Abstract
Background
Bullous pemphigoid (BP) is an autoimmune blistering disease that is associated with an increased mortality rate.
Objective
To determine the incidence and mortality rate of patients with bullous pemphigoid.
Methods
Eighty-seven residents of Olmsted County, Minnesota, were identified who had their first lifetime diagnosis of BP from January 1960 – December 2009. Incidence and mortality rate were compared to age- and sex-matched control patients from the same geographic area.
Results
The adjusted incidence of BP was 2.4 per 100,000 person-years (95% CI, 1.9–2.9). Incidence of BP increased significantly with age (P<.001) and over time (P=0.034). Trend tests indicate increased diagnosis of localized disease (P=.006) may be a contributing factor. Survival observed in the incident BP cohort was significantly poorer than expected (P<.001). Survival was not different among patients with multisite vs localized disease (P=.90).
Limitations
Retrospective study design and study population from a small geographic area.
Conclusion
Incidence of BP in the United States is comparable to that found in Europe and Asia. The mortality rate of BP is lower in the United States than Europe, but higher than previous estimates.
Keywords: Bullous pemphigoid, autoimmune blistering disorder, epidemiology, incidence, mortality, geriatric
Introduction
Bullous pemphigoid (BP) is the most common autoimmune blistering dermatosis1 and has increased prevalence in the elderly population.2 Most large population studies of BP incidence and mortality rate have been performed in Europe,3 but there is a paucity of comparable data from the United States.4–6
The incidence of BP appears to be increasing, with previous values approximating 6 or 7 cases per 1 million persons per year in Europe;7,8 vs more recent numbers ranging from 10 to 43 cases per 1 million persons per year9–11. A French cohort of nearly 4 million persons, showed 21.7 cases per 1 million persons per year—a 3-fold increase in incidence during the past 15 years.1,3 No analogous US studies have assessed BP incidence.
Multiple case series have shown increased mortality rates in patients with BP compared with an age-, sex- and location-matched population, beginning with early series, such as Savin12,13 and Roujeau et al,14 but controversy continues over whether this is a true association with the disease or is due to multiple confounding factors associated with an aging population (eg, medical comorbidities, infection, hospitalization, exposure to certain medications).
Previous US data have showed lower mortality rates associated with BP in the United States than in Europe. The largest US-based series,5 which evaluated 223 patients, did not find an increased mortality rate in BP patients. However, the control group was based solely on age-matched US population control subjects, whose characteristics may have differed substantially from the regional study cohort.16 Another series used International Classification of Diseases (ICD)-9 and ICD-10 codes as listed on death certificates from a publicly available national database but did not verify diagnosis through a case or chart review.6
The purpose of the present study was to determine the age-stratified incidence of BP in Olmsted County, Minnesota, from 1960 through 2009 and to compare survival of these patients with that of an analogous age- and sex-matched population in Minnesota.
Materials and Methods
This study was approved by the institutional review boards of Olmsted Medical Center and Mayo Clinic and Declaration of Helsinki protocols were followed. We used the Rochester Epidemiology Project, a centralized, medical records linkage system containing medical diagnoses for nearly all patients in Olmsted County. This population has been shown to be demographically similar to the general US white population.45–47 It also has a relatively low emigration rate,45 which facilitates acquisition of complete patient follow-up data.
Through the Rochester Epidemiology Project, all medical records for all patients receiving a first-ever diagnosis of BP (based on search with terms “bullous pemphigoid” and “pemphigoid”) between January 1, 1960, and December 31, 2009, were identified and retrieved. Diagnosis of BP was confirmed based on combined review of the clinical presentation and laboratory evidence, including any of the following: 1) histopathologic findings, 2) direct immunofluorescence study showing linear deposition of antibody or complement (ie, immunoglobulin G [IgG] or C3, or both), 3) indirect immunofluorescence detecting circulating IgG antibodies against basement membrane proteins, or 4) positive BP180 or BP230 IgG antibody measured with enzyme-linked immunosorbent assay (ELISA). Cases in which the clinical presentation was atypical (eg, urticarial, erosion, crust/scale) were included when there was sufficient supporting objective laboratory data to support BP. Cases of cicatricial pemphigoid were excluded. Patients with oral disease or predominant oral disease only were excluded to avoid including bullous diseases caused by different auto-antibodies.
