Abstract
Great progress has recently been made in structural and functional research of phospholipase C (PLC)-β. We now understand how PLC-β isoforms (β1-β4) are activated by GTP-bound Gαq downstream of G protein-coupled receptors. Numerous studies indicate that PLC-βs participate in the differentiation and activation of immune cells that control both the innate and adaptive immune systems. The PLC-β3 isoform also interplays with tyrosine kinase-based signaling pathways, to inhibit Stat5 activation by recruiting the protein-tyrosine phosphatase SHP-1, with which PLC-β3 and Stat5 form a multi-molecular signaling platform, named SPS complex. The SPS complex has important regulatory roles in tumorigenesis and immune cell activation.
Keywords: PLC-β, G protein, lymphocyte, macrophage, neutrophil, mast cell
Introduction
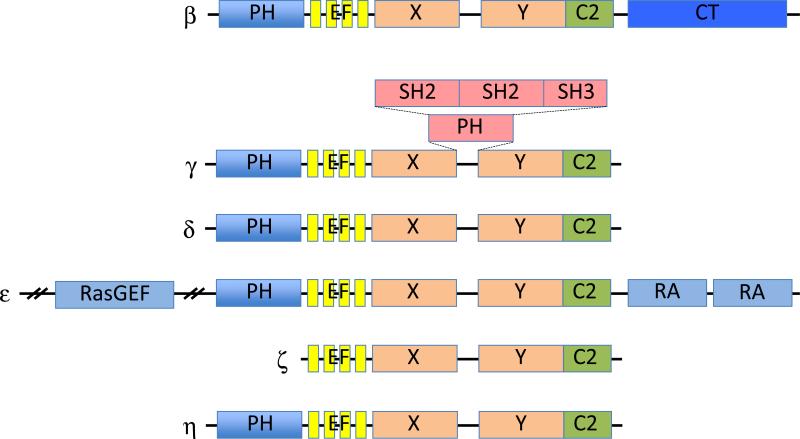
PLC is a family of enzymes that catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) (Rhee, 2001). IP3 induces calcium release from intracellular stores and DAG can initiate activation of Ras proteins via the RasGRP family of guanine nucleotide exchange factors. Ca2+ and DAG also activate protein kinase C (PKC), among other targets (Bunney and Katan, 2011, Rhee, 2001). There are 13 mammalian PLC isozymes that can be classified into six subfamilies (PLC-β, PLC-γ, PLC-δ, PLC-ε, PLC-ζ and PLC-η) (Suh et al. , 2008). Members of the PLC-β subfamily (β1~ β4) share a well-conserved architecture consisting of an N-terminal pleckstrin homology domain, four EF hands, split X+Y catalytic domain, C2 domain, and C-terminal (CT) domain (Figure 1). PLC-βs show different tissue expression and G protein regulation. PLC-β1 and PLC-β3 are expressed in a wide range of tissues and cell types, whereas PLC-β2 and PLC-β4 have been found only in hematopoietic and neuronal tissues, respectively. All PLC-β isoforms are activated by the Gα subunits of the Gq class. PLC-β2 and PLC-β3 can also be activated by βγ subunits of the Gαi/o family of G proteins and the small GTPases such as Rac and Cdc42. In addition, PLC-βs are GTPase-activating proteins (GAPs) for the Gαq proteins that activate them. G proteins cycle from the GDP-bound inactive to the GTP-bound active state. GTP-bound G proteins activate the effectors such as PLC-β. G protein-coupled receptors (GPCRs) act by catalyzing the release of GDP and binding of GTP. GAPs accelerate the deactivation process, which would otherwise be very slow. In contrast to PLC-β isoforms, PLC-γ isoforms are regulated by receptor and non-receptor tyrosine kinases. PLC-ε is regulated directly by small GTPases of the Ras and Rho families, as well as subunits of G proteins (Bunney et al. , 2006, Harden et al. , 2009, Harden and Sondek, 2006). Crystal structures of human PLC-β3 (enzyme core or full-length) in complex with Gαq were recently solved, revealing novel structural insights into the function of each domain involved in Gαq-induced activation of PLC-β3 (Lyon et al. , 2013, Waldo et al. , 2010). Structure and regulation of PLC by GPCRs and other means have been recently reviewed (Gresset et al. , 2012, Kadamur and Ross, 2013). Here we focus on recent progresses in research of the role of PLC-β in the regulation of immune cells.
Figure 1. The domain structure of PLCs.
