Abstract
Phosphate is a key element for several physiological pathways, such as skeletal development, bone mineralization, membrane composition, nucleotide structure, maintenance of plasma pH, and cellular signaling. The kidneys have a key role in phosphate homeostasis with three hormones playing important roles in renal phosphate handling (i.e., parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), and 1-25 dihydroxy-vitamin D).
Independently of the genetic diseases affecting the FGF23 pathway (such as hypophosphatemic rickets), hypophosphatemia is a frequent condition in daily practice, and untreated severe hypophosphatemia can induce hemolysis, rhabdomyolysis, respiratory failure, cardiac dysfunction and neurological impairment, thus requiring a rapid correction to avoid severe complications.
The aims of this case report are to summarize the etiologies and the biological evaluation of hypophosphatemia in adults, and to provide an overview of our current understanding of phosphate metabolism.
Keywords: FGF23, hypophosphatemia, hypophosphatemic rickets, tumor-induced osteomalacia
Introduction
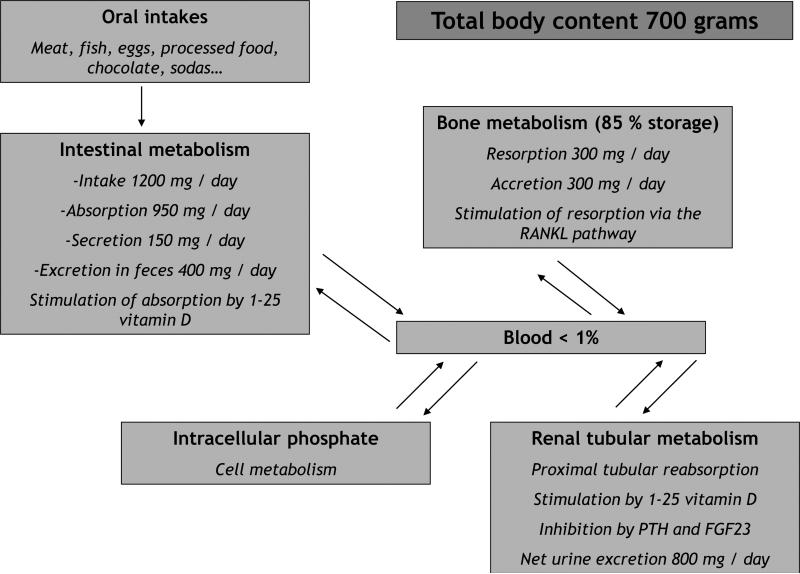
Phosphate is a key element for several physiological pathways, such as skeletal development, bone mineralization, membrane composition, nucleotide structure, maintenance of plasma pH, and cellular signaling (1). The vast majority of phosphate is in bone, but the kidneys have a key role in phosphate homeostasis with two hormones playing important roles in renal phosphate handling: parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23). Both hormones have hypophosphatemic effects by decreasing tubular phosphate reabsorption, but opposite effects on the regulation of 1,25 dihydroxivitamin D. The third main regulator of phosphate metabolism is 1-25 dihydroxyvitamin D via an increased phosphate intestinal absorption and an inhibition of PTH synthesis. An overview of phosphate physiology, with intestinal absorption, renal excretion and bone metabolism is provided in Figure 1.
Figure 1.
Overview of phosphate physiology in adults
PTH: parathyroid hormone
FGF23: Fibroblast Growth Factor 23
RANKL: Receptor Activator for Nuclear Factor κ B Ligand
Hypophosphatemia is defined in adults by a serum phosphate level below 2.5 mg/dL (0.8 mmol/L) and a severe hypophosphatemia by a serum phosphate level below 1 mg/dL (0.3 mmol/L) (2); in children hypophosphatemia is defined according to age-related normal range (3). Severe hypophosphatemia can induce proximal muscular weakness, respiratory failure, rhabdomyolysis, neurological impairment (e.g, irritability, paresthesias, seizures and coma), cardiovascular dysfunction (e.g., arrhythmia, congestive heart failure, cardiomyopathy), platelet dysfunction (e.g., thrombocytopenia, hemorrhage), hemolysis, metabolic acidosis and osteomalacia (4); in children with defects of tubular reabsorption of phosphorus (e.g., hypophosphatemic rickets (HR), proximal tubulopathy such as De Toni Debre Fanconi syndrome or Dent disease), hypophosphatemia can also induce growth retardation, bone deformaties and rickets. In the early 2000's, the key role of FGF23 and its regulators in phosphate physiology was discovered: its overexpression was initially described in pediatric patients with autosomal dominant HR (5), but it was also rapidly associated with tumor-induced osteomalacia (TIO) in adults and other types of HR in children (6).
