Abstract
Background
Complete axillary lymph node dissection (ALND) after a positive sentinel lymph node biopsy (SLNB) remains the standard practice. As nodal surgery has long been considered a staging procedure without a clear survival benefit, the need for ALND in all patients is debatable. The purpose of this study was to examine differences in survival for patients undergoing SLNB alone versus SLNB with complete ALND.
Methods
Patients with breast cancer who underwent SLNB and were found to have nodal metastases were identified from the Surveillance, Epidemiology, and End Results database (1998–2004). Clinicopathologic and outcomes data were examined for patients who underwent SLNB alone versus SLNB with ALND.
Results
We identified 26,986 patients with disease-positive lymph nodes; 4,425 (16.4%) underwent SLNB alone, and 22,561 (83.6%) underwent SLNB with ALND. Patients were significantly more likely to undergo SLNB alone if they were older (median 59 years old) or if the tumor was low grade and estrogen receptor positive. From 1998 to 2004, the proportion of patients with micrometastasis in the sentinel lymph nodes who underwent SLNB alone increased from 21.0 to 37.8% (P < 0.001). At a median follow-up of 50 months, there were no statistically significant differences in overall survival (OS) between patients who underwent SLNB alone versus complete ALND.
Conclusions
There is an increasing trend toward omitting ALND in patients with micrometastatic nodal disease identified by SLNB. Compared with SLNB alone, completion ALND does not seem to be associated with improved survival for breast cancer patients with micrometastasis in the sentinel lymph nodes.
Sentinel lymph node (SLN) biopsy (SLNB) has become a widely accepted method of nodal staging for patients with clinically lymph node-negative breast cancer.1,2 Current guidelines from the American Society of Clinical Oncology and National Comprehensive Cancer Network recommend complete axillary lymph node dissection (ALND) for patients who have SLN metastases of >0.2 mm identified in a SLN by any method of detection.2,3 Because complete ALND often requires a second surgery and is potentially associated with marked morbidity, some have questioned the need for ALND in all patients with SLN metastases.4–9 Some investigators have suggested that ALND may be beneficial for only selected SLN-positive patients, because in 40–60% of patients, the SLN is the only positive lymph node.10 Others assert that routine use of ALND can improve survival by ensuring regional control.11
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial was a randomized trial designed to compare overall survival (OS) in SLN-positive patients who did and did not undergo complete ALND.12 The Z0011 trial was closed early as a result of slow accrual (total accrual 891 of a planned 1900 patients) and a lower-than-expected event rate in both study groups.13,14 The study investigators recently reported that they found no differences in local or regional recurrence rates between the two study arms at a median follow-up of 5.9 years.15
Without definitive results from the Z0011 trial, there is no level 1 evidence to guide clinicians and patients regarding the importance of ALND in SLN positive patients. The rate of adherence to national guidelines for providing complete ALND for SLN-positive breast cancer is largely unknown. A recent study that used data from the National Cancer Data Base (NCDB) assessed national practice patterns for evaluating nodes and examined the differences in recurrence and survival between patients undergoing SLNB alone and those undergoing SLNB with complete ALND.16 That study reported that compared with SLNB alone, SLNB with complete ALND did not seem to be associated with greatly improved survival for breast cancer patients with micrometastasis or macrometastasis in the SLNs.16
The objectives of our study were to determine how often patients in the United States with SLN-positive breast cancer undergo SLNB alone without complete ALND, to assess the factors associated with undergoing SLNB alone, and to determine whether SLNB alone versus SLNB with complete ALND is associated with differences in the survival of patients with positive SLNs.
PATIENTS AND METHODS
Data Acquisition and Patient Selection
We used the Surveillance Epidemiology, and End Results (SEER) database of the National Cancer Institute to identify breast cancer patients who underwent SLNB and had positive SLNs found between 1998 and 2004. Data were obtained from all 17 U.S. cancer registries participating in the SEER program using SEER*Stat version 6.5.2 (http://seer.cancer.gov/seerstat). The SEER registries routinely collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment, and follow-up vital status. The geographic scope of the current SEER database has been reported previously.17–19
We identified 771,436 women older than 18 years diagnosed with primary breast cancer from January 1, 1998, to November 30, 2004, based on International Classification of Diseases for Oncology and histology codes. Patients were categorized according to their primary surgical procedure (breast-conservation surgery or mastectomy) and according to their axillary lymph node assessment as follows: no nodal evaluation, ALND only, SLNB only (without complete ALND), and SLNB with complete ALND. If a patient underwent SLNB and a modified radical mastectomy, the patient was categorized as having undergone complete ALND. Patients were excluded if they underwent ALND only (without SLNB), did not undergo a lymph node evaluation or if evaluation status was not specified in SEER data, or did not undergo primary surgery. Patients were excluded if they had stage IV disease. Additional exclusions were made for patients with a follow-up time of <24 months. We used the American Joint Committee on Cancer pathologic N category subclassifications to categorize patients according to the degree of nodal metastatic disease as follows: macrometastatic disease (>2.0 mm) and micrometastatic disease (>0.2–2.0 mm).20 Patients reported only as N1 were classified as having macrometastatic nodal disease.