PLC-β consists of an N-terminal PH domain, four EF hands, a catalytic TIM barrel (X + Y), a C2 domain, and a carboxy-terminal (CT) domain. The second PH domain of PLC-γ is split to allow an insert of two SH2 domains and one SH3 domain between X and Y domains. SH2, Src homology 2; SH3, Src homology 3; RA, Ras association; RasGEF, Ras GDP/GTP exchange factor
PLC-β in lymphocytes
1. T cells
T cell activation via T cell receptor (TCR) is the essential initial process of adaptive immunity. Following the antigen recognition by TCR, Lck, a Src family tyrosine kinase associated with coreceptors CD4 and CD8, is activated as one of the earliest activation events (Abraham and Weiss, 2004, Smith-Garvin et al. , 2009). Lck phosphorylates the immunoreceptor tyrosine-based activation motif (ITAMs) on the ε, δ, γ, and ζ subunits of the TCR/CD3 complex, generating the binding sites for ZAP-70, a Syk family tyrosine kinase. ZAP-70 phosphorylates tyrosine residues in the cytoplasmic portion of the transmembrane adaptor LAT. LAT acts as an adaptor/scaffold for the assembly of signaling complexes. These ‘signalosomes’ trigger multiple downstream pathways that eventually activate transcription factors, leading to gene transcription characteristic of activated T cells. In this activation scheme, PLC-γ1 is critical for generation of IP3 and DAG and Ca2+ mobilization (Yablonski et al. , 1998), but PLC-β subfamily appears to be nonessential ((Bach et al. , 2007) and our unpublished data).
Unlike this canonical TCR signaling, bacterial superantigens can activate an alternative pathway that does require TCR but does not involve Lck, ZAP-70 or PLC-γ1. Using superantigens derived from Staphylococcus and Streptococcus bacteria, Madrenas and associates showed that neither CD4 nor Lck is required for superantigen-induced T cell activation (Bueno et al. , 2006). PLC-β inhibitor U73122 inhibited superantigen-induced T cell activation. Furthermore, experiments with PLC-β1 specific siRNA showed that PLC-β1 (and probably other PLC-β isoforms as well) is part of the superantigen-induced signaling pathway. Consistent with the general view that the PLC-β family is downstream of G proteins, a dominant negative Gα11 mutant, but not pertussis toxin (Gαi inhibitor), inhibited superantigen-induced T cell activation. Therefore, the triggering of this Gα11-PLC-β dependent alternative pathway by superantigens suggests that these toxins use a GPCR as a coreceptor on T cells. Interestingly, the canonical T cell activation is sensitive to glucocorticoids, whereas superantigens induce a state of steroid resistance in activated T cells (Hauk et al. , 2000). The glucocorticoid receptor is present in a TCR-associated complex and glucocorticoids rapidly dissociate Lck from the TCR complex, resulting in the inhibition of the canonical Lck- PLC-γ-dependent TCR signaling (Lowenberg et al. , 2005, Lowenberg et al. , 2006). A recent study showed that staphylococcal enterotoxin B activates a Gαq and PLC-β2-dependent pathway in human T cells, rendering superantigen-stimulated T cells insensitive to glucocorticoids (Verhaar et al. , 2013). T cell proliferation induced by PMA and ionomycin, which bypassed the TCR-Lck-PLC-γ signaling, was completely resistant to steroids. Thus, the corticosteroid effects on T cell activation were mainly nongenomic or nontranscriptional. Toxic shock syndrome caused by auperantigens are resistant to steroids, but a combination of steroids and PLC-β inhibitor might be effective.
Chemokines acting through GPCRs play an essential role in the immune response, as lymphocyte traffic is a key element in immune surveillance (Cyster, 2005, Rot and von Andrian, 2004). PLC-β has been studied in the regulation of chemokine-mediated T cell migration (Bach, Chen, 2007). Abrams and associates demonstrated that loss of both PLC-β2 and PLC-β3 significantly impaired T cell migration. T cell migration induced by stromal cell-derived factor-1α (SDF-1α/CXCL12), the sole ligand of CXCR4, was inhibited by chelation of Ca2+. Ca2+ influx induced by SDF-1α was undetectable in PLC-β2−/−; PLC-β3−/− double knockout (dko) lymphocytes, suggesting that the migration defect is due to the impaired ability to increase intracellular Ca2+. This study, together with another study showing that human T cell migration through a chemokine receptor CXCR3 is sensitive to PLC inhibitor (Smit et al. , 2003), demonstrated that phospholipid second messengers generated by PLC-β play a critical role in T lymphocyte chemotaxis.
Upon SDF-1α binding to CXCR4, CXCR4 heterodimerizes with the TCR (Kumar et al. , 2006). The CXCR4-TCR heterodimer stimulates increased intracellular Ca2+ concentrations, prolonged ERK activation, gene transcription and cytokine production. These responses involve several traditional TCR signaling molecules including ZAP-70 and SLP-76 as well as Gi-type G proteins (Kremer et al. , 2003). Furthermore, in Jurkat T cells, SDF-1α signaling via the CXCR4-TCR heterodimer uses PLC-β3 to activate the Ras-ERK pathway and increase intracellular Ca2+ concentrations, whereas PLC-γ1 is dispensable for these events. In contrast, PLC-γ1, but not PLC-β3, is required for SDF-1α-mediated migration via a mechanism independent of LAT (Kremer et al. , 2011). These results characterize new roles for PLC-β3 and PLC-γ1 in T cells, and suggest that multiple PLCs may also be activated downstream of chemokine receptors to distinctly regulate migration versus other signaling functions. The lack of effects of PLC-β3 in SDF-1α mediated migration of Jurkat T cells might be explained by the redundancy of other PLC-β members, probably PLC-β2.
