the globe. Evolution and Human Behavior. 1999;20:309–324. [Google Scholar]
- Cleveland MJ, Gibbons FX, Gerrard M, Pomery EA, Brody GH. The impact of parenting on risk cognitions and risk behavior: A study of mediation and moderation in a panel of African American adolescents. Child Development. 2005;76:900–916. doi: 10.1111/j.1467-8624.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, MacKillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: A DRD2 Taq1 A and DRD4 48-bp VNTR association study. Behavioral and Brain Functions. 2007;3:Article 2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Stam CJ, Hendriks VM, van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003;170:205–212. doi: 10.1007/s00213-003-1542-7. [DOI] [PubMed] [Google Scholar]
- Gore SL, Aseltine RH., Jr Racial and ethnic differences in depressed mood following the transition form high school. Journal of Health and Social Behavior. 2003;44:370–390. [PubMed] [Google Scholar]
- Howe GW, Reiss D, Yuh J. Can prevention trials test theories of etiology? Development and Psychopathology. 2002;14:673–694. doi: 10.1017/s0954579402004029. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the Future national survey results on drug use, 1975–1999. Volume I: Secondary school students (NIH Publication No. 00-4802) Bethesda, MD: National Institute on Drug Abuse; 2000. [Google Scholar]
- Kegel CAT, Bus AG, van IJzendoorn MH. Differential susceptibility in early literacy instruction through computer games: The role of the dopamine D4 receptor gene (DRD4) Mind, Brain, and Education. 2011;5:71–78. [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kurdek LA. Conflict resolution styles in gay, lesbian, heterosexual nonparent, and heterosexual parent couples. Journal of Marriage and the Family. 1994;56:705–722. [Google Scholar]
- Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Little TD, Bovaird JA, Card NA, editors. Modeling contextual effects in longitudinal studies. Mahwah, NJ: Erlbaum; 2007. [Google Scholar]
- Matheny AP, Jr, Wachs TD, Ludwig JL, Phillips K. Bringing order out of chaos: Psychometric characteristics of the Confusion, Hubbub, and Order Scale. Journal of Applied Developmental Psychology. 1995;16:429–444. [Google Scholar]
- McGeary J. The DRD4 exon 3 VNTR polymorphism and addiction-related phenotypes: A review. Pharmacology, Biochemistry and Behavior. 2009;93:222–229. doi: 10.1016/j.pbb.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén BO. Second-generation structural equation modeling with a combination of categorical and continuous latent variables: New opportunities for latent class/latent growth modeling. In: Collins LM, Sayer A, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 291–322. [Google Scholar]
- Muthén BO, Muthén LK. Mplus users’ guide (Version 7) Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Muthén LK, Curran PJ. General longitudinal modeling of individual differences in experimental designs: A latent variable framework for analysis and power estimation. Psychological Methods. 1997;2:371–402. [Google Scholar]
- O’Connell ME, Boat T, Warner KE. Preventing mental, emotional, and behavioral disorders among young people: Progress and possibilities. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Pauli-Pott U, Friedl S, Hinney A, Hebebrand J. Serotonin transporter gene polymorphism (5-HTTLPR), environmental conditions, and developing negative emotionality and fear in early childhood. Journal of Neural Transmission. 2009;116:503–512. doi: 10.1007/s00702-008-0171-z. [DOI] [PubMed] [Google Scholar]
- Pomery EA, Gibbons FX, Reis-Bergan M, Gerrard M. From willingness to intention: Experience moderates the shift from reactive to reasoned behavior. Personality and Social Psychology Bulletin. 2009;35:894–908. doi: 10.1177/0146167209335166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O’Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother–adolescent dyads. Journal of Applied Behavior Analysis. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES–D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–336. [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Rutter ML. Environmentally mediated risks for psychopathology: Research strategies and findings. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HHM. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenetics Journal. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Stone AL, Becker LG, Huber AM, Catalano RF. Review of risk and protective factors of substance use and problem use in emerging adulthood. Addictive Behaviors. 2012;37:747–775. doi: 10.1016/j.addbeh.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH. Genetic differential susceptibility experiments. Paper presented at the Jacobs Foundation Conference on Genetic Moderation of Intervention Effects; Marbach Castle, Öhningen, Germany. 2013. Apr, [Google Scholar]
- Warshaw PR, Davis FD. Disentangling behavioral intention and behavioral expectation. Journal of Experimental Social Psychology. 1985;21:213–228. [Google Scholar]