The SEER database does not include information about recurrence. To evaluate ipsilateral breast cancer events and regional events after surgery, we identified patients with two or more registered entries after primary surgery; if the same breast was affected, the cancer was categorized as an ipsilateral breast cancer event, and if nodes were also involved, it was categorized as an ipsilateral regional event. The final sample included 26,986 patients.
Statistical Analysis
We compared the differences in categorical variables and proportions between patients who underwent SLNB alone and SLNB with complete ALND by χ2 testing. Preliminary analyses of the management and outcomes were compared between patients undergoing SLNB alone and those undergoing SLNB with complete ALND in all patients. Similar analyses were also performed in patients who had micrometastasis and patients who had macrometastasis in the SLNs. Survival was calculated as the number of months between the date of diagnosis and the date of death, date last known to be alive, or November 30, 2006, whichever occurred first. The survival end points for the present study were OS and disease-specific survival (DSS). Patients who were lost to follow-up or who survived beyond November 30, 2006, were coded as censored observations.
We performed univariate analyses to determine the influence of patient, tumor, and treatment factors of known or potential prognostic value on OS and DSS determined by the Kaplan-Meier method. Statistical differences between survival curves were assessed by the log-rank test. Variables subjected to univariate analysis included age (median split, ≤55 vs. >55 years), tumor grade (low/intermediate vs. high), tumor size (T1–T3), estrogen receptor (ER) and progesterone receptor (PR) status (positive, negative, unknown), use of radiotherapy after surgery (yes vs. no), and use of ALND.
Significant factors from the univariate analysis were included in a multivariate Cox proportional hazard model to identify significant predictors of OS and DSS. The covariates age and tumor size were analyzed as continuous variables in the multivariate models. Estimated risks of death were calculated as hazard ratios (HRs) with 95% confidence intervals. Stata SE version 9.0 statistical software (StataCorp, College Station, TX) was used for statistical analyses. All tests were two-tailed, and statistical significance was set at P < 0.05.
RESULTS
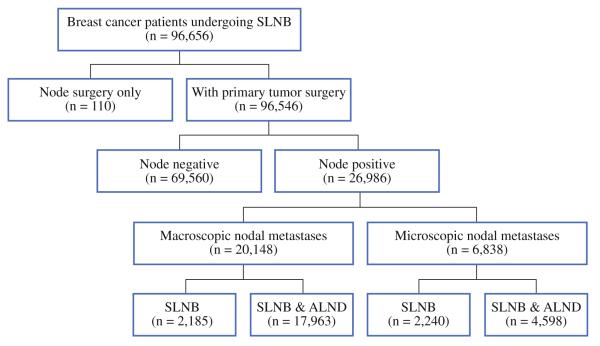
Of the 96,656 women in the SEER database who underwent SLNB as part of their surgical treatment for breast cancer from 1998 to 2004, 26,986 (28.0%) had nodal metastases and comprised the cohort we analyzed. Of the patients in the cohort, 4,425 (16.4%) underwent SLNB alone, and 22,561 (83.6%) underwent SLNB with a complete ALND (Fig. 1). Most patients (n = 20,148) had macrometastasis, and 6,838 patients had micrometastasis. From 1998 to 2004, the proportion of patients who underwent SLNB alone for micrometastases continued to increase (21.1–37.6%; P < 0.001; Fig. 2). However, in patients with macrometastases, the proportion of patients who underwent SLNB alone increased from 1998 to 2002 (5.7–13.1%, P < 0.001) and then decreased from 2002 to 2004 (13.1–10.6%, P < 0.001). A higher proportion of patients underwent SLNB alone if they were diagnosed after 2000 (23.2 vs. 16.8%).
FIG. 1.
Nodal management of breast cancer patients in the Surveillance, Epidemiology, and End Results Program (SEER) database who underwent SLNB and/or complete ALND, during the period 1998–2004
FIG. 2.