Human T cells express PLC-β and -γ isoforms (Di Pietro and Rana, 1998). Interestingly, PLC-β2 expression is reduced in T cells from elderly people, suggesting that an impaired expression of PLC-β2 in aged T lymphocytes might explain the age-related defect of PLC activity and contribute to immune suppression in this group of people (Di Pietro et al. , 2000).
2. B cells
Chemokines are critical for B cell development, homeostasis, activation, and effector function. For example, CXCR4 is important for B cell lymphopoiesis (Nagasawa et al. , 1996) and the organization of germinal centers (Allen et al. , 2004); CXCR5 is required for the localization of B cells in B cell follicles (Forster et al. , 1996) and germinal centers (Allen, Ansel, 2004). Therefore, PLC-βs are important players in chemoattractant-induced integrin activation, cell-substrate adhesion, and migration in B cells. Interestingly, B cell activation by LPS reduces the expression of PLC-β2, but not PLC-β1, PLC-β3, or PLC-γ2, despite the increased expression of CCR6 and CCR7, indicating a novel mechanism for controlling chemokine-induced signals (Shirakawa et al. , 2010).
Antibody production by B cells is regulated by PLC-βs. PLC-β2−/−;PLC-β3−/− dko mice generate greater amounts of λ light chain-containing antibodies than did wild-type mice, when immunized with T cell-independent (TI) antigen hydroxylnitrophenyl (NP)-Ficoll. Enhancement in TI-Igλ production appeared to be primarily dependent on PLC-β3 deficiency. PLC-β2 or PLC-β3 deficiency did not affect the production of TI-Igκ or of T cell-dependent antigen NP-chicken gamma globulin (NP-CCG)-specific antibodies. Together these data suggest that the production of TI-Igλ may be subjected to regulation by G protein-mediated signaling pathways (Li et al. , 2000). A recent study showed that the Igk locus of B cell progenitors in IL-7-rich environment is epigenetically repressed by Stat5 tetramer-mediated recruitment of the histone methyltransferase Ezh2 (Mandal et al. , 2011). Thus, if Stat5 activity is high in PLC-β3−/− B cell progenitors as expected from our study (Xiao et al. , 2009), Igκ production might be suppressed relative to Igλ production in PLC-β3−/− mice. When these mice become aged, both the Th1- and Th2-associated antibodies are increased in their serum, suggesting that PLC-β3 is a brake in antibody production (our unpublished data).
PLC-β in myeloid cells
1. Macrophages
Macrophages are innate immune cells with well-established roles in the primary response to pathogens, tissue homeostasis, coordination of the adaptive immune response, inflammation, resolution, and repair (Pollard, 2009). Activated macrophages are classified into classically activated (M1) and alternatively activated (M2) macrophages. M1 macrophages are anti-inflammatory, but M2 macrophages are pro-inflammatory, but immunomodulatory and angiogenic (Gordon and Martinez, 2010). PLC-βs regulate both the function and differentiation of macrophages. LPS, together with adenosine A2A receptor (A2AR) agonists, downregulates expression of tumor necrosis factor α (TNFα) and interleukin (IL)-12, and upregulates expression of vascular endothelial growth factor (VEGF) and IL-10, thus switching macrophages from M1 to M2 phenotype (Grinberg et al. , 2009). This change is accompanied by down-regulation of PLC-β1 and -β2 expression due to destabilization of their mRNAs. LPS also suppresses PLC-β1 and -β2 expression in vivo. Expression of M2 markers such as VEGF is increased in macrophages treated with PLC-β inhibitors, or a small-interfering RNA specific to PLC-β2 but not PLC-β1, and in macrophages from PLC-β2−/− mice, suggesting that PLC-β2 downregulation plays an important role in switching M1 macrophages into an M2 state. In line with this, the number of alveolar macrophages that express Ym-1 and arginase I, markers of M2 macrophages, is increased in PLC-β3−/− mice (Xiao et al. , 2010), suggesting that PLC-β3, similar to PLC-β2, might inhibit the polarization of macrophages into M2 phenotype.
Atherosclerosis is an inflammatory disease that is associated with monocyte recruitment and subsequent differentiation into lipid-laden macrophages at sites of arterial lesions, leading to the development of atherosclerotic plaques (Moore and Tabas, 2011). PLC-β is a key member of signaling pathways initiated by GPCR ligands in macrophages. Wu and associates found that deficiency in PLC-β3, the major functional PLC-β isoform in murine macrophages, rendered macrophages highly sensitive to multiple inducers of apoptosis (Wang et al. , 2008). PLC-β3 appeared to regulate this sensitivity via PKC-dependent upregulation of Bcl-XL, an antiapoptotic member of the Bcl-2 family. The significance of PLC-β signaling in vivo was examined using the apoE-deficient mouse model of atherosclerosis. Mice deficient in both PLC-β3 and apoE exhibited fewer total macrophages and increased macrophage apoptosis in atherosclerotic lesions, as well as reduced atherosclerotic lesion size when compared with mice lacking only apoE. These results demonstrate an important role for PLC-β3 activity in promoting macrophage survival in atherosclerotic plaques and identify PLC-β3 as a potential target for the treatment of atherosclerosis.