Incidence rates were obtained by considering incident cases of BP as the numerator and age- and sex-specific population counts from Olmsted County, Minnesota, as the denominator. Population counts for 1960 through 2000 were estimated using census data from 1960, 1970, 1980, 1990, and 2000, with linear interpolation for intercensal years. The populations at risk for 2001 through 2009 were obtained from US Intercensal Estimates.48 Because nearly all of the population of Olmsted County is white, incidence rates were directly age- and sex-adjusted to the structure of the US white population in the year 2000. Incident cases were grouped into intervals on the basis of age at diagnosis (0–39, 40–49, 50–59, 60–69, 70–79, 80–89, and ≥90 years) and the year of diagnosis (1960–1969, 1970–1979, 1980–1989, 1990–1999, and 2000–2009). The relations of age at diagnosis, sex, and year of diagnosis with the incidence of BP were assessed by fitting Poisson regression models (GENMOD procedure; SAS Institute Inc).
Overall survival was estimated with the Kaplan-Meier method and compared among groups through log-rank tests. The duration of follow-up was calculated from the date of diagnosis to the date of death or last follow-up. Overall survival was compared with the survival expected in the Minnesota white population on the basis of age at diagnosis, sex, and year of diagnosis with the cohort method.49
Localized disease was characterized as single site (eg, scalp, neck, limbs, chest). Generalized disease was characterized as involvement at more than 1 site. Trends in the diagnosis of localized disease and atypical clinical presentations over time were evaluated using Cochran-Armitage trend tests.
Statistical analyses were performed with a software package (SAS Institute Inc). All tests were 2-sided, and P values less than .05 were considered statistically significant.
Results
BP Incidence
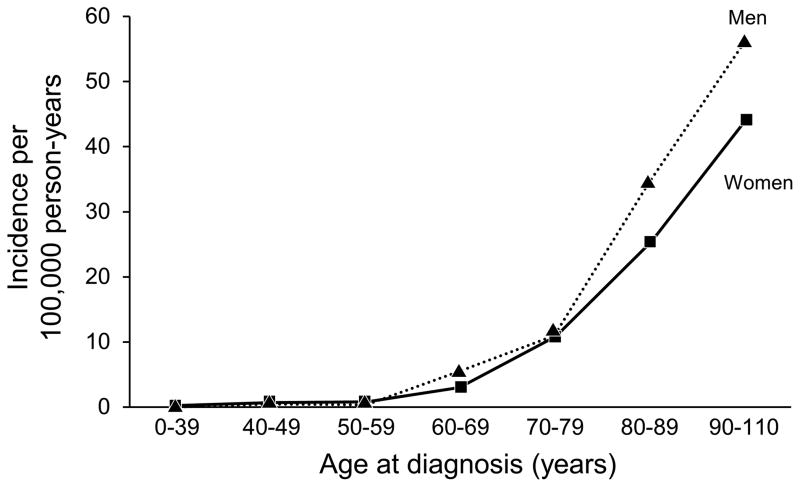
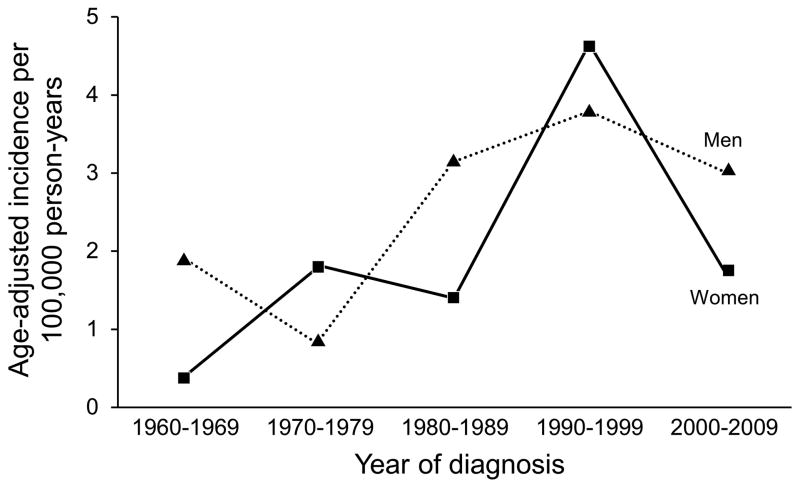
Characteristics collected from the 87 incident cases of BP are summarized in Table 1. The mean age at diagnosis was 74.5 years. The age- and sex-adjusted incidence of BP was 2.4 per 100,000 person-years (95% CI 1.9–2.9). The age-adjusted incidence was 2.2 per 100,000 person-years (95% CI, 1.6–2.8) for women compared with 2.8 per 100,000 person-years (95% CI, 1.8–3.7) for men (P=.25). The incidence of BP increased significantly with age at diagnosis (P<.001) (Figure 1) and over time (P=.034) (Table 2 and Figure 2). There was no statistically significant evidence that the increase in incidence over time differed between men and women (P=.93) or by age (P=.47). There was not a statistically significant difference in age at diagnosis among the time periods of the study (p=0.82). A trend test for localized vs generalized disease by diagnosis year indicated that the diagnosis of localized disease became more common over time (P=.006). In looking at bullous presentation vs atypical clinical presentations, a trend test was not significant for nonbullous presentations being diagnosed more frequently over time (P=.13).