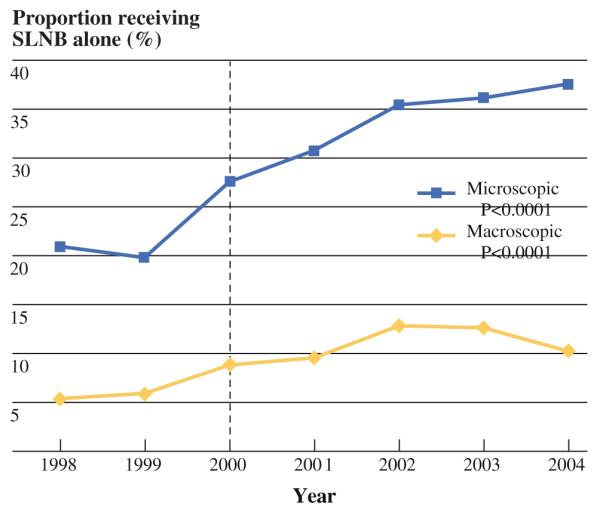
Use over time of SLNB alone for sentinel lymph node-positive breast cancer
Patients who underwent SLNB alone rather than SLNB with complete ALND were more likely to be older (median age, 59 vs. 56 years, respectively), or to have smaller (median tumor size, 16 vs. 20 mm) or low-grade tumors (21% low/intermediate vs. 13.3% high grade) (Table 1). Most patients (78.8%) who underwent SLNB alone had breast-conservation surgery. The median number of lymph nodes removed during surgery was 3 in the patients who underwent SLNB alone and 13 in those who underwent SLNB with complete ALND (P < 0.0001). The mean number of lymph nodes removed was 5 (range 1–44) lymph nodes removed in the SLNB-alone group. This statistically significant range suggests that at least some of these patients were misclassified in the database as having a SLNB only. To address this problem, this study was additionally constrained by giving upper-end and lower-end nodal counts for the SLNB-alone group and the ALND group, respectively. Patients were considered to have undergone a SLNB alone if a SLNB was reported and if they had five or fewer nodes examined. Patients were considered to have undergone a SLNB with completion ALND if a completion ALND was reported and if they had nine or more nodes examined. By using nodal evaluation with lymph node count thresholds, the differences between patients who underwent SLNB alone and patients who underwent SLNB with ALND were similar as reported by nodal evaluation.
TABLE 1.
Comparison of patient and tumor characteristics between patients who underwent SLNB alone and patients who underwent SLNB and ALND in patients with positive SLNs (n = 26,986)
| Characteristic | Reported nodal evaluation |
Nodal evaluation with lymph node count threshold |
||||
|---|---|---|---|---|---|---|
| SLNB (n = 4,425) |
SLNB and ALND (n = 22,561) |
P value | SLNBa (n = 3,240) |
SLNB and ALNDb (n = 16,432) |
P value | |
| Age (years) | <0.0001c | <0.0001c | ||||
| Mean | 60.1 | 56.8 | 60.7 | 56.3 | ||
| Median (range) | 59 (22–96) | 56 (18–99) | 60 (24–96) | 55 (19–99) | ||
| Race, n (%) | 0.5 | 0.3 | ||||
| White | 3,804 (86.0) | 19,277 (85.4) | 2,762 (85.3) | 13,979 (85.1) | ||
| Black | 305 (6.9) | 1,659 (7.4) | 227 (7.0) | 1,253 (7.6) | ||
| Other | 316 (7.1) | 1,625 (7.2) | 251 (7.8) | 1,200 (7.3) | ||
| Year of diagnosis, n (%) | <0.0001 | <0.0001 | ||||
| Before 2000 | 743 (16.8) | 5,224 (23.2) | 483 (14.9) | 3,967 (24.1) | ||
| After 2000 | 3,682 (83.2) | 17,337 (76.8) | 2,757 (85.1) | 12,465 (75.9) | ||
| Surgery type, n (%) | <0.0001 | <0.0001 | ||||
| Segmental mastectomy | 3,488 (78.8) | 12,250 (54.3) | 2,250 (78.7) | 8,860 (53.9) | ||
| Total mastectomy | 937 (21.2) | 10,311 (45.7) | 690 (21.3) | 7,572 (46.1) | ||
| AJCC TNM stage, n (%) | <0.0001 | <0.0001 | ||||
