Abstract
Background
Hernia formation is common following abdominal operations and transplant patients are at increased risk due to their need for postoperative immunosuppression. The purpose of this study is to estimate the incidence of incisional hernia formation following primary abdominal solid organ transplantation and identify clinical risk factors for hernia formation.
Methods
We performed a single-institution retrospective review of a prospectively collected database to evaluate all patients who underwent primary liver, kidney, or pancreas transplantation between 2000 and 2011. The primary outcome measure was hernia formation at the transplant incision. Univariate and multivariate Cox proportional hazards models were used to identify risk factors for incisional hernia formation.
Results
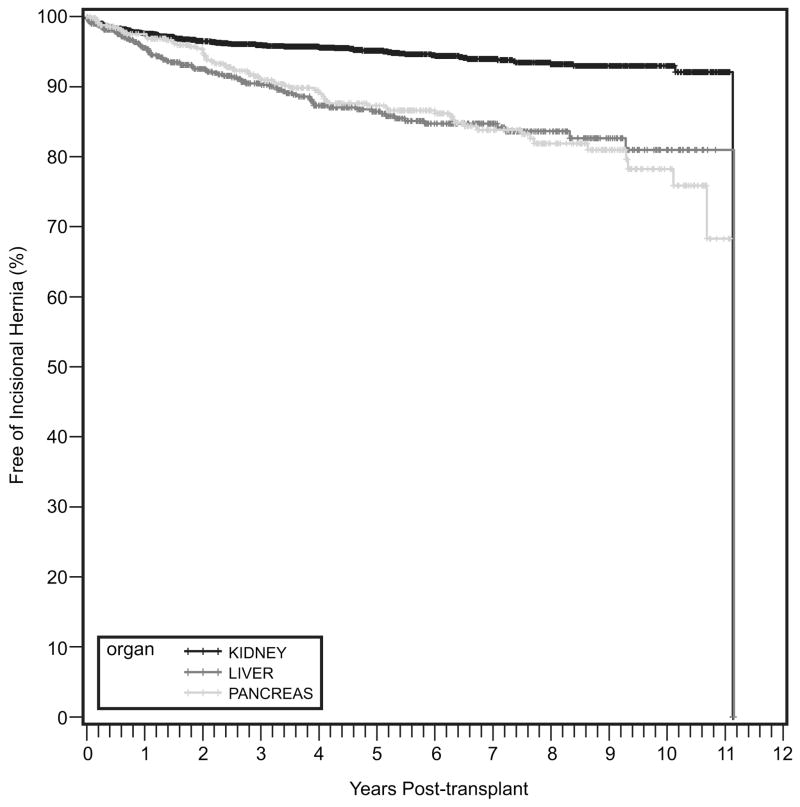
3460 transplants were performed during the study period: 2247 kidney only, 718 liver only, and 495 pancreas or simultaneous pancreas and kidney (pancreas group). The overall incisional hernia rate was 7.5%. The Kaplan-Meier rates of hernia formation at 1, 5, and 10 years were 2.5%, 4.9%, and 7.0% for kidney; 4.5%, 13.6%, and 19.0% for liver; and 2.5%, 12.7%, and 21.8% for the pancreas groups. On univariate analysis, surgical site infection (SSI), body mass index (BMI) >25, delayed graft function (DGF), and absence of a calcineurin inhibitor or mycophenolic acid (MMF) were associated with hernia formation in the kidney group. SSI and BMI>25 were associated with hernia formation in the liver group. In the pancreas group, SSI, the use of cyclosporine, and lack of MMF were all associated with hernia formation. On multivariate analysis, SSI was strongly associated with hernia formation in all groups (Hazard Ratio (HR): Kidney = 24.71, p<0.001; Liver = 12.0, p<0.001, Pancreas = 12.95, p=0.001).
Conclusion
Incisional hernias are common following abdominal organ transplant with nearly one in five patients developing an incisional hernia five years after liver of pancreas transplantation. Strategies focusing on prevention and early treatment of SSI may help to decrease the risk of incisional hernia formation following abdominal organ transplantation.