Table 1.
Summary of 87 Incident Cases of Bullous Pemphigoid
| Characteristics | Value | No. of Patients |
|---|---|---|
| Age at diagnosis, y | ||
| Mean (SD) | 74.5 (12.2) | |
| Median (range) | 79 (41–100) | |
| Time from symptom onset to diagnosis, mo (n=86) | ||
| Mean (SD) | 7.2 (14.8) | |
| Median (range) | 2 (0–76) | |
| Weight, kg (n=86) | ||
| Mean (SD) | 75.0 (15.6) | |
| Median (range) | 73 (48–135) | |
| Age at diagnosis, y | ||
| 0–39 | 0 | |
| 40–49 | 3 | |
| 50–59 | 3 | |
| 60–69 | 14 | |
| 70–79 | 24 | |
| 80–89 | 32 | |
| ≥90 | 11 | |
| Sex | ||
| Female | 50 | |
| Male | 37 | |
| Year of diagnosis | ||
| 1960–1969 | 4 | |
| 1970–1979 | 8 | |
| 1980–1989 | 13 | |
| 1990–1999 | 36 | |
| 2000–2009 | 26 | |
| Race/ethnicity (n=84) | ||
| White | 78 | |
| African American | 4 | |
| Other | 2 | |
| Residency at diagnosis | ||
| Rochester | 84 | |
| Balance of Olmsted County | 3 | |
| Clinical department that made diagnosis (n=85) | ||
| Dermatology | 82 | |
| General medicine | 2 | |
| Other | 1 | |
| Referral to dermatology service (n=65) | ||
| No | 11 | |
| Yes | 54 | |
| Method of diagnosis | ||
| Histology + direct IM + indirect IM | 42 | |
| Histology + direct IM | 29 | |
| Direct IM | 4 | |
| Histology | 3 | |
| Histology + indirect IM | 3 | |
| Histology + direct IM + indirect IM + BP180/BP230 | 2 | |
| Indirect IM | 1 | |
| Direct IM + indirect IM | 1 | |
| Indirect IM + BP180/BP230 | 1 | |
| Histology + direct IM + BP180/BP230 | 1 | |
| Direct IM (n=83) | ||
| Positive | 79 | |
| Negative | 4 | |
| Indirect IM (n=57) | ||
| Positive | 57 | |
| Negative | 0 | |
| Exposure to therapy (n=86) | ||
| Taking medications, but none listed previously | 58 | |
| Furosemide | 10 | |
| Penicillin | 8 | |
| Captopril | 3 | |
| Sulfa | 3 | |
| None | 2 | |
| UV radiation | 2 | |
| Initial extent of disease (n=85) | ||
| Limbs | 74 | |
| Flexure | 58 | |
| Chest | 48 | |
| Back | 35 | |
| Neck | 22 | |
| Scalp | 9 | |
| Face | 9 | |
| Genitals | 6 | |
| Oral cavity | 5 | |
| Initial extent of disease (n=85) | ||
| Localized | 12 | |
| Generalized | 73 | |
| Predominant appearance of lesions | ||
| Blistered and denuded | 76 | |
| Urticarial | 6 | |
| Erosion | 4 | |
| Crusted or scaly | 1 | |
Figure 1.
Bullous Pemphigoid. Incidence of BP Showing Significant Increases With Age at Diagnosis.
Table 2.
