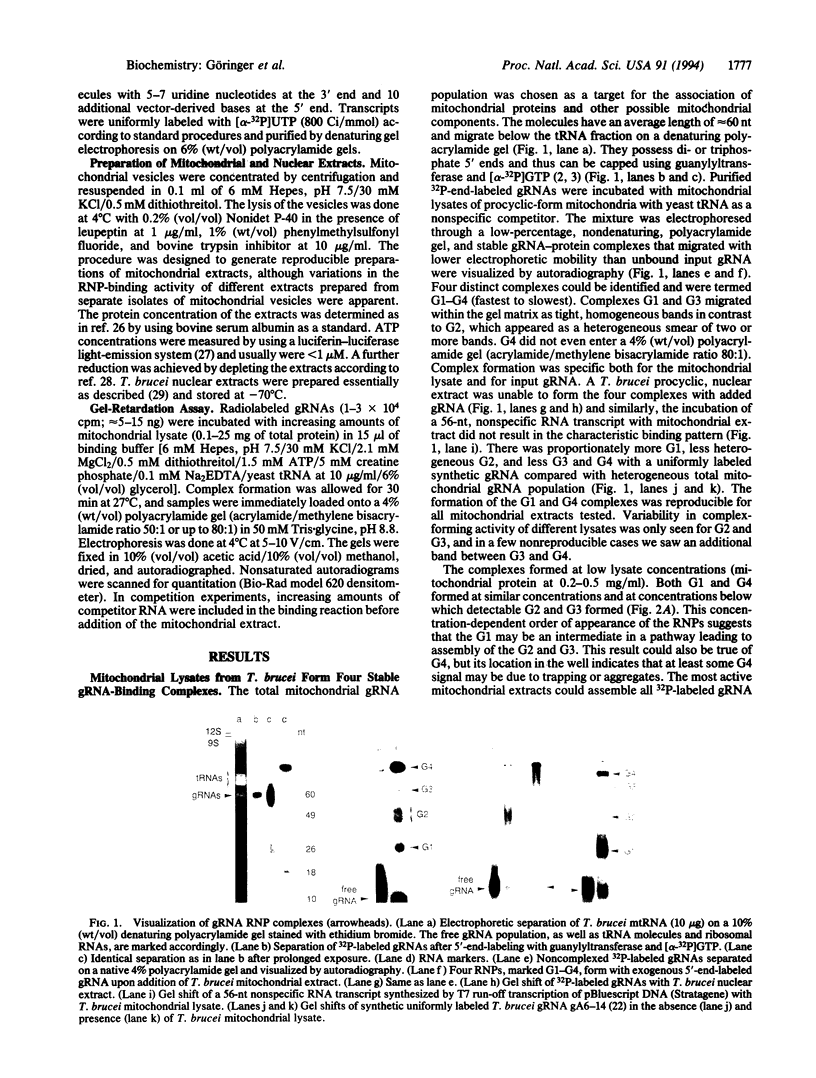
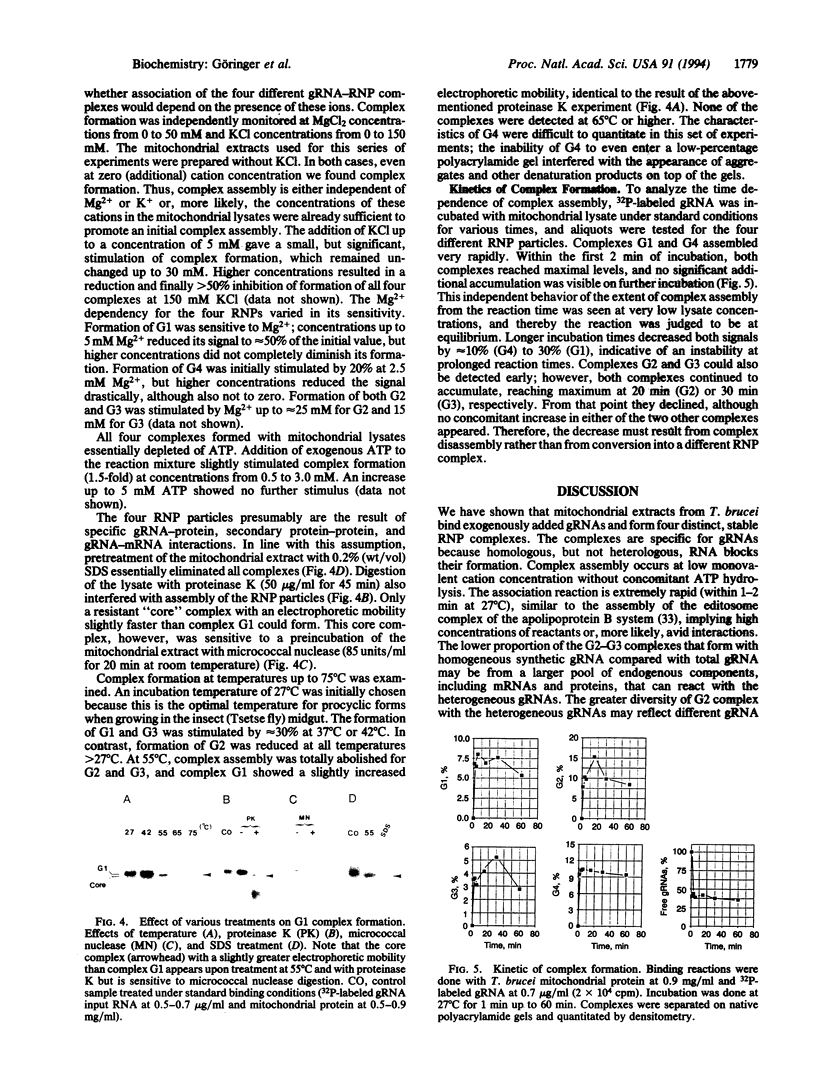
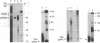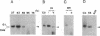Abstract
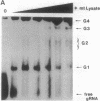
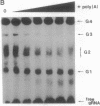
Transcripts from mitochondrial (maxicircle) genes of kinetoplastid organisms undergo RNA editing characterized by a series of reactions that insert and delete uridine nucleotides within the sequence of the pre-mRNAs. Guide RNAs, which complement fully edited mRNAs, provide the information for the edited sequence by an unknown mechanism. We report here that guide RNA molecules associate with other mitochondrial components to form four specific, stable ribonucleoprotein complexes. The complexes form very rapidly at a low monovalent cation concentration, and their formation is blocked by heparin or pretreatment of the mitochondrial lysate with SDS. ATP hydrolysis is not required but slightly stimulates complex association up to concentrations of 5 mM. The results are suggestive of a sequential assembly of the ribonucleoprotein complexes, and their possible involvement during the kinetoplastid RNA editing is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alden C. J., Kim S. H. Solvent-accessible surfaces of nucleic acids. J Mol Biol. 1979 Aug 15;132(3):411–434. doi: 10.1016/0022-2836(79)90268-7. [DOI] [PubMed] [Google Scholar]
- Backus J. W., Smith H. C. Apolipoprotein B mRNA sequences 3' of the editing site are necessary and sufficient for editing and editosome assembly. Nucleic Acids Res. 1991 Dec 25;19(24):6781–6786. doi: 10.1093/nar/19.24.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R. RNA-editing in trypanosome mitochondria. Biochim Biophys Acta. 1989 Mar 1;1007(2):131–139. doi: 10.1016/0167-4781(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Black D. L., Pinto A. L. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989 Aug;9(8):3350–3359. doi: 10.1128/mcb.9.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum B., Bakalara N., Simpson L. A model for RNA editing in kinetoplastid mitochondria: "guide" RNA molecules transcribed from maxicircle DNA provide the edited information. Cell. 1990 Jan 26;60(2):189–198. doi: 10.1016/0092-8674(90)90735-w. [DOI] [PubMed] [Google Scholar]