Keywords: Incisional Hernia, Abdominal Transplant, Risk Factors
Introduction
Incisional hernia formation is a major cause of postoperative morbidity following open abdominal operations. The Centers for Disease Control estimates that roughly 4–5 million laparotomies are performed annually in the US [1]. Rates of incisional hernia formation range from 2 to 20% following laparotomy [2, 3]. There are approximately 350,000 ventral hernia repairs done in the US each year and as many as 200,000 are associated with a prior incision [3–6]. Hernias following abdominal organ transplantation are of particular concern as patients are placed on immunosuppressive medications postoperatively, which may increase the risk of incisional hernia formation due to an associated impairment in the wound healing process.
More than 22,000 solid organ transplants were performed in the United States in 2013, including 15,416 kidney transplants, 5,913 liver transplants and 953 pancreas and simultaneous pancreas and kidney transplants [7]. Previous studies have examined the incidence and risk factors for incisional hernia formation after abdominal organ transplantation. Estimates of hernia formation after kidney transplantation range from 1.6 – 18% [8–10]. Hernia formation after liver and pancreas transplantation tends to be more common, with estimates ranging from 1.7–32.4% [11–17] and 13–34.8% [18, 19], respectively.
While previous studies have examined incisional hernia formation after specific transplants, such as kidney transplant, no study to our knowledge has analyzed potential risk factors for kidney, liver, and pancreas transplantation. The goal of this study is to estimate the incidence of and risk factors for incisional hernia formation following primary abdominal solid organ transplantation.
Materials and Methods
Data Source
The Division of Transplant Surgery at the University of Wisconsin maintains a longitudinal database of all transplant recipients. We performed a single institution retrospective review of this prospectively collected database to evaluate all patients who underwent primary liver, kidney, or pancreas transplantation between 2000 and 2011.
Study Population
All patients undergoing abdominal solid organ transplantation during the study period were included in the analysis. Patients who developed an incisional hernia at their transplant incision were identified using coding data. Incisional hernias at any site other than the transplant incision, port-site hernias, stoma hernias, internal hernias, and umbilical hernias were excluded. The electronic medical record for all patients with identified hernias was reviewed to identify the location of the incision during surgery and to ensure the site of hernia corresponded with the incision used for transplantation.
Data Collection
Baseline demographics including age, sex, ethnicity, and body mass index (BMI) were included for all patients. We also collected data on the specific immunosuppressive medications used for induction and maintenance, presence of diabetes, presence of wound infection, primary and secondary cause for transplant organ failure, model end-organ liver disease (MELD) score for liver recipients, and development of delayed graft function for kidney recipients, which was defined as the need for dialysis within the first seven days after transplant. The primary outcome was formation of incisional hernia at the transplant site.
Statistical Analysis
Incisional hernia and incisional hernia repair rates were estimated utilizing the methods of Kaplan and Meier. Potential risk factors for 1) the formation of incisional hernia and 2) those requiring incisional hernia repair were evaluated with Cox proportional hazards models. An indicator variable representing post-transplant wound infection was evaluated as a time-varying covariate. P-values less than 0.05 were considered as significant. Variables deemed to be significant in univariate models were included in a multivariable model. All analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 3,460 transplants were performed in the study period. Twenty-two hundred, forty-seven patients underwent kidney transplant, 718 received liver transplants, and 495 underwent either pancreas or simultaneous pancreas and kidney (SPK) transplantation (pancreas group). Median follow-up was 4.6, 4.2 and 5.6 years in the kidney, liver, and pancreas groups, respectively. Baseline demographics and immunosuppression regimen are shown in Table 1. The rate of incisional hernia formation for the entire cohort was 7.5% (n=258). The Kaplan-Meier rates of hernia formation at 1, 5 and 10 years are displayed in Figure 1 and were 2.5%, 4.9%, and 7.0% for kidney; 4.5%, 13.6%, and 19.0% for liver; and 2.5%, 12.7%, and 21.8% for the pancreas group. Estimates for repair of incisional hernia were slightly less with rates at 1, 5, and 10 years of 2.1%, 4.2% and 6.1% for kidney; 3.4%, 12.1% and 16.2% for liver; and 2.1%, 10.6% and 19.4% for pancreas groups.
Table 1.