Incidence of Bullous Pemphigoid in Olmsted County, Minnesota, 1960–2009
| Year of Diagnosis | Female Patients
|
Male Patients
|
Total Patients
|
||||
|---|---|---|---|---|---|---|---|
| No. | Ratea | No. | Ratea | No. | Rateb | 95% CI | |
| 1960–1969 | 1 | 0.4 | 3 | 1.9 | 4 | 0.9 | 0.0–1.8 |
| 1970–1979 | 6 | 1.8 | 2 | 0.8 | 8 | 1.6 | 0.5–2.6 |
| 1980–1989 | 6 | 1.4 | 7 | 3.1 | 13 | 2.0 | 0.9–3.2 |
| 1990–1999 | 25 | 4.6 | 11 | 3.8 | 36 | 4.2 | 2.8–5.6 |
| 2000–2009 | 12 | 1.8 | 14 | 3.0 | 26 | 2.2 | 1.4–3.1 |
Incidence per 100,000 person-years, age-adjusted to US white population in the year 2000.
Incidence per 100,000 person-years, age- and sex-adjusted to US white population in the year 2000.
Figure 2.
Bullous Pemphigoid. Incidence of BP Showing Significant Increase Over Time.
Mortality Data
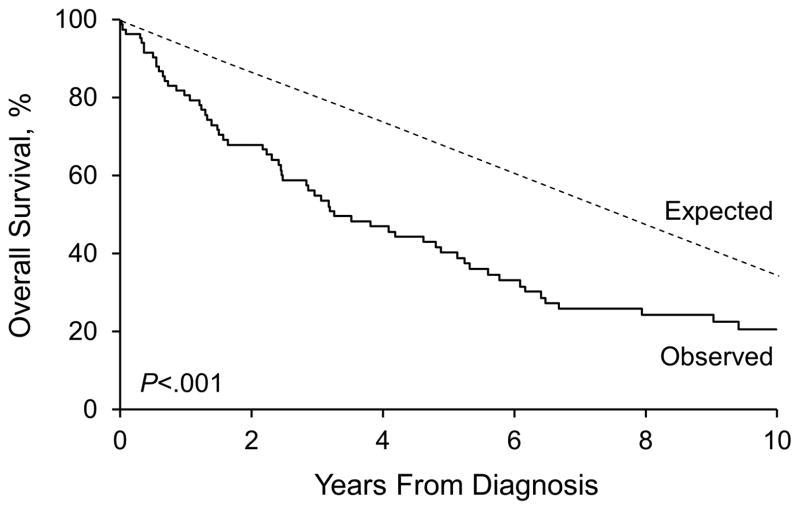
At last follow-up, 66 patients had died, at a mean of 4.5 years after BP diagnosis (median [range], 2.6 years [6 days–37 years]). The mean duration of follow-up for the 21 patients who were still alive at last follow-up was 6.7 years (median [range], 5.0 years [1 month-21 years]). Of the 21 patients who were still alive at last follow-up, 6 had fewer than 1 year of follow-up. Estimated overall survival rates (95% CI; number still at risk) at 1, 2, 4, 6, 8, and 10 years after the diagnosis were 81% (73%–90%; 65), 68% (59%–79%; 53), 47% (37%–60%; 36), 33% (24%–46%; 23), 25% (16%–37%; 16), and 21% (13%–33%; 11), respectively. By comparison, survival rates at these time points expected in the Minnesota white population were 92%, 84%, 71%, 58%, 48%, and 39%, respectively. The survival observed in the incident BP cohort was significantly poorer than expected (P<.001) (Figure 3). Given the same distributions of age and sex, about 35 deaths would have been expected in the Minnesota white population, resulting in a standardized mortality ratio of 1.90 (95% CI, 1.47–2.42).
Figure 3.
Bullous Pemphigoid. Survival in the Incident BP Cohort With Significantly Poorer Than Expected Results.
Estimated overall survival rates (95% CI; number still at risk) at 1, 2, 4, 6, and 8 years after diagnosis for the 12 patients with localized disease were 91% (75%–100%; 8), 80% (58%–100%; 6), 48% (22%–100%; 3), 32% (10%–98%; 2), and 32% (10%–98%; 2), respectively. Estimated overall survival rates (95% CI; number still at risk) at 1, 2, 4, 6, 8, and 10 years after diagnosis for the 73 patients with generalized disease were 79% (70%–89%; 55), 67% (57%–79%; 46), 47% (36%–60%; 32), 33% (24%–46%; 21), 23% (15%–36%; 14), and 22% (14%–35%; 11), respectively. There was not a statistically significant difference in overall survival among patients with localized vs those with generalized disease (P=.90).