The role of PLC-β in atherosclerosis was further investigated in two mouse models with hyperlipidemia and genetically imposed hypercoagulability, i.e., TMPro/Pro ApoE−/− and FVLQ/Q ApoE−/− mice (Seehaus et al. , 2009). In both models, hypercoagulability resulted in larger plaques, but vascular stenosis was not enhanced secondary to positive vascular remodeling. Plaque stability was increased in hypercoagulable mice with less necrotic cores, more extracellular matrix, more smooth muscle cells, and fewer macrophages. The reduced frequency of intraplaque macrophages in hypercoagulable mice is due to thrombin-induced inhibitory effect on monocyte transendothelial migration. In this context, thrombin/protease-activated receptor-1 mediated signaling in macrophages is blocked by PLC-β inhibitor U73122, suggesting that PLC-β might have a protective role against atherosclerosis under hypercoagulable status. However, given the unwanted side effects of U73122 (Klein et al. , 2011) and the usefulness of specific inhibitory effects of CT domains of PLC-βs (Adjobo-Hermans et al. , 2013), it is advisable to readdress the role of PLC-β in atherosclerosis in the above models using better inhibitors.
2. Neutrophils
Wu and associates showed that chemokine-induced IP3 production and Ca2+ signaling are reduced in PLC-β2−/− neutrophils (Jiang et al. , 1997) and are abrogated in PLC-β2−/−;PLC-β3−/− dko neutrophils (Li, Jiang, 2000). Downstream of IP3 and Ca2+, PKC phosphorylation and associated p47phox translocation and JNK phosphorylation were not detected in fMLP (a GPCR agonist)-stimulated dko neutrophils. Thus, PLC-β2 and PLC-β3 are essential in chemoattractant-induced neutrophil activation. On the other hand, the dko neutrophils showed enhanced chemotactic activities in response to the CC chemokine MIP-1α both in vitro and in vivo (Li, Jiang, 2000), suggesting that PLC-β rather has a negative regulatory role in neutrophil functions under certain circumstances.
PLC-β might also be involved in the development of neutrophils. According to a genetic study of Japanese subjects (Okada et al. , 2010), two gene loci were significantly associated with neutrophil count (rs4794822 in PSMD3–CSF3 and rs2072910 in PLC-Β4), but no association with the counts of other WBCs (lymphocytes, monocytes, eosinophils and basophils) was found. The subjects who were homozygous for ‘neutrophil-increasing alleles’ in both of the SNPs (T alleles for rs4794822 and rs2072910) had 1.17-fold higher neutrophil counts when compared with the subjects homozygous for ‘neutrophil-decreasing alleles’ (C alleles for rs4794822 and rs2072910). Therefore, both PSMD3–CSF3 and PLC-β4 might regulate neutrophil production.
Consistent with the possible role of PLC-β in neutrophil development, we found that PLC-β3−/− mice develop myeloproliferative neoplasm (MPN) with increased mature neutrophils. This was unique to PLC-β3−/− mice as PLC-β2−/− mice did not develop MPN. Aged PLC-β3−/− mice typically have increased numbers of hematopoietic stem cells (HSCs) and myeloid progenitors in the bone marrow and spleens. These HSCs and myeloid progenitors had an increased predisposition to differentiate into granulocytes, and were hypersensitive to cytokines such as GM-CSF and IL-3, a hallmark of human MPNs. PLC-β3−/− HSCs demonstrated increased proliferation and reduced apoptosis compared to wild-type controls. These abnormal HSCs can gave rise to a Stat5-dependent MPN when adoptively transferred into lethally irradiated wild-type mice. Therefore, PLC-β3 regulates neutrophil differentiation and production at the level of HSCs via Stat5 inhibition (Xiao, Hong, 2009). As a biochemical basis for this function of PLC-β3, the CT domain of PLC-β3 can recruit Stat5 and SHP-1 to a multimolecular complex termed SPS complex, and within this complex, SHP-1 dephosphorylates Stat5 and thus inhibits Stat5 activity. Thus, the CT domain of PLC-β3 functions as a growth inhibitory adaptor. Fine mapping of the Stat5- and SHP-1-binding sites showed that three noncontiguous regions, designated a (positions 917-943), b (positions 983-1000), and c (positions 1032-1069), bound to Stat5 and two regions, designated c (positions 1032-1069) and d (positions 1182-1209) bound to SHP-1 (Yasudo et al. , 2011). Interestingly, an 11-residue peptide (peptide b11; positions 988-998), a part of the Dα3 helix (residues 960-1008) in the CT domain (Lyon, Dutta, 2013), induced growth inhibition and reduced Stat5 phosphorylation at Tyr694 in peptide b11-expressing cells (Kawakami et al. , 2012).