Baseline patient characteristics
| All (n = 3,460) | Kidney (n = 2,247) | Liver (n = 718) | Pancreas/SPK (n = 495) | |
|---|---|---|---|---|
| Age (y) | 49.9 +/− 12.4 | 50.9 +/− 13.0 | 53.6 +/− 9.7 | 40.1 +/− 7.6 |
| Body Mass Index (kg/m2) | 27.6 +/− 5.5 | 27.8 +/− 5.3 | 28.9 +/− 6.3 | 24.9 +/− 3.8 |
| Female (%) | 39.1 | 39.5 | 36.5 | 40.9 |
| African American (%) | 7.2 | 9.5 | 2.1 | 4.2 |
| Diabetes (%) | 31.7 | 26.8 | 0 | 99.8 |
| Cyclosporine (%) | 28.3 | 40.1 | 8.1 | 3.8 |
| Tacrolimus (%) | 61.3 | 45.4 | 89.1 | 92.9 |
| Azathiopurine (%) | 0.4 | 0.4 | 0.6 | 0.2 |
| Mycophenolate mofetil (%) | 89.5 | 94.9 | 67.7 | 96.4 |
| Delayed Graft Function (%) | NA | 17.5 | NA | NA |
| MELDa | NA | NA | 20.46 +/− 9.08 | NA |
| Simultaneous Pancreas and Kidney (%) | NA | NA | NA | 90.5 |
MELD - Model End Organ Liver Disease,
NA=Not Applicable)
Figure 1.
The results of our univariate analysis are summarized in Table 2. Surgical site infection (SSI) was found to be a strong predictor of hernia formation in all three groups. Hazard ratios (HR) were 28.8 for kidney (p<0.001), 13.2 for liver (p<0.001), and 13.0 for the pancreas group (p<0.001). BMI greater than 25 kg/m2 was associated with hernia formation in both the kidney and liver groups with HR of 1.8 (p=0.015) and 1.9 (p=0.044), respectively. This was not the case in the pancreas group, which had a lower average BMI (HR=1.1, p=0.81). BMI less than 18.5 was not predictive of hernia formation. DGF was a significant risk factor in the kidney group, (HR=1.9, p=0.003). Failure to initiate treatment with a calcineurin inhibitor during the initial hospitalization was a significant risk factor for hernia formation in the kidney group (HR=2.3, p=0.002) and the pancreas group (HR=3.6, p<0.001). Additionally, failure to initiate MMF during initial hospital stay was also a risk factor in both of these groups (HR=2.5, p=0.001 and 3.1, p=0.004, respectively). Age, race, and gender were not statistically significant risk factors for hernia formation in any of the groups. MELD score was not associated with an increased hernia risk in the liver group. Neither the presence of diabetes nor the choice of agent used for induction immunosuppression therapy was predictive of hernia formation for any group.
Table 2.
Univariate analysis of risk factors associated with hernia formation
| Kidney (n=2,247) | Liver (n=718) | Pancreas (n=495) | ||||
|---|---|---|---|---|---|---|
| HR | P value | HR | P value | HR | P value | |
| Age 18–34 | 0.7 | 0.32 | 0.4 | 0.24 | 0.9 | 0.682 |
| Age >65 | 1.0 | 0.91 | 0.8 | 0.64 | NA | NA |
| Male | 1.0 | 0.10 | 1.5 | 0.10 | 1.0 | 0.90 |
| African American | 0.5 | 0.13 | 0.5 | 0.50 | 0.3 | 0.28 |
| MELD | NA | NA | 1.0 | 0.693 | NA | NA |
| Diabetes | 0.9 | 0.58 | NA | NA | NA | NA |
| Body Mass Index <18.5 | 1.3 | 0.76 | 1.2 | 0.781 | NA | 0.98 |
| Body Mass Index >25 | 1.8 | 0.02 | 1.9 | 0.04 | 1.1 | 0.81 |
| Surgical Site Infection | 28.8 | <0.001 | 13.2 | <0.001 | 13.0 | <0.001 |
| Delayed Graft Function | 1.9 | 0.003 | NA | NA | NA | NA |
| Cyclosporine | 1.4 | 0.15 | 0.8 | 0.63 | 1.8 | 0.28 |
| No Calcineurin Inhibitor | 2.3 | 0.002 | 1.1 | 0.91 | 3.6 | <0.001 |
| No Mycophenolate mofetil | 2.5 | 0.001 | 0.9 | 0.53 | 3.1 | 0.004 |
significant values in bold
NA = Not applicable.