Estimated overall survival rates (95% CI; number still at risk) at 1 year following diagnosis were 75% (43%–100%; 3), 75% (50%–100%; 5), 85% (67%–100%; 11), 78% (65%–93%; 28), and 87% (73%–100%; 18) for patients who had the diagnosis in 1960 through 1969, 1970 through 1979, 1980 through 1989, 1990 to 1999, and 2000 through 2009, respectively (P=.77).
Two patient were missing treatment information; of the other patients, 62 (73%) were taking systemic immunosuppressive agents.
Discussion
BP Incidence
Whereas the incidence of BP appears to be increasing in Europe,1,3,17,18 there are no prior incidence studies reported in the United States. Our data show the incidence of BP at 2.4 cases per 100,000 person-years, which is on par with or higher than most of the recent European reports. It has been proposed by other investigators that the increased BP incidence is attributable to a greater proportion of older persons in the general population.3,11 We found the age-adjusted incidence increased over time across all age-groups, arguing against the hypothesis that the increasing proportion of elderly persons is the sole reason for increased incidence of BP.
Another proposed explanation for the increase in BP incidence is the concomitant increase in the prevalence of neurodegenerative disorders,19 because many reports have implicated disorders such as dementia, stroke, and Parkinson disease as risk factors for BP development.20,21 It is conceivable that increased use of medications such as diuretics and neuroleptics, which are often implicated as triggers for BP, could also contribute, although this hypothesis was not evaluated in this study. In addition, many other autoimmune diseases have increased in incidence in recent decades, such as rheumatoid arthritis in the same Olmsted County population,22 diabetes mellitus type 1,23 and myasthenia gravis.24
Rarer forms of BP, such as pemphigoid nodularis, eczema-type, dyshidrosiform-type, and others, comprised 21% of the cases reported by Joly et al.3 In the present study, all 6 patients without classic clinical findings of BP received the diagnosis in the 1990s. The increased incidence seems less likely due to increased sensitivity of laboratory testing.11,25 Only 4 patients in this study had ELISA performed, so our findings are not likely attributable to enhanced detection through ELISA methods.26 Moreover, it may be debated whether diagnostic sensitivity with NC16A-directed BP180 ELISA testing is superior to clinical criteria plus direct immunofluorescence or indirect immunofluorescence, or both, in some cases.27,28
Some authors have studied the rate of diagnosis of BP based on tissue specimen diagnosis as a proxy for clinical diagnosis and observed no change in incidence over time.29 Although that study design was a simple way to address the question of BP incidence, it is unclear whether tissue specimen diagnosis correlates directly with clinical disease incidence.
There are several limitations to this study. The study population is from a small geographic area that is predominantly white and may not be generalizable. Given the long time frame of the study, knowledge and recognition of BP and treatment regimens have changed over time. Increasing awareness of the disease entity and therefore increased diagnosis may be contributing to the increasing incidence.
Mortality Rate
Our data showed a 19% 1-year mortality rate for patients with BP during the previous 50 years, which straddles previous reports from Europe (13%–41%) and the United States (11%–23%). Table 3 summarizes findings from large mortality studies from the past 40 years. It is notable that the most recent mortality figures from France3 are nearly double that observed in our study. As hypothesized previously, older age at diagnosis (74.5 years in our series vs 82 years) and poorer general medical condition may be to blame for the greater mortality rate reported in Europe.3 This hypothesis is supported by Rzany et al,30 who found that increased age (average of 80 years), greater dosage of oral glucocorticoids at hospital discharge, and low serum albumin level as a proxy measure for overall medical condition31 were associated with a significantly higher fatality rate within the first year following hospitalization. Some authors have also asserted that patient selection bias has led to the differences in reported mortality rates32 and cite also the lack of age- and comorbidity-matched control subjects as limitations to estimating actual disease-specific mortality rate.33 Selection bias inherent in studies examining patients at major tertiary referral centers was averted in our study with the use of a population-based study design.4
Table 3.