| II | 4,208 (95.1) | 19,878 (88.1) | 3,102 (95.7) | 14,249 (86.7) | ||
| III | 217 (4.9) | 2,683 (11.9) | 138 (4.3) | 2,183 (13.3) | ||
| Tumor size (mm), n (%) | <0.0001c | <0.0001c | ||||
| Mean | 24.6 | 30.7 | 24.3 | 31.1 | ||
| Median (range) | 16 (1–998) | 20 (1–998) | 16 (1–998) | 20 (1–998) | ||
| T stage, n (%) | <0.0001d | <0.0001 | ||||
| T1 | 2,987 (67.5) | 11,764 (52.1) | 2,219 (68.6) | 8,252 (50.4) | ||
| T2 | 1,280 (28.9) | 9,315 (41.3) | 912 (28.2) | 7,046 (43.0) | ||
| T3 | 150 (3.4) | 1,421 (6.3) | 103 (3.2) | 1,088 (6.7) | ||
| Positive lymph node diameter, n (%) | <0.0001 | <0.0001 | ||||
| Micrometastasis (>0.2–2.0 mm) | 2,240 (50.6) | 4,598 (20.4) | 1,767 (54.5) | 2,832 (17.2) | ||
| Macrometastasis (>2.0 mm) | 2,185 (49.4) | 17,963 (79.6) | 1,473 (45.5) | 13,600 (82.8) | ||
| No. of positive LNs removed | <0.0001c | <0.0001c | ||||
| Mean | 1.3 | 2.9 | 1.1 | 3.4 | ||
| Median (range) | 1 (1–24) | 2 (1–54) | 1 (1–5) | 2 (1–54) | ||
| No. of LNs removed | <0.0001c | <0.0001c | ||||
| Mean | 5.1 | 13.3 | 2.3 | 16.3 | ||
| Median (range) | 3 (1–44) | 13 (1–64) | 2 (1–5) | 15 (9–64) | ||
| Grade, n (%) | <0.0001d | <0.0001d | ||||
| Low/intermediate | 934 (21.1) | 3,001 (13.3) | 704 (23.0) | 2,044 (13.1) | ||
| High | 3,246 (73.4) | 18,459 (81.8) | 2,361 (77.0) | 13,601 (86.9) | ||
| Unknown | 245 (5.5) | 1,101 (4.9) | <0.0001d | |||
| Estrogen receptor status, n (%) | <0.0001d | |||||
| Positive | 3,407 (77.0) | 16,682 (73.9) | 2,513 (87.5) | 11,985 (80.8) | ||
| Negative | 527 (11.9) | 3,699 (16.4) | 359 (12.5) | 2,841 (19.2) | ||
| Unknown | 491 (11.1) | 2,180 (9.7) | <0.0001d | |||
| Progesterone receptor status, n (%) | <0.0001d | |||||
| Positive | 2,850 (64.4) | 13,911 (61.7) | 2,098 (75.0) | 10,002 (69.4) | ||
| Negative | 991 (22.4) | 5,936 (26.3) | 700 (25.0) | 4,417 (30.6) | ||
| Unknown | 584 (13.2) | 2,714 (12.0) | ||||
| Radiotherapy, n (%) | <0.0001 | <0.0001 | ||||
| None/before surgery | 1,757 (39.7) | 10,548 (46.8) | 1,299 (40.1) | 7,633 (46.4) | ||
| After surgery | 2,668 (60.3) | 12,013 (53.2) | 1,941 (59.9) | 8,799 (53.6) | ||
SLN sentinel lymph node; SLNB sentinel lymph node biopsy; ALND complete axillary lymph node dissection; AJCC American Joint Committee on Cancer; TNM tumor, node, metastasis system
Threshold of five or fewer nodes
Threshold of nine or more nodes
Wilcoxon rank-sum test
Excluded unknown category
Of the patients with micrometastases, 50.6% underwent SLNB alone, compared with only 20.4% of patients with macrometastases. Patients with micrometastases were far more likely to undergo SLNB alone than SLNB with complete ALND if they were older, had smaller tumors, or were diagnosed after the year 2000. Patients with macrometastases were also more likely to undergo SLNB alone than SLNB with complete ALND if they were older, had smaller or low-grade tumors, or were diagnosed after the year 2000 (Table 2).
TABLE 2.