- Blum B., Simpson L. Guide RNAs in kinetoplastid mitochondria have a nonencoded 3' oligo(U) tail involved in recognition of the preedited region. Cell. 1990 Jul 27;62(2):391–397. doi: 10.1016/0092-8674(90)90375-o. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Cech T. R. RNA editing: world's smallest introns? Cell. 1991 Feb 22;64(4):667–669. doi: 10.1016/0092-8674(91)90494-j. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B. Ras C-terminal processing enzymes--new drug targets? Cell. 1991 Apr 5;65(1):1–4. doi: 10.1016/0092-8674(91)90352-y. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Harris M. E., Hajduk S. L. Kinetoplastid RNA editing: in vitro formation of cytochrome b gRNA-mRNA chimeras from synthetic substrate RNAs. Cell. 1992 Mar 20;68(6):1091–1099. doi: 10.1016/0092-8674(92)90080-v. [DOI] [PubMed] [Google Scholar]
- Harris M. E., Moore D. R., Hajduk S. L. Addition of uridines to edited RNAs in trypanosome mitochondria occurs independently of transcription. J Biol Chem. 1990 Jul 5;265(19):11368–11376. [PubMed] [Google Scholar]
- Harris S. G., Sabio I., Mayer E., Steinberg M. F., Backus J. W., Sparks J. D., Sparks C. E., Smith H. C. Extract-specific heterogeneity in high-order complexes containing apolipoprotein B mRNA editing activity and RNA-binding proteins. J Biol Chem. 1993 Apr 5;268(10):7382–7392. [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNAs. Cell. 1986 Sep 12;46(6):845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- Koslowsky D. J., Göringer H. U., Morales T. H., Stuart K. In vitro guide RNA/mRNA chimaera formation in Trypanosoma brucei RNA editing. Nature. 1992 Apr 30;356(6372):807–809. doi: 10.1038/356807a0. [DOI] [PubMed] [Google Scholar]
- Koslowsky D. J., Riley G. R., Feagin J. E., Stuart K. Guide RNAs for transcripts with developmentally regulated RNA editing are present in both life cycle stages of Trypanosoma brucei. Mol Cell Biol. 1992 May;12(5):2043–2049. doi: 10.1128/mcb.12.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard V. W., Hajduk S. L. Trypanosoma equiperdum minicircles encode three distinct primary transcripts which exhibit guide RNA characteristics. Mol Cell Biol. 1991 Mar;11(3):1668–1675. doi: 10.1128/mcb.11.3.