On multivariate analysis, SSI was an independent risk factor for incisional hernia formation in all groups (Table 3). Failure to initiate MMF therapy during the initial hospitalization was predictive of hernia formation in the kidney group (HR=2.9, p<0.001) as was initiation of cyclosporine in the pancreas group (HR=3.7, p<0.001).
Table 3.
Multivariate analysis of risk factors associated with hernia formation
| Kidney (n=2,247) | Liver (n=718) | Pancreas (n=495) | ||||
|---|---|---|---|---|---|---|
| HR | P value | HR | P value | HR | P value | |
| Body Mass Index <18.5 | 1.3 | 0.70 | 1.3 | 0.77 | - | - |
| Body Mass Index>25 | 1.5 | 0.08 | 1.6 | 0.15 | - | - |
| Surgical Site Infection | 24.7 | <0.001 | 12.0 | <0.001 | 13.0 | 0.001 |
| Delayed Graft Function | 1.6 | 0.70 | - | - | - | - |
| No Calcineurin Inhibitor | 1.4 | 0.23 | - | - | 1.3 | 0.62 |
| Cyclosporine | 1.4 | 0.18 | - | - | 3.7 | <0.001 |
| No Mycophenolate mofetil | 2.9 | <0.001 | - | - | 1.6 | 0.45 |
significant values in bold.
NA = Not Applicable
Discussion
In this study we report the incidence and risk factors for incisional hernia formation after abdominal solid organ transplant. The Kaplan-Meier estimates indicate that hernias continue to form as far out as 10 years after surgery with one in five patients forming an incisional hernia after liver or pancreas transplantation. Not surprisingly, we found that the presence of surgical site infection is a significant risk factor for hernia formation. This is of particular importance in this patient population as transplant patients are at increased risk for this complication given their baseline organ dysfunction and need for immunosuppression postoperatively. Initial choice of immunosuppression agent also seems to have an effect on postoperative hernia formation. The lack of MMF or calcineurin inhibitors in the initial immunosuppressive regimen was associated with increased hernia risk.
Incisional hernias are a major source of postoperative morbidity as most require surgical repair at some point. The rate of hernia formation following solid organ transplantation has been previously studied. Estimates of hernia formation after kidney transplantation range from 1.6 – 18% [8–10]. Humar and colleagues found 73 hernias in 2013 patients undergoing renal transplantation for a rate of 3.6% [20]. They found that the use of MMF and obesity were both significant risk factors for hernia formation. Nanni and colleagues compared incision types in renal transplant and showed a reduction in hernia formation by switching to an oblique incision from a J-shaped flank incision [21]. Mahdavi found BMI, age and female gender to be risk factors in a study of 589 kidney recipients with 16 incisional hernias (3%) [22]. This is similar to rates reported by Mazzucchi (3.8%) [8]. Several other studies report rates as low at 1.6% [9, 23]. One author reported incisional hernia rates of 18% with the use of Sirolimus [10].
Hernia formation after liver transplantation tends to be higher, with estimates ranging from 1.7 – 32.4% [11–17]. A German study in 2002 reported a 17% rate of incisional hernia formation in 290 liver transplants over 10 years. They identified acute rejection (treated with steroids), postoperative thrombocytopenia, and Mercedes type incision as risk factors [14]. Other studies have implicated steroids as a risk factor for hernia formation as well [24]. The use of the Mercedes type incision was also associated with hernia in Piazzese’s study, which reported a low rate of incisional hernia at 4.9% [11]. A separate study of almost 1000 patients reported incisional hernia rates below 5% with exclusive use of the Mercedes incision [12]. In a letter to the editor, Gastaca reported a low rate of hernia formation (1.7%) in over 600 patients with the use of a bilateral subcostal incision without midline extension [17]. Montalti reported a rate of 32.4% and identified the mTOR inhibitor rapamycin as a risk factor along with male sex, higher BMI, and MELD > 22 [15]. Fikatas and colleagues found the use of MMF to be a risk factor for hernia formation in over 800 patients undergoing liver transplant [25]. A study by Kahn also shows MMF to be a risk factor [13]. They reported a higher hernia rate at over 23% and included Sirolimus as part of the immunosuppression regimen in almost 20% of those with hernias. This study also implicated end stage liver cirrhosis when compared with hepatocellular carcinoma.