The aims of this case report are to summarize the etiologies and the biological evaluation of hypophosphatemia in adults, and to provide an overview of our current understanding of phosphate metabolism.
Case report
Clinical history and initial laboratory data
A 27-year old female was diagnosed with HR at the age of 2 years: she presented with growth retardation (worsening since the beginning of walking), bowing of the legs and hypophosphatemia. There was a negative familial history for phosphorus disorders, and a genetic analysis of the main genes involved in HR has not been performed. Therapy was initiated with both phosphate supplementation and alfacalcidol. Her final adult height was 154 cm; leg deformities were very moderate, without need for surgical correction. There were no dental problems.
Additional investigations
A mild but stable nephrocalcinosis appeared at the age of 16 years; renal function remained normal during follow-up. However, at the age of 21 years, while she was still receiving phosphate supplements (20 mg/kg per day) and alfacalcidol (2 μg per day), PTH levels began to rise (i.e., 80 pg/ml, upper normal range of the assay of 65 pg/ml); treatment was continued without modifications and PTH levels continued to increase (PTH: 115 pg/ml). Phosphate supplements and alfacalcidol were therefore tapered and eventually discontinued. However, clinical symptoms (i.e., muscular and bone pain, asthenia) recurred with serum phosphorus levels of 1.50 mg/dl three months after the initial therapeutic withdrawal, leading to the reintroduction of phosphorus (20 mg/kg per day) and alfacalcidol (1 μg per day), in addition to a native vitamin D supplementation (cholecalciferol, 100 000 units monthly).
Diagnosis
Thus, after an initial diagnosis of HR during early childhood based on the association between bone deformaties and hypophosphatemia, the patient received long-term treatment with phosphate supplementation and active vitamin D sterol (alfacalcidol) and as a young adult, the presents with the two main long-term complications secondary to therapies often observed in HR: secondary hyperparathyroidism and nephrocalcinosis.
Clinical follow-up
At age xx years, , the patient was asymptomatic but PTH levels rose again (from 89 to 111 pg/mL) with normal serum calcium, decreased serum phosphorus (1.50 mg/dl, values range between 1.9 and 2.2 mg/dl), 25(OH)D level of 25 ng/mL within the lower limit, urine calcium/creatinine ratio of 0.59 within the upper limit and increased bone biomarkers (crosslaps 10 times, osteocalcin 1.5 times and bone alkaline phosphatase 9 times the upper normal value, respectively; total bone alkaline phosphatase within the upper normal level). Parathyroid ultrasounds and scintigraphy were normal. Of note, glycosuria was present in the absence of diabetes mellitus; indeed, the development of glycosuria is a rare feature of X-linked HR, but its pathogenesis is not well defined (7, 8).
Discussion
In adults, hypophosphatemia is relatively frequent in daily medical practice, affecting up to 5 % of hospitalized patients and up to 30-50 % if patients are alcoholic, septic or hospitalized in intensive care units (1, 2). Indeed, hypophosphatemia will become clinically significant when there is an underlying phosphate depletion state that can be silent at baseline in chronically-malnourished patients but with a tendency to worsen in case of acute stress. Starvation is associated with phosphate depletion and urinary loss of phosphorus; at the same time, IV carbohydrate infusions induce transcellular phosphorus shifts that worsen the hypophosphatemic state (9). In these critically-ill patients, respiratory alkalosis, inadequate amount of phosphate intake and increased glycolysis (by leading to the formation of phosphorylated glucose compounds) all contribute to hypophosphatemia. For example, in alcoholic patients, poor nutrition prior to admission, transcellular shifts secondary to intravenous fluids containing carbohydrates, and hyperventilation often observed in acute alcohol withdrawal are thought to play a critical role in the pathogenesis of hypophosphatemia in hospitalized alcoholics patients, furthermore, chronic alcoholism increases phosphaturia (9).