Factors associated with undergoing SLNB alone according to size of SLN metastasis in women with SLN-positive breast cancer
| Characteristic | Diameter of nodal metastasesa |
|||
|---|---|---|---|---|
| Micrometastasis (n = 6,838) |
Macrometastasis (n = 20,148) |
|||
| SLNB alone (%) |
P value | SLNB alone (%) |
P value | |
| Age (years) | <0.0001 | <0.0001 | ||
| <55 | 28.6 | 9.0 | ||
| ≥55 | 35.9 | 12.4 | ||
| Year of diagnosis | <0.0001 | <0.0001 | ||
| 1998–2000 | 24.9 | 8.0 | ||
| 2001–2004 | 35.1 | 11.9 | ||
| T stage | <0.0001b | <0.0001b | ||
| T1 | 35.2 | 13.4 | ||
| T2 | 28.1 | 8.3 | ||
| T3 | 21.2 | 8.0 | ||
| Grade | <0.0001b | <0.0001b | ||
| Low/intermediate | 36.9 | 17.1 | ||
| High | 31.7 | 9.7 | ||
| Estrogen receptor status | <0.0001b | <0.0001b | ||
| Positive | 33.7 | 11.0 | ||
| Negative | 27.1 | 8.7 | ||
| Progesterone receptor status | 00.01b | 0.016b | ||
| Positive | 33.7 | 11.0 | ||
| Negative | 30.1 | 9.8 | ||
SLN sentinel lymph node; SLNB sentinel lymph node biopsy
Macrometastasis is >2.0 mm; micrometastasis is >0.2–2.0 mm
Excluded unknown category
Table 3 shows a multivariate analysis for factors associated with undergoing SLNB alone. Patients were more likely to undergo SLNB alone compared with SLNB with complete ALND if they were older (≥55), had smaller or low-grade tumors, had micrometastases, were of positive ER status, and were undergoing segmental mastectomy.
TABLE 3.
Multivariate analysis of factors associated with undergoing SLNB alone
| Characteristic | Odds ratio (95% CI) | P value |
|---|---|---|
| Age (years) | ||
| <55 | Reference | |
| ≥55 | 1.4 (1.3–1.5) | <0.0001 |
| Surgery type | ||
| Total mastectomy | Reference | |
| Segmental mastectomy | 2.8 (2.5–3.0) | <0.0001 |
| T stage | ||
| T2/T3 | Reference | |
| T1 | 1.2 (1.1–1.3) | <0.0001 |
| Positive lymph node diameter | ||
| Macrometastasis | ||
| Micrometastasis | 3.8 (3.5–4.1) | <0.0001 |
| Grade | ||
| High | ||
| Low/intermediate | 1.4 (1.3–1.5) | <0.0001 |
| Estrogen receptor status | ||
| Negative | ||
| Positive | 1.2 (1.1–1.3) | 0.001 |
SLNB sentinel lymph node biopsy; 95% CI 95% confidence interval
At a median follow-up time of 50 months, 184 (0.7%) ipsilateral breast cancer events and 25 (0.1%) ipsilateral regional events were reported after surgery. Of the study population, 2293 patients (8.5%) had died, and 1460 patients (5.4%) had died of breast cancer. Overall survival was not greatly different for patients undergoing SLNB alone compared with those undergoing SLNB with complete ALND in the entire cohort and in patients with micrometastases or macrometastases. In patients with micrometastases (n = 6,838), there were no statistically significant differences in the occurrence of ipsilateral regional events between those who underwent SLNB alone and those who underwent complete ALND. Patients with macrometastases (n = 20,148) had a significantly lower risk of developing ipsilateral regional events after complete ALND than after SLNB alone (0.08 vs. 0.2%; HR, 0.30; P = 0.02).
Table 4 shows the clinical and pathological factors affecting OS and DSS. Patients who were older or who had macrometastases, high-grade tumors, larger tumors, negative ER or PR status, or who had more positive lymph nodes found during surgery had reduced OS. Whether patients underwent complete ALND after SLNB did not affect the OS. Patients who were older or who had macrometastases, high-grade tumors, larger tumors, negative ER or PR status, who underwent complete ALND after SLNB, or who had more positive lymph nodes found during surgery had reduced DSS. However, in patients with micrometastases, whether patients underwent complete ALND after SLNB did not affect the breast cancer DSS (HR, 1.2; P = 0.3).
TABLE 4.