Rates of incisional hernias after pancreas transplantation are also high, with estimates ranging from 13 to 34.8% [18, 19]. Hanish specifically looked at BMI as a risk factor [19]. Although BMI may prove to be a significant risk factor, our study would suggest that only a small percentage of pancreas transplant patients may fall in the obese or even overweight classification. The only other report of incisional hernia rates following pancreas transplant was a small series of 23 patients with a hernia rate of 34.8% [18].
We found rates of hernia formation at 10 years in the kidney, liver, and pancreas group of 7.04%, 19.04%, and 21.76%, respectively. These rates fall within estimates from previous studies [8–25]. Our data further corroborates pervious findings that patients may continue to form hernias many years after the index operation [26, 27]. This supports the belief that long-term follow up is needed for true estimates of hernia formation. Similar to other previous studies [32–36] we also found that SSI was the strongest risk factor for hernia formation. This is likely related to bacterial proliferation evoking an immune response and altering normal collagen synthesis and wound healing. With underlying organ dysfunction and immunosuppressive medications, transplant patients are at increased risk for SSI [28] and, subsequently, incisional hernias. Thus efforts to 1) recognize and aggressively treat SSI and 2) reduce infections would likely have secondary benefits of reducing the risk of hernia formation.
As suggested in many studies cited above, our study corroborates the findings that obesity is also a risk factor for hernia formation. Our univariate analysis demonstrated that obesity was a significant risk factor for hernia formation in both the kidney and liver groups, but on multivariate analysis obesity remained a significant risk factor only for patients undergoing liver transplantation. While the relationship between obesity and hernia formation may be related to increased intraabdominal pressure placing mechanical stress on the incision, obese patients may also be at increased risk of SSI, which might explain the reduced association in our multivariate analysis. We did not find an association in the pancreas group, likely due to a lower mean BMI in this group.
Our findings also indicate that the immunosuppression regimen may be an important and modifiable risk factor for hernia formation. Several previous studies have found variable results but seem to implicate high dose steroids and Sirolimus with higher rates of hernia formation [10, 13, 14, 24]. One study from 2002 compared MMF and Sirolimus directly and found significantly more wound complications with Sirolimus [29], a finding supported by a similar study in heart transplant patients [30]. We were surprised to find that failure to initiate therapy with MMF or calcineurin inhibitors was associated with a higher risk of hernia formation, as other studies have found MMF to be a risk factor for hernia formation [13, 25]. The decision to withhold calcineurin inhibitors and MMF during the initial hospitalization involves many potential clinical factors that likely include DGF of the transplanted kidney, presence of infections, and severe side effects of the medications. Thus it is not clear whether the increased risk associated with their non-use is directly due to the medication or some other confounding variable that was not included in our dataset. We suspect that the non-use of MMF and calcineurin inhibitors is likely a surrogate for other clinical factors not studied in our analysis. Further research in this area is clearly needed.
While our study is one of the largest to examine hernia formation in a diverse population of abdominal organ transplant patients, there are several limitations. First, all data was from a single institution and may not be broadly generalizable. Additionally, while our database is maintained by trained abstracters it is possible that there were coding errors in some of the variables. For example, our dataset did not include laboratory values for immunosuppressive regimens and thus adherence to immunosuppressive regimens could not be measured. Lastly, the diagnosis of incisional hernia was largely made clinically and not with routine radiographic studies. Thus, the overall rates of hernia formation may be underestimated as some subclinical hernias may have been missed.
In conclusion, we found that BMI and SSI are significant risk factors for incisional hernia formation following abdominal solid organ transplantation. Efforts to reduce SSI may provide the most significant reduction in hernia formation in this patient population. Additionally, the choice of the initial immunosuppressant agent may also play a role in hernia formation but further research is needed to definitively determine which agent may be the most appropriate.
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427.