Compilation of Large Prospective and Retrospective Studies on Mortality Rate in Bullous Pemphigoid
| Authors of Study (Year) | Location | No. of Patients | Average Age, y | 1-y Mortality Rate, % | Oral Corticosteroids, % of Patients |
|---|---|---|---|---|---|
| Europe and Asia | |||||
| Venning and Wojnarowska,38 1992 | United Kingdom | 82 | 74 | 19 | 70 |
| Bernard et al,1 1995 | France | 78 | 80 | 38 | NA |
| Roujeau et al,14 1998 | France | 217 | 79 | 41 | 79 |
| Joly et al,15 2002 | France | 341 | 81 | 30 | 51 |
| Rzany et al,30 2002 | Germany | 369 | 77 | 29 | 86 |
| Garcia-Doval et al,35 2005 | Spain | 26 | 77 | 40 | 54 |
| Gudi et al,18 2005 | Scotland | 83 | 79 | 25 | NA |
| Joly et al,34 2005 | France | 170 | 83 | 26 | 0 |
| Cortés et al,39 2011 | Switzerland | 115 | 80 | 21 | 66 |
| Joly et al,3 2012 | France | 312 | 82 | 38 | NA |
| Cortés et al,40 2012 | Switzerland | 60 | 80 | 27 | 89 |
| Gual et al,41 2012 | Spain | 101 | 78 | 13 | 77a |
| Zhang et al,42 2013 | China | 94 | NA | 23 | 85 |
| Li et al,43 2013 | China | 140 | 64 | 13 | 72 |
| United States | |||||
| Fivenson et al,44 1994 | Cincinnati, Ohio, Detroit, Michigan | 18 | 78 | 12 | NA |
| Colbert et al,4 2004 | Milwaukee, Wisconsin | 37 | 77 | 11 | 76 |
| Parker et al,5 2008 | United States | 223 | 75 | 23 | 19b |
| Brick et al (present study) | Olmsted County, Minnesota | 87 | 75 | 19 | 58c |
Abbreviation: NA, not available.
Of patients who died during the study, 77% (10/13) were receiving immunosuppressants.
Among patients, 19% took systemic corticosteroids alone; 60% took corticosteroid-sparing agents, but it is unclear which of these patients were also taking corticosteroids.
All 62 patients taking a systemic immunosuppressant medication were included in the data analysis.
In previous studies, no factors directly related to BP, such as extent of lesions, were found to affect overall survival. Actual survival predictors were related more to underlying demographic characteristics, including older age or female sex, and associated medical conditions, such as cardiac insufficiency, history of stroke, and dementia, along with a low Karnofsky performance score.34 We also did not find a statistically significant difference in overall survival among patients with localized disease vs those with generalized disease. However, it is noted that those patients with generalized disease had a higher 1-year mortality rate.
Death due to sepsis in a more frequently hospitalized, immunosuppressed elderly population has also been proposed as a reason for the increased mortality rate in Europe.4 This reason was refuted by a population-based study in Spain, in which only 2 of 11 patients died of sepsis during the study period and neither death was within 6 weeks of initial hospitalization for BP.35 In addition, there was no difference in the length of hospitalization for the 11 patients who died, making it unlikely that these parameters could account for greater mortality rates in all settings36 —although this may have been the case in other European series.15 However, data on patients from the same Olmsted County population as the present study have showed that death due to sepsis was significantly more likely to occur in patients with BP than with matched control subjects.37
Increased mortality rate due to oral corticosteroid use at dosages greater than 0.5 mg/kg per day (and concomitant longer hospital stays) in comparison with topical corticosteroid use has also been reported.7,15 We were not able to compare mortality rates in patient on immunosuppressive medications versus other treatments in the current study. The present population-based, longitudinal study provides evidence for the reported increased incidence of BP over time. Although explanation for the increased incidence is not readily identified, our findings raise the possibility that increased diagnosis of localized disease over time may have a role. We found that extent of disease did not contribute to differences in overall survival.
Acknowledgments
Funding: This study was made possible by the Rochester Epidemiology Project (Grant Number R01 AG034676 from the National Institute on Aging).
Abbreviations
- BP
bullous pemphigoid
- ELISA
enzyme-linked immunosorbent assay
- ICD
International Classification of Diseases
- IgG
immunoglobulin G
Footnotes
Conflict of Interest: The authors state no conflict of interest.