Results of Cox proportional hazard analyses of OS and breast cancer-specific survival
| Variable | OS survival | Breast cancer-specific survival in all patients |
Breast cancer-specific survival in patients with micrometastasis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | P | 95% CI | HR | P | 95% CI | HR | P | 95% CI | |
| LN category | |||||||||
| Micrometastasis (>0.2–2.0 mm) | Reference | Reference | |||||||
| Macrometastasis (>2.0 mm) | 1.2 | <0.0001 | 1.1–1.4 | 1.5 | <0.0001 | 1.3–1.8 | |||
| Grade | |||||||||
| Low/intermediate | Reference | Reference | Reference | ||||||
| High | 1.5 | <0.0001 | 1.3–1.8 | 2.9 | <0.0001 | 2.2–3.8 | 2.4 | 0.004 | 1.3–4.4 |
| Axillary LN surgery | |||||||||
| SLNB only | Reference | Reference | Reference | ||||||
| SLNB and ALND | 1.0 | 0.6 | 0.9–1.2 | 1.3 | 0.003 | 1.1–1.6 | 1.2 | 0.3 | 0.9–1.7 |
| Age (years) | 1.04 | <0.0001 | 1.04–1.05 | 1.01 | <0.0001 | 1.01–1.02 | 1.02 | 0.001 | 1.01–1.03 |
| T stage | |||||||||
| T1 | Reference | Reference | Reference | ||||||
| T2 | 1.8 | <0.0001 | 1.6–2.0 | 2.2 | <0.0001 | 1.9–2.5 | 2.4 | <0.0001 | 1.8–3.2 |
| T3 | 2.3 | <0.0001 | 2.0–2.7 | 3.7 | <0.0001 | 3.1–4.4 | 2.6 | 0.005 | 1.3–5.0 |
| Estrogen receptor status | |||||||||
| Positive | Reference | Reference | Reference | ||||||
| Negative | 1.8 | <0.0001 | 1.6–2.1 | 2.0 | <0.0001 | 1.7–2.4 | 2.2 | <0.0001 | 1.4–3.3 |
| Progesterone receptor status | |||||||||
| Positive | Reference | Reference | Reference | ||||||
| Negative | 1.2 | <0.0001 | 1.04–1.3 | 1.4 | <0.0001 | 1.2–1.7 | 1.5 | 0.05 | 1.0–2.2 |
| No. of positive LNs | 1.1 | <0.0001 | 1.04–1.06 | 1.1 | <0.0001 | 1.01–1.02 | 1.2 | <0.0001 | 1.1–1.2 |
SLNB sentinel lymph node biopsy; ALND complete axillary lymph node dissection; LN lymph node; HR hazard ratio; 95% CI 95% confidence interval
DISCUSSION
Similar to the findings from the NCDB reported by Bilimoria et al.16, our study using the SEER database suggests an increasing trend toward omitting ALND when SLNB reveals only micrometastases. The proportion of patients with micrometastases who underwent SLNB alone increased from 21.0 to 37.8% during the study period (P < 0.001). At a median follow-up of 50 months, the use of ALND in SLN-positive patients did not seem to be associated with improved survival outcomes for breast cancer patients with micrometastases in the SLNs.
Bilimoria et al.16 recently reported that the proportion of patients in the NCDB who did not undergo complete ALND declined slightly from 1998 to 2005 in cases of macroscopic SLN disease but increased dramatically in cases of microscopic SLN disease. We found a similar trend in the SEER database in the proportion of patients with microscopic SLN disease who did not undergo complete ALND increased dramatically from 1998 to 2004. This was concurrent with the Z0011 trial, which randomized patients with positive SLNs to ALND versus no further surgery. During the conduct of this trial, physicians may have been comfortable omitting the use of ALND in patients with low-volume disease in the SLNs. In the subset of patients with macroscopic SLN disease, the proportion of those who did not undergo complete ALND increased from 1998 to 2001 and then declined slightly from 2002 to 2004.
Similar to the NCDB study, we also found that patients were more likely to undergo SLNB alone if they were older and had smaller primary tumors.16 The increased likelihood that patients would undergo SLNB alone if they were diagnosed after 2000 may have also been influenced by the availability of various nomograms to predict non-SLN involvement. Another alternative to ALND for axillary treatment of lymph node-positive patients is the use of nodal radiation. In the current study, we noted that patients undergoing breast-conservation therapy were more likely to undergo SLNB alone. This trend may be related to the fact that clinicians have become comfortable recommending radiotherapy instead of ALND. Because patients undergoing breast-conservation therapy would have already been scheduled to receive adjuvant radiation, these physicians might have more readily accepted the use of high tangents or the addition of an axillary field to avoid a second surgical procedure.