As summarized in Table 1, hypophosphatemia can have a variety of etiologies (1, 4, 10, 11). Furthermore, several medical conditions can interfere with serum phosphate determinations, such as hemolysis, hyperbilirubinemia, hyperlipemia and hyperglobulinemia (1, 4, 12).
Table 1.
FGF23 disorders and other regulators in humans
| Disease | Involved genes | |
|---|---|---|
| Hypophosphatemia | Hypophosphatemia rickets | Activating mutation of FGF23 *** |
| Inactivating mutation of PHEX *** | ||
| Inactivating mutation of DMP 1 *** | ||
| Inactivating mutation of ENPP1 *** | ||
| Inactivating mutation of Npt2c ** | ||
| Activating translocation of Klotho * / ** | ||
| With renal lithiasis and/or osteopenia and/or hypercalciuria | Inactivating mutation of Npt2a ** | |
| Inactivating mutation of Npt2c ** | ||
| Inactivating mutation of NHERF1 | ||
| Mac Cune Albright / fibrous dysplasia of bone | Overexpression of FGF23, GNAS *** | |
| Tumor-induced osteomalacia | Overexpression of FGF23, MEPE, FGF7 and/or FRP4 *** | |
| Epidermal naevus syndrome | FGF-R3 *** | |
| Hyperphosphatemia | Familial tumoral calcinosis | Inactivating mutation of FGF23 ** |
| Inactivating mutation of Klotho *** | ||
| Inactivating mutation of GALNT3 ** | ||
FGF: fibroblast growth factor
PHEX: phosphate-regulating gene with homologies to endopeptidases on the X chromosome
DMP1: Dentin matrix protein 1
ENPP1 : ecto-nucleotide pyrophosphatase / phosphodiesterase 1
Npt2a: type IIa sodium-phosphate cotransporter (SLC34A1)
Npt2c: type IIc sodium-phosphate cotransporter (SLC34A3)
MEPE: matrix extracellular phosphoglycoprotein
FRP4: frizzled related protein 4
FGF-R3: fibroblast growth factor receptor 3
GALNT3: UDP-N-acetyl-a-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3
with associated hyperparathyroidism
disease associated with a low FGF23 serum level
disease associated with a high FGF23 serum level
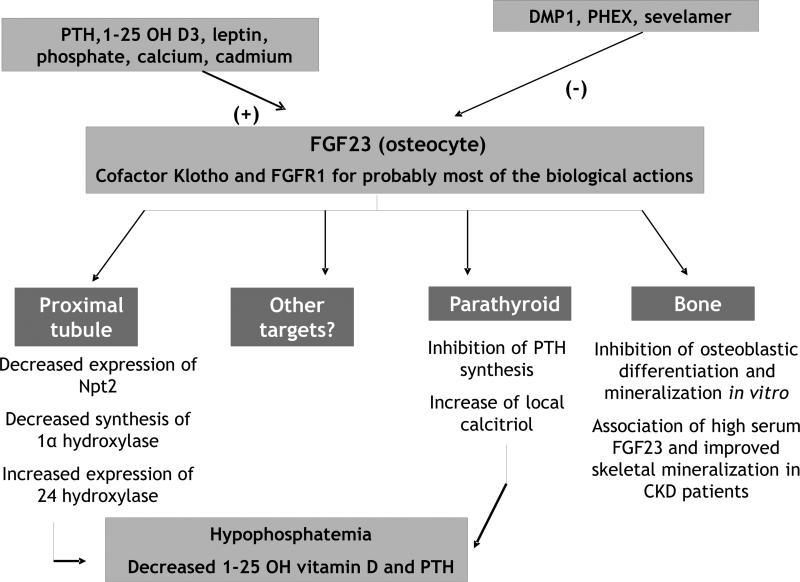
Since FGF23 has been recently shown to be a cornerstone of phosphate/calcium/vitamin D metabolism, an overview of its physiology is shown in Figure 2. Mutations of FGF23 and most of its regulators, that account for either hypophosphatemic or hyperphosphatemic disorders, are summarized in Table 2 (5, 13-16). FGF23 is a protein synthesized by osteocytes and osteoblasts that mainly acts as a phosphaturic factor and an inhibitory regulator of 1,25 dihydroxy-vitamin D; it may also regulate PTH synthesis and secretion (17). Indeed, it inhibits the expression of type IIa and IIc sodium-phosphate co-transporters on the apical membrane of proximal tubular cells, thus leading to an inhibition of tubular phosphate reabsorption. Moreover, it also inhibits the 1α-hydroxylase activity whereas it stimulates the 24 hydroxylase activity, therefore leading to a decreased 1,25 dihydroxyvitamin D serum level (18). FGF23 binds with only modest affinity to multiple receptors belonging to the family of FGF receptors (FGF-R, mainly types 1, 3 and 4); however, Klotho, a single-pass transmembrane anti-aging protein, can serve as a