Potentially similar to the SPS complex, a recent study reported a complex formation of PLC-β1 and SHP-2 (Calo et al. , 2011). SHP-2 and PLC-β1 were present as a preformed complex. Angiotensin II increased SHP-2-PLC-β1 complexes and caused complex-associated PLC-β1 tyrosine phosphorylation to decline while complex-associated SHP-2's tyrosine phosphorylation increased. However, no functional studies have been attempted on this complex in immune or nonimmune cells.
3. Mast cells
Mast cells are the major effector cells in immediate hypersensitivity and chronic allergic reactions, and contribute to asthma and other allergic diseases (Galli and Tsai, 2012). They are also important in the host protection against certain bacteria, helminthes, and viruses, as well as bee and snake venoms (Abraham and St John, 2010, Metz et al. , 2006). The major mechanism of mast cell activation in immediate hypersensitivity and allergic reactions is via IgE/antigen-mediated cross-linking of the FcεRI (high-affinity IgE receptors) on mast cells (Kalesnikoff and Galli, 2008). FcεRI in mast cells consists of an IgE-binding α subunit, a signal-amplifying β subunit, and a dimer of signal-generating γ subunits (Kinet, 1999). Cross-linking of FcεRI (Metzger, 1992) elicits the enzymatic activation of receptor-bound Src family PTKs such as Lyn, which phosphorylates tyrosine residues in the ITAMs of the signaling β and γ subunits of receptor (Eiseman and Bolen, 1992, Jouvin et al. , 1994). Tyrosine-phosphorylated ITAMs recruit Src and Syk family PTKs through Src homology 2 (SH2) domain-phosphotyrosine interactions and activate these kinases (Benhamou et al. , 1993, Jouvin, Adamczewski, 1994). Syk phosphorylates a number of adaptors (including LAT) and enzymatic signaling molecules, leading to degranulation, release of lipid mediators, and production and secretion of various cytokines, chemokines, and growth factors. Thus, the principles of FcεRI-mediated signaling pathways are essentially identical to those of TCR (and BCR) signaling pathways. However, some details are unique to each system: ZAP-70 is important in the TCR system, whereas Syk is important in FcεRI and BCR systems; PLC-γ1 is critical for generation of IP3 and DAG and Ca2+ mobilization in T cells, whereas PLC-γ2 is important in B cells and both PLC-γ1 and PLC-γ2 are important in mast cells.
FcεRI-dependent signals can be synergized with signals derived from other receptors expressed on mast cells and the latter receptors may thus contribute to the allergic responses. One such receptor family is the GPCRs (Kuehn and Gilfillan, 2007). For example, prostaglandin (PG) E2, adenosine, sphingosine 1-phosphate, MIP-1α (CCL3), and RANTES (CCL5) can augment IgE/antigen-mediated degranulation in mast cells. This enhancement, which is the most robust by PGE2, and the ability of PGE2 to amplify IgE/antigen-induced calcium mobilization, was linked to a pertussis toxin-sensitive synergistic membrane translocation of PLC-γ and PLC-β (i.e., β2 and β3) and tyrosine phosphorylation of PLC-γ1 and PLC-γ2 (Kuehn et al. , 2008). This ‘trans-synergistic’ activation of PLC-β and PLC-γ, in turn, enhanced production of IP3, store-operated calcium entry, and activation of PKC-α and PKC-β. These responses were critical for degranulation. Therefore, GPCR-linked PLC-β can activate mast cells synergistically with FcεRI-linked PLC-γ. In contrast, adenosine- and FcεRI-mediated signals are integrated at PI3Kγ, but not PLC (Laffargue et al. , 2002). Adenosine, acting through the A3 receptor (A3R) as well as other agonists of Gαi-coupled GPCRs, increased PI(3,4,5)P3 via PI3Kγ. PI3Kγ-derived PI(3,4,5)P3 was instrumental for initiating a sustained influx of external Ca2+, which might be mediated by Btk and other Tec family kinases (Scharenberg et al. , 1998), and degranulation.
PLC-β3 appears to be the major functional isoform in mast cells as well (Xiao et al. , 2011). In a passive cutaneous anaphylaxis model, intradermal injection of antigen induces rapid and robust increase in vascular permeability and skin edema in IgE-sensitized mice. This acute reaction is dependent on histamine and serotonin released from activated mast cells (Inagaki et al. , 1986). PLC-β3−/− and wild-type mice showed similar acute responses, which is consistent with the normal degranulation of PLC-β3−/− mast cells. However, late-phase reactions are dependent on mast cell-derived cytokines (particularly TNFα and IL-33) (Hsu et al. , 2010, Wershil et al. , 1991). PLC-β3−/− mice have blunted FcεRI-dependent late-phase anaphylactic responses. Consistent with this, FcεRI stimulation of PLC-β3−/− mast cells exhibited substantially reduced cytokine production. In a mast cell-dependent airway inflammation model, PLC-β3−/− mice showed significantly reduced response. Therefore, PLC-β3 is indispensible for IgE/antigen-induced cytokine production in mast cells, and thus is important for mast cell-mediated late phase anaphylaxis and airway inflammation.