Footnotes
Disclosures:
Carter T Smith, MD
Micah G Katz
David Foley, MD
Bridget Welch
Glen E Leverson, PhD
Luke Funk, MD, MPH = One-time honorarium as a consultant for Bard-Davol
Jacob A Greenberg, MD, EdM = Consultant for Bard-Davol and Covidien
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
References
- 1.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. National Center for Health Statistics; 1998. [PubMed] [Google Scholar]
- 2.Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 2010;251:843–856. doi: 10.1097/SLA.0b013e3181d973e4. [DOI] [PubMed] [Google Scholar]
- 3.Burger JWA, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. doi: 10.1097/01.sla.0000141193.08524.e7. discussion 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis RT, Wiegand FM. Natural history of vertical abdominal parietal closure: Prolene versus Dexon. Can J Surg J Can Chir. 1989;32:196–200. [PubMed] [Google Scholar]
- 5.Sugerman HJ, Kellum JM, Jr, Reines HD, DeMaria EJ, Newsome HH, Lowry JW. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171:80–84. doi: 10.1016/S0002-9610(99)80078-6. [DOI] [PubMed] [Google Scholar]
- 6.Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia J Hernias Abdom Wall Surg. 2012;16:179–183. doi: 10.1007/s10029-011-0879-9. [DOI] [PubMed] [Google Scholar]
- 7.Based on OPTN data as of February 14, 2014 OPTN. Organ Procurement and Transplantation Network; [Accessed 23 Feb 2014]. http://optn.transplant.hrsa.gov/latestData/rptData.asp. [Google Scholar]
- 8.Mazzucchi E, Nahas WC, Antonopoulos I, Ianhez LE, Arap S. Incisional hernia and its repair with polypropylene mesh in renal transplant recipients. J Urol. 2001;166:816–819. [PubMed] [Google Scholar]
- 9.Varga M, Matia I, Kucera M, Oliverius M, Adamec M. Polypropylene mesh repair of incisional hernia after kidney transplantation: single-center experience and review of the literature. Ann Transplant Q Pol Transplant Soc. 2011;16:121–125. doi: 10.12659/aot.882004. [DOI] [PubMed] [Google Scholar]
- 10.Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, Welsh M, Kerman RH, Podder H, Buren CTV, Katz SM, Kahan BD. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007;21:460–465. doi: 10.1111/j.1399-0012.2007.00668.x. [DOI] [PubMed] [Google Scholar]
- 11.Piazzese E, Montalti R, Beltempo P, Bertelli R, Puviani L, Pacilè V, Nardo B, Cavallari A. Incidence, predisposing factors, and results of surgical treatment of incisional hernia after orthotopic liver transplantation. Transplant Proc. 2004;36:3097–3098. doi: 10.1016/j.transproceed.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Vardanian AJ, Farmer DG, Ghobrial RM, Busuttil RW, Hiatt JR. Incisional hernia after liver transplantation. J Am Coll Surg. 2006;203:421–425. doi: 10.1016/j.jamcollsurg.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Kahn J, Müller H, Iberer F, Kniepeiss D, Duller D, Rehak P, Tscheliessnigg K. Incisional hernia following liver transplantation: incidence and predisposing factors. Clin Transplant. 2007;21:423–426. doi: 10.1111/j.1399-0012.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- 14.Janßen H, Lange R, Erhard J, Malagó M, Eigler FW, Broelsch CE. Causative factors, surgical treatment and outcome of incisional hernia after liver transplantation. Br J Surg. 2002;89:1049–1054. doi: 10.1046/j.1365-2168.2002.02165.x. [DOI] [PubMed] [Google Scholar]
- 15.Montalti R, Mimmo A, Rompianesi G, Serra V, Cautero N, Ballarin R, De Ruvo N, Cunningham Gerring R, Enrico Gerunda G, Di Benedetto F. Early use of mammalian target of rapamycin inhibitors is an independent risk factor for incisional hernia development after liver transplantation. Liver Transpl. 2012;18:188–194. doi: 10.1002/lt.22445. [DOI] [PubMed] [Google Scholar]