co-receptor that increases the affinity and specificity of FGF binding to FGFRs thereby allowing FGF23-mediated receptor activation (19). Klotho/FGF-R complexes are expressed in the parathyroid and the kidney, but the highest expression of this complex is in the distal tubule whereas the major biologic effects of FGF23 are located in the proximal tubule (20); this discrepancy is not explained to date, but recent reports have described an expression of Klotho in the proximal tubule, with a direct phosphaturic effect by itself (21). Moreover, Klotho can also function as an enzyme modifying the sugar chains of transient receptor potential vanilloid type 5 (TRPV5) in the distal tubule, preventing the calcium channel from internalization and inactivation, thus leading to an increased calcium reabsorption (22). FGF23 is regulated by systemic and local bone-derived factors involving both transcriptional and post-translational mechanisms. In healthy volunteers, oral phosphorus loading stimulates FGF23 synthesis while it is the contrary for dietary phosphorus restriction (23). PTH, vitamin D, phosphate and calcium stimulate FGF23 synthesis whereas glycophosphoproteins synthesized by osteocytes can activate or inhibit FGF23 secretion (e.g., Matrix Extracellular Phosphoglycoprotein MEPE and Dentin Matrix Protein 1 DMP1, respectively) (24). These two proteins are strongly involved in bone mineralization, but MEPE has also been recently described as a phosphaturic factor in the renal tubules (25). The specific role of FGF23 on bone remains to be defined since Klotho is not expressed in bone cells; however recent studies have demonstrated that FGF23 overexpression in vitro can suppress not only osteoblast differentiation but also matrix mineralization, independently of its systemic effect on phosphate metabolism (26). In contrast, other authors have reported a positive effect of FGF23 on bone: indeed, Wesseling-Perry et al. have reported an association between high levels of circulating FGF23 and improved indices of skeletal mineralization in pediatric patients undergoing peritoneal dialysis (27); moreover, bone FGF23 is up-regulated in patients with early stages of chronic kidney disease (27). In medical conditions such as hypophosphatemic rickets or TIO, to our knowledge, the specific role of FGF23 on bone has not been yet established. However, Carpenter et al have recently shown that patients with X-linked HR had elevated FGF23 circulating levels in comparison to healthy controls, and that these values were higher in the treated patients; in contrast, Klotho levels were not modified when compared to healthy controls (21).
Figure 2.
Overview of FGF23 physiology
(+) corresponding to a positive effect
(−) corresponding to an inhibitory effect DMP1: dental matrix protein 1
FGF23: fibroblast growth factor 23
FGFR1: fibroblast growth factor receptor type 1
PHEX: phosphate-regulating gene with homologies to endopeptidases on X chromosome
PTH: parathyroid hormone
Npt 2: type II sodium-phosphate cotransporters (type a and c)
Table 2.