Mechanistically, reduced cytokine production in PLC-β3−/− mast cells could be accounted for by increased activity of the negative regulator Lyn (although Lyn initiates the FcεRI-mediated activation, Lyn plays a predominantly negative regulatory role under most activation conditions (Xiao et al. , 2005)) and reduced activities of the positive regulatory MAPKs. Furthermore, PLC-β3 constitutively interacts with Lyn and SHP-1, and these interactions were increased by FcεRI stimulation. SHP-1 likely recognizes its substrates Lyn and MAPKs via the recently described kinase tyrosine-based inhibitory motif, KTIM (Abu-Dayyeh et al. , 2010, Abu-Dayyeh et al. , 2008). Consistent with PLC-β3/SHP-1-mediated repression of Lyn kinase activity by dephosphorylation of Lyn-Tyr396 (in the activation loop of Lyn), FcεRI-mediated phenotypes were similar in PLC-β3−/− and SHP-1-mutant mast cells. Interestingly, PLC-β3 bound to FcεRI, suggesting that PLC-β3, along with Lyn and SHP-1, regulates early signaling events of mast cell activation. In contrast, PLC-β2 deficiency did not affect the FcεRI phenotype in mast cells. Thus, PLC-β3 plays a unique role in FcεRI signaling probably because of its ability to interact with Lyn, SHP-1, and FcεRI.
Conclusion
Ample evidence has been provided that PLC-βs are crucial players in immune regulation. Many examples indicate the involvement of GPCRs, the canonical receptors that use PLC-βs as their major nodes for signal transduction. Recent progresses in structural analysis of PLC-β3-Gαq complexes have provided insights into the molecular details of the process of signal relays (Lyon, Dutta, 2013, Lyon et al. , 2011, Waldo, Ricks, 2010). The interplay between GPCR-mediated signaling pathways and tyrosine kinase-based TCR and FcεRI signaling pathways converges at the levels of PLC-βs (or other lipid enzymes such as PI3K-γ). These synergistic cross-talks between different pathways probably have more pathophysiological significance than previously thought. Although we have an improved understanding of the molecular details of enzymatic regulation of PLC-βs, several lines of evidence suggest that more complicated signaling mechanisms are to be uncovered. The newly identified function of PLC-β3 CT domain and its resulting SPS multi-molecular signaling platform have shed new insights into the complexity of PLC-β molecules. Along this line, the CT domain was shown to important for subcellular localization of PLC-βs and interaction with Gaq or other proteins (Adjobo-Hermans, Crosby, 2013). Further study on the roles of PLC-βs in immune cells will be important to help us understand and combat immune-mediated diseases and malignancies.
Acknowledgement
The study in the Kawakami laboratory is funded in part by the National Institutes of Health and the MPN Foundation. This study is publication No. 1618 from the La Jolla Institute for Allergy and Immunology.
Abbreviations
- BCR
B cell receptor
- CT
C-terminal
- DAG
diacylglycerol
- dko
double knockout
- GAP
GTPase-activating protein
- GPCR
G-protein-coupled receptor
- HSC
hematopoietic stem cell
- IL
interleukin
- IP3
inositol 1,4,5-trisphosphate
- ITAM
immunoreceptor tyrosine-based activation motif
- NP
hydroxylnitrophenyl
- PG
prostaglandin
- PKC
protein kinase C
- PLC
phospholipase C
- SH2
Src homology 2
- TCR
T cell receptor
- TI
T cell-independent
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest in the preparation of this review.