References
- 1.Bernard P, Vaillant L, Labeille B, et al. Bullous Diseases French Study Group. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Arch Dermatol. 1995;131:48–52. [PubMed] [Google Scholar]
- 2.Jung M, Kippes W, Messer G, et al. Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol. 1999;41(2 Pt 1):266–8. doi: 10.1016/s0190-9622(99)70061-7. [DOI] [PubMed] [Google Scholar]
- 3.Joly P, Baricault S, Sparsa A, et al. Incidence and mortality of bullous pemphigoid in France. J Invest Dermatol. 2012;132:1998–2004. doi: 10.1038/jid.2012.35. Epub 2012 Mar 15. [DOI] [PubMed] [Google Scholar]
- 4.Colbert RL, Allen DM, Eastwood D, et al. Mortality rate of bullous pemphigoid in a US medical center. J Invest Dermatol. 2004;122:1091–5. doi: 10.1111/j.0022-202X.2004.22504.x. [DOI] [PubMed] [Google Scholar]
- 5.Parker SR, Dyson S, Brisman S, et al. Mortality of bullous pemphigoid: an evaluation of 223 patients and comparison with the mortality in the general population in the United States. J Am Acad Dermatol. 2008;59:582–8. doi: 10.1016/j.jaad.2008.07.022. Epub 2008 Aug 15. [DOI] [PubMed] [Google Scholar]
- 6.Risser J, Lewis K, Weinstock MA. Mortality of bullous skin disorders from 1979 through 2002 in the United States. Arch Dermatol. 2009;45:1005–8. doi: 10.1001/archdermatol.2009.205. [DOI] [PubMed] [Google Scholar]
- 7.Bernard P, Charneux J. Bullous pemphigoid: a review [French] Ann Dermatol Venereol. 2011;138:173–81. doi: 10.1016/j.annder.2011.01.004. Epub 2011 Feb 16. [DOI] [PubMed] [Google Scholar]
- 8.Zillikens D, Wever S, Roth A, et al. Incidence of autoimmune subepidermal blistering dermatoses in a region of central Germany. Arch Dermatol. 1995;131:957–8. doi: 10.1001/archderm.131.8.957. [DOI] [PubMed] [Google Scholar]
- 9.Cozzani E, Parodi A, Rebora A, et al. Gruppo Ligure di Studi in Dermatologia (GLISID) Bullous pemphigoid in Liguria: a 2-year survey. J Eur Acad Dermatol Venereol. 2001;15:317–9. [PubMed] [Google Scholar]
- 10.Langan SM, Smeeth L, Hubbard R, et al. Bullous pemphigoid and pemphigus vulgaris: incidence and mortality in the UK: population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertram F, Brocker EB, Zillikens D, et al. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7:434–40. doi: 10.1111/j.1610-0387.2008.06976.x. Epub 2009 Jan 19. [DOI] [PubMed] [Google Scholar]
- 12.Savin JA. Some factors affecting prognosis in pemphigus vulgaris and pemphigoid. Br J Dermatol. 1981;104:415–20. doi: 10.1111/j.1365-2133.1981.tb15311.x. [DOI] [PubMed] [Google Scholar]
- 13.Savin JA. Death in bullous pemphigoid. Clin Dermatol. 1987;5:52–9. doi: 10.1016/0738-081x(87)90049-6. [DOI] [PubMed] [Google Scholar]
- 14.Roujeau JC, Lok C, Bastuji-Garin S, et al. High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol. 1998;134:465–9. doi: 10.1001/archderm.134.4.465. [DOI] [PubMed] [Google Scholar]
- 15.Joly P, Roujeau JC, Benichou J, et al. Bullous Diseases French Study Group . A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321–7. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 16.Hogan DJ. Mortality of bullous pemphigoid. J Am Acad Dermatol. 2009;60:704. doi: 10.1016/j.jaad.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Grattan CE. Evidence of an association between bullous pemphigoid and psoriasis. Br J Dermatol. 1985;113:281–3. doi: 10.1111/j.1365-2133.1985.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 18.Gudi VS, White MI, Cruickshank N, et al. Annual incidence and mortality of bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol. 2005;153:424–7. doi: 10.1111/j.1365-2133.2005.06662.x. [DOI] [PubMed] [Google Scholar]
- 19.Kukull WA, Ganguli M. Epidemiology of dementia: concepts and overview. Neurol Clin. 2000;18:923–50. doi: 10.1016/s0733-8619(05)70233-4. [DOI] [PubMed] [Google Scholar]