The use of SLNB for lymph node staging has spared many breast cancer patients the need for ALND and the resultant morbidity when the SLN has been found to be negative for metastatic disease. Although many physicians still consider ALND to be mandatory for SLN-positive patients, others have questioned whether it is necessary to subject all SLN-positive patients to the short-term and long-term morbidity of the procedure. Many studies have reported that ALND recovered no additional metastases in up to 50% of SLN-positive patients.6,21 The patients without additional nodal metastases are unlikely to receive any therapeutic benefit from ALND. Studies have shown that there is an 11–20% chance of having non-SLN involvement after finding micrometastatic disease in the SLN.6,7,22 We and others have published low locoregional failure rates in selected SLN-positive patients who did not undergo ALND.4,5,7–10,23–25
The benefits of avoiding the morbidity of ALND must be weighed against the risk of harboring axillary metastasis that may potentially seed occult metastatic disease. Clinical context, with consideration of a patient’s expected life span and associated health problems, may affect the definition of “minimal acceptable risk.” From our survival analyses, we can see the DSS was actually worse for patients undergoing SLNB with ALND. That is likely because patients who underwent SLNB with ALND had more advanced disease (larger tumors, macrometastases, higher tumor grade, and negative ER and PR status). When we looked only at patients with micrometastases, the DSS was similar between patients who underwent SLNB alone and patients who underwent SLNB with ALND. A meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group of 78 randomized trials from the pre-SLN era (comprising >42,000 patients) indicates that differences in local control of >10–20% at 5 years’ follow-up translate into statistically significant differences (a 5.0% absolute reduction) in survival at 15 years.26 To reach this level of difference in local recurrence by omitting axillary dissection, the risk of additional axillary nodal involvement would need to exceed 30%, which is unlikely to be realized in patients with micrometastases in the SLNs.27 On the basis of these points and the results from our study, we conclude that axillary dissection seems to be of little benefit to most patients with micrometastases beyond providing additional prognostic information.
Several single-institution reports have described the outcomes of patients with metastases that were identified by SLNB who did not undergo complete ALND.4,5,7–9,23,24 Most of the studies reported that the proportion of patients with axillary recurrence was <2%, and many observed no axillary recurrences in patients who underwent SLNB alone. In the study that used the NCDB, the proportion of patients with axillary recurrence was approximately 1%, with no marked differences in axillary recurrence or survival between patients who underwent SLNB alone and those who underwent SLNB with complete ALND.16 Because the SEER database does not include recurrence information, we looked at ipsilateral regional events after surgery (0.1%) for indirect information about regional control. We found that patients with macrometastases who underwent SLNB with complete ALND had a lower risk of ipsilateral regional events than those who underwent SLNB alone. Although we did not find a statistically significant difference in survival between the two groups, omitting ALND in patients with macrometastases may be associated with a higher regional recurrence rate and thus be undesirable from a clinical standpoint.
There are several obvious limitations to this study. First, as in any other retrospective investigation, it was subjected to selection bias. However, studies have demonstrated that retrospective estimates of treatment effects in observational studies often are not qualitatively different than those obtained from randomized trials.1,28 Second, analyses are limited by the data collected by cancer registries. The SEER database does not contain data regarding recurrence, the method used for pathologically evaluating lymph node specimens, the inclusion or exclusion of the axilla in the radiation field, or the administration of neoadjuvant or adjuvant systemic therapies. The use of ipsilateral regional events after surgery as one of our outcomes, instead of axillary recurrence, almost certainly implies that the rate of recurrence was underestimated because ipsilateral regional events represent only a proportion of axillary recurrence.
In conclusion, although the use of complete ALND in SLN-positive breast cancer patients decreased between 1998 and 2004, it does not seem that the departure from the more radical surgery has compromised outcomes.16,29 Nevertheless, most U.S. women with SLN-positive breast cancer do undergo complete ALND.16 Patients with small primary tumors, positive ER status, and micrometastases in the SLN, and who underwent segmental mastectomy were more likely to undergo SLNB alone. This study offers evidence that survival is comparable for those who undergo SLNB alone and those who undergo SLNB with complete ALND for patients with micrometastases.
Footnotes
DISCLOSURE The authors have no commercial interest in the subject of study and the source of financial or material support.
REFERENCES
- 1.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. Am J Ophthalmol. 2000;130:688. doi: 10.1016/s0002-9394(00)00754-6. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: breast [Accessed on 1 Mar 2010];2008 http://www.nccn.org/professionals/physiciangls/PDF/breast.pdf.