References
- Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nature reviews Immunology. 2004;4:301–8. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nature reviews Immunology. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Dayyeh I, Ralph B, Grayfer L, Belosevic M, Cousineau B, Oliver M. Identification of key cytosolic kinases containing evolutionarily conserved kinase tyrosine-based inhibitory motifs (KTIMs). Developmental and Comparative Immunology. 2010;34:481–4. doi: 10.1016/j.dci.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Abu-Dayyeh I, Shio MT, Sato S, Akira S, Cousineau B, Olivier M. Leishmania-induced IRAK-1 inactivation is mediated by SHP-1 interacting with an evolutionarily conserved KTIM motif. PLoS Negl Trop Dis. 2008;2:e305. doi: 10.1371/journal.pntd.0000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjobo-Hermans MJ, Crosby KC, Putyrski M, Bhageloe A, van Weeren L, Schultz C, et al. PLCbeta isoforms differ in their subcellular location and their CT-domain dependent interaction with Galphaq. Cellular signalling. 2013;25:255–63. doi: 10.1016/j.cellsig.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nature immunology. 2004;5:943–52. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- Bach TL, Chen QM, Kerr WT, Wang Y, Lian L, Choi JK, et al. Phospholipase cbeta is critical for T cell chemotaxis. Journal of immunology. 2007;179:2223–7. doi: 10.4049/jimmunol.179.4.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M, Ryba NJ, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor gamma chain after receptor aggregation. The Journal of biological chemistry. 1993;268:23318–24. [PubMed] [Google Scholar]
- Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, et al. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Galpha11-dependent, PLC-beta-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Harris R, Gandarillas NL, Josephs MB, Roe SM, Sorli SC, et al. Structural and mechanistic insights into ras association domains of phospholipase C epsilon. Molecular cell. 2006;21:495–507. doi: 10.1016/j.molcel.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. PLC regulation: emerging pictures for molecular mechanisms. Trends in biochemical sciences. 2011;36:88–96. doi: 10.1016/j.tibs.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Calo LA, Bordin L, Davis PA, Pagnin E, Dal Maso L, Rossi GP, et al. PLCbeta1-SHP-2 complex, PLCbeta1 tyrosine dephosphorylation and SHP-2 phosphatase activity: a new part of Angiotensin II signaling? Journal of biomedical science. 2011;18:38. doi: 10.1186/1423-0127-18-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual review of immunology. 2005;23:127–59. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- Di Pietro R, Miscia S, Cataldi A, Rana R. Age-dependent variations in the expression of PLC isoforms upon mitogenic stimulation of peripheral blood T cells from healthy donors. British journal of haematology. 2000;111:1209–14. doi: 10.1046/j.1365-2141.2000.02492.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro R, Rana R. Age-related defect of phospholipase C activity, differential expression of the beta 2 isoform in active T lymphocytes from aged humans. Human immunology. 1998;59:25–8. doi: 10.1016/s0198-8859(97)00228-0. [DOI] [PubMed] [Google Scholar]
- Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–47. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nature medicine. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gresset A, Sondek J, Harden TK. The phospholipase C isozymes and their regulation. Sub-cellular biochemistry. 2012;58:61–94. doi: 10.1007/978-94-007-3012-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg S, Hasko G, Wu D, Leibovich SJ. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. The American journal of pathology. 2009;175:2439–53. doi: 10.2353/ajpath.2009.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Hicks SN, Sondek J. Phospholipase C isozymes as effectors of Ras superfamily GTPases. Journal of lipid research. 2009;50(Suppl):S243–8. doi: 10.1194/jlr.R800045-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden TK, Sondek J. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annual review of pharmacology and toxicology. 2006;46:355–79. doi: 10.1146/annurev.pharmtox.46.120604.141223. [DOI] [PubMed] [Google Scholar]
- Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. The Journal of allergy and clinical immunology. 2000;105:782–7. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE- dependent inflammation. PLoS One. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Goto S, Yamasaki M, Nagai H, Koda A. Studies on vascular permeability increasing factors involved in 48-hour homologous PCA in the mouse ear. Int Arch Allergy Appl Immunol. 1986;80:285–90. doi: 10.1159/000234067. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kuang Y, Wu Y, Xie W, Simon MI, Wu D. Roles of phospholipase C beta2 in chemoattractant-elicited responses. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7971–5. doi: 10.1073/pnas.94.15.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvin MH, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet JP. Differential control of the tyrosine kinases Lyn and Syk by the two signaling chains of the high affinity immunoglobulin E receptor. The Journal of biological chemistry. 1994;269:5918–25. [PubMed] [Google Scholar]