Etiologies of hypophosphatemia
| Acute hypophosphatemia | Chronic hypophosphatemia | |
|---|---|---|
| Decreased phosphate intake | Inadequate parenteral nutrition Nutritional defects |
|
| Decreased intestinal absorption | Vitamin D deficiency Vitamin D-dependent rickets Chronic anti-acid therapy Intestinal malabsorption Chronic liver disease Alcoholism |
|
| Increased renal wasting of primary renal disease | After renal transplantation (first weeks) Acute volume expansion |
De De Toni Debre Fanconi syndrome Dent disease Toxic (ifosfamide, platin salts, diuretics, glucocorticoids, retroviral therapies in HIV patients, acetazolamide) |
| Hyperparathyroidism | Primary and secondary hyperparathyroidisms | |
| Increased FGF23 serum levels | Hypophosphatemic rickets Tumor-induced osteomalacia Fibrous dysplasia Mac Cune Albright syndrome Toxic (saccharated ferric oxide) |
|
| Redistribution of phosphate between the different compartments | After bone marrow transplantation Acute leukemia and lymphoma Correction of diabetic ketoacidosis Refeeding Acute respiratory alkalosis Hungry bone syndrome |
Treatment with erythropoiesis-stimulating agents in patients with cirrhosis |
| Miscellaneous | Severe sepsis Extensive burns Inadequate dialysis Acute paracetamol overdose Salicylate poisoning Hypothermia |
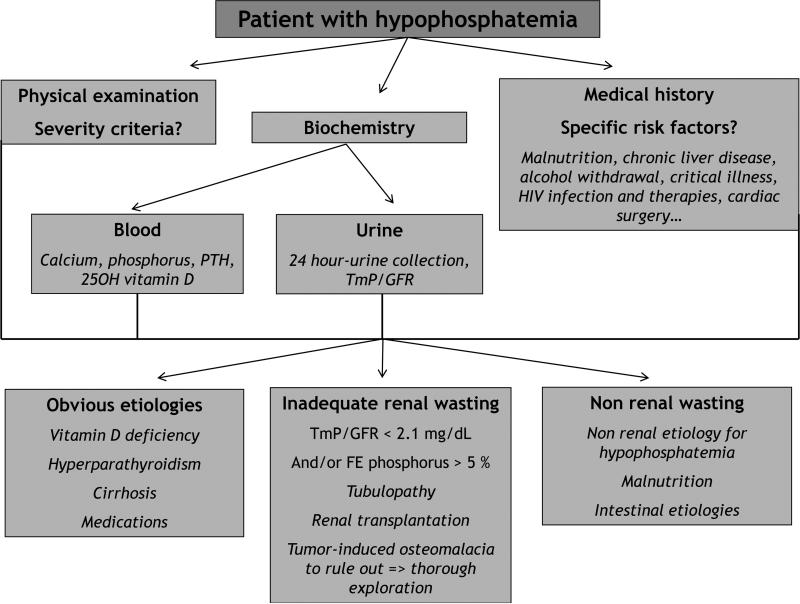
When evaluating a patient with hypophosphatemia (Figure 3), serum phosphorus levels should be interpreted with concomitant evaluations of serum total and ionized calcium, PTH and 25-OH vitamin D, as well as urine phosphate and calcium excretion since isolated serum phosphate levels are not reliable indicators of total body phosphate content (28). The combination of medical history, physical examination and laboratory tests should identify the etiology of hypophosphatemia in most cases, and will help to determine whether the hypophosphatemia is the consequence of renal wasting, a decreased intestinal absorption or a repartitioning among the different body compartments (28). Urine phosphate excretion can be evaluated by the 24-hour excretion of phosphate (U24P), the calculation of the fractional excretion rate of phosphate (FeP) or the determination of the tubular maximum for phosphate corrected for the glomerular filtration rate (TmP/GFR). When circulating phosphorus levels are below 3.1 mg/dL, all the phosphorus in the glomerular filtrate is reabsorbed and phosphorus should not be present in the urine; above this critical concentration, urine phosphorus concentration reflects phosphorus balance, and therefore dietary intake (29). Thus, in cases of hypophosphatemia, if U24P is above 100 mg and/or if FeP is above 5 % and/or if TmP/GFR is below 2.1mg/dL (normal values according to age and gender), hypophosphatemia will be secondary to excessive renal wasting (2). When a 24 hour-collection is difficult, a spot first urine in the morning before lunch can also be used (30). Although FGF23 assays have been developed a few years ago, they are not available in clinical practice and the use of urinary phosphate indices may reflect FGF23 levels. Following the description of an active and an inactive form of FGF23, two types assays have indeed been developed: the ‘intact’ assay that measures only active FGF23 and the ‘C-terminal’ assay that measures both the active and inactive FGF23. Even though some studies for FGF23 determinations have utilized serum, serum it is not currently recommended because FGF-23 serum samples can be degraded, forming several bands on the Western blots in one hand, and because the FGF23 molecule appears to be unstable (with a resulting decreased immunoreactivity over time). As a consequence, if performed, samples should be collected on EDTA plasma or culture media. As stated earlier, urinary phosphate excretion can be considered as an indirect reflect of FGF23 levels.