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard V. W., Rohrer S. P., Michelotti E. F., Hancock K., Hajduk S. L. Organization of minicircle genes for guide RNAs in Trypanosoma brucei. Cell. 1990 Nov 16;63(4):783–790. doi: 10.1016/0092-8674(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Reyes V. M., Abelson J. N. In vitro synthesis of end-mature, intron-containing transfer RNAs. Methods Enzymol. 1989;180:63–69. doi: 10.1016/0076-6879(89)80092-8. [DOI] [PubMed] [Google Scholar]
- Romaniuk P. J. The role of highly conserved single-stranded nucleotides of Xenopus 5S RNA in the binding of transcription factor IIIA. Biochemistry. 1989 Feb 7;28(3):1388–1395. doi: 10.1021/bi00429a067. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Doxsey S. J. Purification of nuclei from a flagellate protozoan, Trypanosoma brucei. Anal Biochem. 1982 Nov 15;127(1):112–115. doi: 10.1016/0003-2697(82)90152-x. [DOI] [PubMed] [Google Scholar]
- Simpson L. RNA editing--a novel genetic phenomenon? Science. 1990 Oct 26;250(4980):512–513. doi: 10.1126/science.1700474. [DOI] [PubMed] [Google Scholar]
- Smith H. C., Kuo S. R., Backus J. W., Harris S. G., Sparks C. E., Sparks J. D. In vitro apolipoprotein B mRNA editing: identification of a 27S editing complex. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1489–1493. doi: 10.1073/pnas.88.4.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart K., Gobright E., Jenni L., Milhausen M., Thomashow L., Agabian N. The IsTaR 1 serodeme of Trypanosoma brucei: development of a new serodeme. J Parasitol. 1984 Oct;70(5):747–754. [PubMed] [Google Scholar]
- Stuart K. RNA editing in trypanosomatid mitochondria. Annu Rev Microbiol. 1991;45:327–344. doi: 10.1146/annurev.mi.45.100191.001551. [DOI] [PubMed] [Google Scholar]
- Stuart K. RNA editing: new insights into the storage and expression of genetic information. Parasitol Today. 1989 Jan;5(1):5–8. doi: 10.1016/0169-4758(89)90211-1. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Simpson L. Kinetoplast DNA minicircles encode guide RNAs for editing of cytochrome oxidase subunit III mRNA. Cell. 1990 Jun 1;61(5):879–884. doi: 10.1016/0092-8674(90)90198-n. [DOI] [PubMed] [Google Scholar]