- Kadamur G, Ross EM. Mammalian phospholipase C. Annual review of physiology. 2013;75:127–54. doi: 10.1146/annurev-physiol-030212-183750. [DOI] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nature immunology. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Xiao W, Yasudo H, Kawakami Y. Regulation of proliferation, survival, differentiation, and activation by the Signaling Platform for SHP-1 phosphatase. Advances in biological regulation. 2012;52:7–15. doi: 10.1016/j.advenzreg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annual review of immunology. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- Klein RR, Bourdon DM, Costales CL, Wagner CD, White WL, Williams JD, et al. Direct activation of human phospholipase C by its well known inhibitor u73122. The Journal of biological chemistry. 2011;286:12407–16. doi: 10.1074/jbc.M110.191783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer KN, Clift IC, Miamen AG, Bamidele AO, Qian NX, Humphreys TD, et al. Stromal cell-derived factor-1 signaling via the CXCR4-TCR heterodimer requires phospholipase C-beta3 and phospholipase C-gamma1 for distinct cellular responses. Journal of immunology. 2011;187:1440–7. doi: 10.4049/jimmunol.1100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer KN, Humphreys TD, Kumar A, Qian NX, Hedin KE. Distinct role of ZAP-70 and Src homology 2 domain-containing leukocyte protein of 76 kDa in the prolonged activation of extracellular signal-regulated protein kinase by the stromal cell-derived factor-1 alpha/CXCL12 chemokine. Journal of immunology. 2003;171:360–7. doi: 10.4049/jimmunol.171.1.360. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Beaven MA, Ma HT, Kim MS, Metcalfe DD, Gilfillan AM. Synergistic activation of phospholipases Cgamma and Cbeta: a novel mechanism for PI3K-independent enhancement of FcepsilonRI-induced mast cell mediator release. Cellular signalling. 2008;20:625–36. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Humphreys TD, Kremer KN, Bramati PS, Bradfield L, Edgar CE, et al. CXCR4 physically associates with the T cell receptor to signal in T cells. Immunity. 2006;25:213–24. doi: 10.1016/j.immuni.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–51. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, et al. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106:1703–10. doi: 10.1182/blood-2004-12-4790. [DOI] [PubMed] [Google Scholar]
- Lowenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, et al. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO reports. 2006;7:1023–9. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AM, Dutta S, Boguth CA, Skiniotis G, Tesmer JJ. Full-length Galpha(q)- phospholipase C-beta3 structure reveals interfaces of the C-terminal coiled-coil domain. Nature structural & molecular biology. 2013;20:355–62. doi: 10.1038/nsmb.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AM, Tesmer VM, Dhamsania VD, Thal DM, Gutierrez J, Chowdhury S, et al. An autoinhibitory helix in the C-terminal region of phospholipase C-beta mediates Galphaq activation. Nature structural & molecular biology. 2011;18:999–1005. doi: 10.1038/nsmb.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Powers SE, Maienschein-Cline M, Bartom ET, Hamel KM, Kee BL, et al. Epigenetic repression of the Igk locus by STAT5-mediated recruitment of the histone methyltransferase Ezh2. Nature immunology. 2011;12:1212–20. doi: 10.1038/ni.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Okada Y, Kamatani Y, Takahashi A, Matsuda K, Hosono N, Ohmiya H, et al. Common variations in PSMD3-CSF3 and PLCB4 are associated with neutrophil count. Human molecular genetics. 2010;19:2079–85. doi: 10.1093/hmg/ddq080. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nature reviews Immunology. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual review of immunology. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Scharenberg AM, El-Hillal O, Fruman DA, Beitz LO, Li Z, Lin S, et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. Embo J. 1998;17:1961–72. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehaus S, Shahzad K, Kashif M, Vinnikov IA, Schiller M, Wang H, et al. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation. 2009;120:774–84. doi: 10.1161/CIRCULATIONAHA.109.849539. [DOI] [PubMed] [Google Scholar]
- Shirakawa AK, Liao F, Zhang HH, Hedrick MN, Singh SP, Wu D, et al. Pathway- selective suppression of chemokine receptor signaling in B cells by LPS through downregulation of PLC-beta2. Cellular & molecular immunology. 2010;7:428–39. doi: 10.1038/cmi.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, et al. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102:1959–65. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual review of immunology. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh PG, Park JI, Manzoli L, Cocco L, Peak JC, Katan M, et al. Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 2008;41:415–34. doi: 10.5483/bmbrep.2008.41.6.415. [DOI] [PubMed] [Google Scholar]
- Verhaar AP, Wildenberg ME, Duijvestein M, Vos AC, Peppelenbosch MP, Lowenberg M, et al. Superantigen-induced steroid resistance depends on activation of phospholipase cbeta2. Journal of immunology. 2013;190:6589–95. doi: 10.4049/jimmunol.1202898. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, et al. Kinetic scaffolding mediated by a phospholipase C-beta and Gq signaling complex. Science. 2010;330:974–80. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu B, Wang P, Dong X, Fernandez-Hernando C, Li Z, et al. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. The Journal of clinical investigation. 2008;118:195–204. doi: 10.1172/JCI33139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE- dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–53. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Ando T, Wang HY, Kawakami Y, Kawakami T. Lyn- and PLC-beta3-dependent regulation of SHP-1 phosphorylation controls Stat5 activity and myelomonocytic leukemia-like disease. Blood. 2010;116:6003–13. doi: 10.1182/blood-2010-05-283937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Hong H, Kawakami Y, Kato Y, Wu D, Yasudo H, et al. Tumor suppression by phospholipase C-beta3 via SHP-1-mediated dephosphorylation of Stat5. Cancer Cell. 2009;16:161–71. doi: 10.1016/j.ccr.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Kashiwakura J, Hong H, Yasudo H, Ando T, Maeda-Yamamoto M, et al. Phospholipase C-beta3 Regulates FcvarepsilonRI-Mediated Mast Cell Activation by Recruiting the Protein Phosphatase SHP-1. Immunity. 2011;34:893–904. doi: 10.1016/j.immuni.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, et al. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. Journal of immunology. 2005;175:6885–92. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–6. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- Yasudo H, Ando T, Xiao W, Kawakami Y, Kawakami T. Short Stat5-interacting peptide derived from phospholipase C-beta3 inhibits hematopoietic cell proliferation and myeloid differentiation. PloS one. 2011;6:e24995. doi: 10.1371/journal.pone.0024995. [DOI] [PMC free article] [PubMed] [Google Scholar]