Figure 3.
Evaluation of an adult patient with hypophosphatemia
TmP/GFR: tubular maximal reabsorption of phosphorus / glomerular filtration rate FE phosphorus: fractional excretion of phosphorus
PTH: parathyroid hormone
In this case, the patient presented with hypophosphatemia beginning at an early age and investigations revealed evidence for HR. HR correspond to a heterogeneous genetic pathology, affecting 1/20 000 children, resulting from mutations in FGF23, Klotho or their regulators to induce hypophosphatemia, rickets, dental abnormalities (pulp chambers, dental hypoplasia, dentin abnormalities and dental abscesses) and bone deformations (32). Patients present with hypophosphatemia, normal serum calcium, decreased tubular phosphate reabsorption, normal 25OH vitamin D and PTH, and increased alkaline phosphatase levels. Adults with HR can also present with calcific entesopathy in fibrocartilaginous insertion sites (e.g., knees, ankles, pelvis, thoracic spine) with associated osteophyte formation (33).
The therapeutic management combines high oral phosphate intake and active vitamin D therapy, allowing a better growth and correcting bone deformaties, and such combined therapy may lead to long-term complications such as: secondary hyperparathyroidism secondary and nephrocalcinosis (34). While hypophosphatemic rickets is usually diagnosed during early childhood, an acquired disease with the same phenotype can also affect adults (more exceptionally children or teenagers), i.e., tumor-induced osteomalacia (TIO) (35). This clinical entity (TIO) corresponds to mesenchymal tumors, usually benign and located in the appendicular skeleton ; they result from an acquired hypersecretion of phosphatonins (mostly FGF23, sometimes SFRP4, MEPE or FGF7) (31). Patients present with osteomalacia, bone pain, fractures and muscular weakness; a normal phosphate level years or months before the onset of the clinical picture is a strong rationale for an acquired cause of FGF23 hypersecretion. In case of renal wasting with no obvious etiology, a thorough search for TIO is important (i.e., tomography, MRI, scintigraphy with Tc99m and/or octreotide, sometimes PET-Scan with F18-fluorodeoxyglucose), since the removal of the tumor will correct hypophosphatemia and FGF23 production (31).
A few points can briefly be highlighted concerning the management of HR in adults since the two main long-term complications secondary to therapies, i.e., secondary hyperparathyroidism and nephrocalcinosis, were observed in our patient. According to current guidelines (32), the first step would be to decrease PTH levels: an increase of the dose of active vitamin D sterols, as well as a decrease of the phosphate supplement. However, in this case urinary calcium was in the upper normal range and thus limits the use of alfacalcidol. By contrast, correcting 25 OH vitamin D insufficiency and adding new agents utilized for the treatment of primary and secondary hyperparathyroidism such as calcimimetics (as a secondary line) may be potentially beneficial (36). Such therapeutic options have nevertheless not been utilized in this patient but promising results have been reported in these conditions (36, 37). In the future, targeting the FGF23 pathway with specific antibodies will probably be the most physiological approach, but to date, even though results are promising in animal models, no data have been published in humans (38). The treatment of HR patients during adulthood still remains controversial: it seems reasonable to treat symptomatic patients (bone pain, asthenia, depression or muscle weakness) with the lowest doses as possible of active vitamin D (39). Vitamin D deficiency or insufficiency should be corrected in all patients but there are no specific recommendations. The treatment for women with HR during pregnancy is questionable and even though no data are published, 25OH vitamin D supplementation is usually given, as well as oral phosphate according to symptoms. The future mother should be warned that early post-natal diagnosis can be made only after 3 to 6 months of life: indeed, because of the physiological low glomerular filtration rate in newborns, phosphate reabsorption abnormalities may be hidden during the first weeks of life.