- 4.Guenther JM, Hansen NM, DiFronzo LA, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003;138:52–6. doi: 10.1001/archsurg.138.1.52. [DOI] [PubMed] [Google Scholar]
- 5.Jeruss JS, Winchester DJ, Sener SF, et al. Axillary recurrence after sentinel node biopsy. Ann Surg Oncol. 2005;12:34–40. doi: 10.1007/s10434-004-1164-2. [DOI] [PubMed] [Google Scholar]
- 6.Chu KU, Turner RR, Hansen NM, et al. Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536–41. doi: 10.1097/00000658-199904000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007;110:723–30. doi: 10.1002/cncr.22847. [DOI] [PubMed] [Google Scholar]
- 8.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Fant JS, Grant MD, Knox SM, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003;10:126–30. doi: 10.1245/aso.2003.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Grube BJ, Giuliano AE. Observation of the breast cancer patient with a tumor-positive sentinel node: implications of the ACOSOG Z0011 trial. Semin Surg Oncol. 2001;20:230–7. doi: 10.1002/ssu.1038. [DOI] [PubMed] [Google Scholar]
- 11.Moore MP, Kinne DW. Axillary lymphadenectomy: a diagnostic and therapeutic procedure. J Surg Oncol. 1997;66:2–6. doi: 10.1002/(sici)1096-9098(199709)66:1<2::aid-jso2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed on 1 Mar 2010];ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1 or T2 N0 M0 breast cancer who have a positive sentinel node. https://www.acosog.org/studies/synopses/Z0011_Synopsis.pdf.
- 13.ACOSOGZ0011 suspension of registration [Accessed on 1 Mar 2010]; https://www.acosog.org/announcements/announcement_display.jsp?id=c0875d5e-062a-4901-ba65-926a7637c451.
- 14.Leitch AM, McCall L, Beitsch P. Factors influencing accrual to ACOSOG Z0011, a randomized phase III trial of axillary dissection vs observation for sentinel node positive breast cancer (abstract 601) J Clin Oncol. 2006;24(Suppl):28s. [Google Scholar]
- 15.Giuliano AE, McCall L, Beitsch P, et al. Local and regional control in breast cancer after sentinel node biopsy without axillary lymph node dissection: results from a randomized trial. Paper presented at: American Surgical Association 130th annual meeting; 2010; http://www.americansurgical.info/abstracts/2010/1.cgi. [Google Scholar]
- 16.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–53. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 17.Leggett MD, Chen SL, Schneider PD, Martinez SR. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2493–9. doi: 10.1245/s10434-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 18.Martinez SR, Robbins AS, Meyers FJ, et al. Racial and ethnic differences in treatment and survival among adults with primary extremity soft-tissue sarcoma. Cancer. 2008;112:1162–8. doi: 10.1002/cncr.23261. [DOI] [PubMed] [Google Scholar]
- 19.Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg. 2008;196:495–9. doi: 10.1016/j.amjsurg.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 6th ed Springer; New York: 2009. pp. 223–40. [Google Scholar]
- 21.McMasters KM, Giuliano AE, Ross MI, et al. Sentinel-lymph-node biopsy for breast cancer—not yet the standard of care. N Engl J Med. 1998;339:990–5. doi: 10.1056/NEJM199810013391410. [DOI] [PubMed] [Google Scholar]
- 22.Cserni G, Gregori D, Merletti F, et al. Meta-analysis of non-sentinel node metastases associated with micrometastatic sentinel nodes in breast cancer. Br J Surg. 2004;91:1245–52. doi: 10.1002/bjs.4725. [DOI] [PubMed] [Google Scholar]
- 23.Katz A, Smith BL, Golshan M, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008;26:2093–8. doi: 10.1200/JCO.2007.11.9479. [DOI] [PubMed] [Google Scholar]
- 24.Naik AM, Fey J, Gemignani M, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462–8. doi: 10.1097/01.sla.0000137130.23530.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straver ME, Meijnen P, van Tienhoven G, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28:731–7. doi: 10.1200/JCO.2008.21.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 27.Morrow M. Patterns of care with a positive sentinel node: echoes of an opportunity missed. Ann Surg Oncol. 2009;16:2429–30. doi: 10.1245/s10434-009-0552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–92. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winchester DP, Trabanino L, Lopez MJ. The evolution of surgery for breast cancer. Surg Oncol Clin N Am. 2005;14:479–98. vi. doi: 10.1016/j.soc.2005.04.006. [DOI] [PubMed] [Google Scholar]