- 16.Shi LW, Verran D, Rao ARN, Stewart GJ, McCaughan GW. Incisional hernia following orthotopic liver transplantation. Transplant Proc. 2003;35:425–426. doi: 10.1016/S0041-1345(02)03930-1. [DOI] [PubMed] [Google Scholar]
- 17.Gastaca M, Valdivieso A, Ruiz P, de Urbina JO. Reducing the incidence of incisional hernia after liver transplantation. Transpl Int Off J Eur Soc Organ Transplant. 2010;23:559–560. doi: 10.1111/j.1432-2277.2009.00992.x. [DOI] [PubMed] [Google Scholar]
- 18.Piros L, Máthé Z, Földes K, Langer RM. Incisional hernia after simultaneous pancreas kidney tranplantation: a single-center experience from Budapest. Transplant Proc. 2011;43:1303–1305. doi: 10.1016/j.transproceed.2011.03.090. [DOI] [PubMed] [Google Scholar]
- 19.Hanish SI, Petersen RP, Collins BH, Tuttle-Newhall J, Marroquin CE, Kuo PC, Butterly DW, Smith SR, Desai DM. Obesity Predicts Increased Overall Complications Following Pancreas Transplantation. Transplant Proc. 2005;37:3564–3566. doi: 10.1016/j.transproceed.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Humar A, Ramcharan T, Denny R, Gillingham KJ, Payne WD, Matas AJ. Are wound complications after a kidney transplant more common with modern immunosuppression? Transplantation. 2001;72:1920–1923. doi: 10.1097/00007890-200112270-00009. [DOI] [PubMed] [Google Scholar]
- 21.Nanni G, Tondolo V, Citterio F, Romagnoli J, Borgetti M, Boldrini G, Castagneto M. Comparison of Oblique Versus Hockey-Stick Surgical Incision for Kidney Transplantation. Transplant Proc. 2005;37:2479–2481. doi: 10.1016/j.transproceed.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 22.Mahdavi R, Mehrabi M. Incisional hernia after renal transplantation and its repair with propylene mesh. Urol J. 2004;1:259–262. [PubMed] [Google Scholar]
- 23.Li EN, Silverman RP, Goldberg NH. Incisional hernia repair in renal transplantation patients. Hernia J Hernias Abdom Wall Surg. 2005;9:231–237. doi: 10.1007/s10029-005-0325-y. [DOI] [PubMed] [Google Scholar]
- 24.Gómez R, Hidalgo M, Marques E, Marin L, Loinaz C, Gonzalez I, Garcia I, Moreno E. Incidence and predisposing factors for incisional hernia in patients with liver transplantation. Hernia J Hernias Abdom Wall Surg. 2001;5:172–176. doi: 10.1007/s10029-001-0032-2. [DOI] [PubMed] [Google Scholar]
- 25.Fikatas P, Schoening W, Lee J-E, Chopra SS, Seehofer D, Guckelberger O, Puhl G, Neuhaus P, Schmidt SC. Incidence, risk factors and management of incisional hernia in a high volume liver transplant center. Ann Transplant Q Pol Transplant Soc. 2013;18:223–230. doi: 10.12659/AOT.883914. [DOI] [PubMed] [Google Scholar]
- 26.Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A, Werner J, Büchler MW, Diener MK. Incisional hernia rate 3 years after midline laparotomy. Br J Surg. 2014;101:51–54. doi: 10.1002/bjs.9364. [DOI] [PubMed] [Google Scholar]
- 27.Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72:70–71. doi: 10.1002/bjs.1800720127. [DOI] [PubMed] [Google Scholar]
- 28.Weiss CA, III, Statz CL, Dahms RA, Remucal MJ, Dunn DL, Beilman GJ. Six years of surgical wound infection surveillance at a tertiary care center: Review of the microbiologic and epidemiological aspects of 20,007 wounds. Arch Surg. 1999;134:1041–1048. doi: 10.1001/archsurg.134.10.1041. [DOI] [PubMed] [Google Scholar]
- 29.Valente JF, Hricik D, Weigel K, Seaman D, Knauss T, Siegel CT, Bodziak K, Schulak JA. Comparison of Sirolimus vs. Mycophenolate Mofetil on Surgical Complications and Wound Healing in Adult Kidney Transplantation. Am J Transplant. 2003;3:1128–1134. doi: 10.1034/j.1600-6143.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuppahally S, Al-Khaldi A, Weisshaar D, Valantine HA, Oyer P, Robbins RC, Hunt SA. Wound Healing Complications with De Novo Sirolimus Versus Mycophenolate Mofetil-Based Regimen in Cardiac Transplant Recipients. Am J Transplant. 2006;6:986–992. doi: 10.1111/j.1600-6143.2006.01282.x. [DOI] [PubMed] [Google Scholar]