In conclusion, hypophosphatemia is common in hospitalized patients, and malnutrition, alcohol withdrawal, critical illness, HIV infection and cardiac surgery, among others, are risk factors for this condition. Untreated severe hypophosphatemia can induce hemolysis, rhabdomyolysis, respiratory failure, cardiac dysfunction and neurological impairment, thus requiring a rapid correction to avoid severe complications.
The recent description of the key role of the FGF23 in the ‘bone-kidney-parathyroid’ axis had led to a better understanding of genetic conditions associated with hypophosphatemia and of the pathophysiology of both phosphate disorders (13). In HR patients, a careful use of phosphate supplements and active vitamin D sterols should be considered to prevent nephrocalcinosis, secondary hyperparathyroidism and soft tissue calcifications. In the future, specific antibodies directed against FGF23 will probably modify dramatically the management of such patients.
Acknowledgements
This work was supported in part by educational grants (Académie Française Jean Walter Zellidja, Réunion Pédiatrique de la Région Rhône Alpes, Société Française de Pédiatrie Evian, Fondation pour la Recherche Médicale and the Philippe Foundation, JB) and by USPHS grants DK 35423, DK 67563 and RR 00865 and funds from the Casey Lee Ball Foundation.
Footnotes
Disclosure of interests: none.
References
- 1.Bugg NC, Jones JA. Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia. 1998 Sep;53(9):895–902. doi: 10.1046/j.1365-2044.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruppe M, Jan de Beur SM. Disorders of phosphate homeostasis. In: Rosen CJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 7 ed. Washington: 2008. pp. 317–24. [Google Scholar]
- 3.Ardeshirpour L, Cole DE, Carpenter TO. Evaluation of bone and mineral disorders. Pediatr Endocrinol Rev. 2007 Oct;5(Suppl 1):584–98. [PubMed] [Google Scholar]
- 4.Hruska H. Hyperphosphatemia and hypophosphatemia. In: Favus M, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6 ed. Washington: 2006. pp. 233–42. [Google Scholar]
- 5.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000 Oct;58(4):1758–64. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 6.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6500–5. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HY, Lee BH, Kang JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005 Aug;58(2):329–33. doi: 10.1203/01.PDR.0000169983.40758.7B. [DOI] [PubMed] [Google Scholar]
- 8.Olauson H, Qureshi AR, Miyamoto T, Barany P, Heimburger O, Lindholm B, et al. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant. Sep;25(9):3033–8. doi: 10.1093/ndt/gfq191. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian R, Khardori R. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore) 2000 Jan;79(1):1–8. doi: 10.1097/00005792-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu Y, Tada Y, Yamauchi M, Okamoto T, Suzuki H, Ito N, et al. Hypophosphatemia induced by intravenous administration of saccharated ferric oxide: another form of FGF23-related hypophosphatemia. Bone. 2009 Oct;45(4):814–6. doi: 10.1016/j.bone.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Bagnis CI, Karie S, Deray G, Essig M. Hypophosphataemia: an easy strategy for diagnosis and treatment in HIV patients. Antivir Ther. 2009;14(4):481–8. [PubMed] [Google Scholar]
- 12.Kerr S, Kindt J, Daram SR. Hypophosphatemia associated with paraproteinemia: a case report and review of the literature. Wmj. 2007 Dec;106(8):490–3. [PubMed] [Google Scholar]
- 13.Razzaque MS. FGF23-mediated regulation of systemic phosphate homeostasis: is Klotho an essential player? Am J Physiol Renal Physiol. 2009 Mar;296(3):F470–6. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prie D, Urena Torres P, Friedlander G. Latest findings in phosphate homeostasis. Kidney Int. 2009 May;75(9):882–9. doi: 10.1038/ki.2008.643. [DOI] [PubMed] [Google Scholar]
- 15.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010 Apr;25(4):591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010 Feb 12;86(2):267–72. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res. 2008 Sep;23(9):1509–18. doi: 10.1359/jbmr.080417. [DOI] [PubMed] [Google Scholar]
- 18.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, et al. The Influence of Glomerular Filtration Rate and Age on Fibroblast Growth Factor 23 Serum Levels in Pediatric Chronic Kidney Disease. J Clin Endocrinol Metab. Feb 15; doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 19.Goetz R, Beenken A, Ibrahimi OA, Kalinina J, Olsen SK, Eliseenkova AV, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007 May;27(9):3417–28. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009 May;20(5):955–60. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010 Apr;95(4):1846–50. doi: 10.1210/jc.2009-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007 Oct;72(8):1004–13. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 23.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006 Aug;21(8):1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 24.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007 Oct;18(10):2683–8. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 25.Shirley DG, Faria NJ, Unwin RJ, Dobbie H. Direct micropuncture evidence that matrix extracellular phosphoglycoprotein inhibits proximal tubular phosphate reabsorption. Nephrol Dial Transplant. 2010 Oct;25(10):3191–5. doi: 10.1093/ndt/gfq263. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008 Jun;23(6):939–48. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 27.Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, et al. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009 Feb;94(2):511–7. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amanzadeh J, Reilly RF., Jr Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006 Mar;2(3):136–48. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 29.Guyton B, Hall J, editors. Textbook of medical physiology. eleventh edition Elsevier Saunders; Philadelphia: 2006. [Google Scholar]
- 30.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. The Journal of clinical investigation. 1987 Oct;80(4):1147–54. doi: 10.1172/JCI113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo VL, Landesberg R, Imel EA, Singer SR, Folpe AL, Econs MJ, et al. Phosphaturic mesenchymal tumor, mixed connective tissue variant, of the mandible: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Dec;108(6):925–32. doi: 10.1016/j.tripleo.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011 Jul;26(7):1381–8. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009 Jan;75(2):176–84. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacchetta J. Cochat P, editor. Rachitisme hypophosphatémique. Progrès en Pédiatrie, Néphrologie Pédiatrique: Doin. 2010 Epub. [Google Scholar]
- 35.Reyes-Mugica M, Arnsmeier SL, Backeljauw PF, Persing J, Ellis B, Carpenter TO. Phosphaturic mesenchymal tumor-induced rickets. Pediatr Dev Pathol. 2000 Jan-Feb;3(1):61–9. doi: 10.1007/s100240050008. [DOI] [PubMed] [Google Scholar]
- 36.Raeder H, Shaw N, Netelenbos C, Bjerknes R. A case of X-linked hypophosphatemic rickets: complications and the therapeutic use of cinacalcet. Eur J Endocrinol. 2008 Dec;159(Suppl 1):S101–5. doi: 10.1530/EJE-08-0383. [DOI] [PubMed] [Google Scholar]
- 37.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008 May;3(3):658–64. doi: 10.2215/CJN.04981107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res. 2009 Nov;24(11):1879–88. doi: 10.1359/jbmr.090509. [DOI] [PubMed] [Google Scholar]
- 39.Holm I, Econs M, Carpenter T. Familial hypophosphatemia and related disorders. In: Glorieux FH, editor. Pediatric Bone, Biology and Diseases. Elsevier; San Diego: 2003. pp. 603–31. [Google Scholar]
- 40.Petje G, Meizer R, Radler C, Aigner N, Grill F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin Orthop Relat Res. 2008 Dec;466(12):3078–85. doi: 10.1007/s11999-008-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fucentese SF, Neuhaus TJ, Ramseier LE, Ulrich Exner G. Metabolic and orthopedic management of X-linked vitamin D-resistant hypophosphatemic rickets. J Child Orthop. 2008 Aug;2(4):285–91. doi: 10.1007/s11832-008-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souza MA, Soares Junior LA, Santos MA, Vaisbich MH. Dental abnormalities and oral health in patients with Hypophosphatemic rickets. Clinics (Sao Paulo) 2010;65(10):1023–6. doi: 10.1590/S1807-59322010001000017. [DOI] [PMC free article] [PubMed] [Google Scholar]