- 20.Taghipour K, Chi CC, Vincent A, et al. The association of bullous pemphigoid with cerebrovascular disease and dementia: a case-control study. Arch Dermatol. 2010;146:1251–4. doi: 10.1001/archdermatol.2010.322. [DOI] [PubMed] [Google Scholar]
- 21.Bastuji-Garin S, Joly P, Lemordant P, et al. French Study Group for Bullous Diseases. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011;131:637–43. doi: 10.1038/jid.2010.301. Epub 2010 Oct 14. [DOI] [PubMed] [Google Scholar]
- 22.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson CC, Gyurus E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of nonuniformity over time in rates of increase. Diabetologia. 2012;55:2142–7. doi: 10.1007/s00125-012-2571-8. Epub 2012 May 26. [DOI] [PubMed] [Google Scholar]
- 24.Casetta I, Groppo E, De Gennaro R, et al. Myasthenia gravis: a changing pattern of incidence. J Neurol. 2010;257:2015–9. doi: 10.1007/s00415-010-5651-z. Epub 2010 Jul 11. [DOI] [PubMed] [Google Scholar]
- 25.Charneux J, Lorin J, Vitry F, et al. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of bullous pemphigoid: a retrospective study of 138 patients. Arch Dermatol. 2011;147:286–91. doi: 10.1001/archdermatol.2011.23. [DOI] [PubMed] [Google Scholar]
- 26.Joly P, Courville P, Lok C, et al. French Bullous Study Group. Clinical criteria for the diagnosis of bullous pemphigoid: a reevaluation according to immunoblot analysis of patient sera. Dermatology. 2004;208:16–20. doi: 10.1159/000075040. [DOI] [PubMed] [Google Scholar]
- 27.Di Zenzo G, Thoma-Uszynski S, Fontao L, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008;128:415–26. doi: 10.1016/j.clim.2008.04.012. Epub 2008 Jun 20. [DOI] [PubMed] [Google Scholar]
- 28.Di Zenzo G, Joly P, Zambruno G, et al. Sensitivity of immunofluorescence studies vs enzyme-linked immunosorbent assay for diagnosis of bullous pemphigoid. Arch Dermatol. 2011;147:1454–6. doi: 10.1001/archderm.147.12.1454-b. [DOI] [PubMed] [Google Scholar]
- 29.Groves RW, Bhogal B, Taghipour K, et al. Bullous pemphigoid: is the incidence of pemphigoid really increasing? BMJ. 2008;337:a1138. doi: 10.1136/bmj.a1138. [DOI] [PubMed] [Google Scholar]
- 30.Rzany B, Partscht K, Jung M, et al. Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol. 2002;138:903–8. doi: 10.1001/archderm.138.7.903. [DOI] [PubMed] [Google Scholar]
- 31.Teno JM, Harrell FE, Jr, Knaus W, et al. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 Suppl):S16–24. doi: 10.1111/j.1532-5415.2000.tb03126.x. [DOI] [PubMed] [Google Scholar]
- 32.Korman NJ. Bullous pemphigoid: the latest in diagnosis, prognosis, and therapy. Arch Dermatol. 1998;134:1137–41. doi: 10.1001/archderm.134.9.1137. [DOI] [PubMed] [Google Scholar]
- 33.Bystryn JC, Rudolph JL. Why is the mortality of bullous pemphigoid greater in Europe than in the US? J Invest Dermatol. 2005;124(3):xx–xxi. doi: 10.1111/j.0022-202X.2005.23677.x. [DOI] [PubMed] [Google Scholar]
- 34.Joly P, Benichou J, Lok C, et al. Prediction of survival for patients with bullous pemphigoid: a prospective study. Arch Dermatol. 2005;141:691–8. doi: 10.1001/archderm.141.6.691. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Doval I, Conde Taboada A, Cruces Prado MJ. Sepsis associated with dermatologic hospitalization is not the cause of high mortality of bullous pemphigoid in Europe. J Invest Dermatol. 2005;124:666–7. doi: 10.1111/j.0022-202X.2005.23628.x. [DOI] [PubMed] [Google Scholar]
- 36.Swerlick RA, Korman NJ. Bullous pemphigoid: what is the prognosis? J Invest Dermatol. 2004;122:XVII–XVIII. doi: 10.1111/j.0022-202X.2004.22538.x. [DOI] [PubMed] [Google Scholar]
- 37.Barrick BJ, Lohse CM, Lehman JS. Specific causes of death in patients with bullous pemphigoid as measured by death certificate data: a retrospective cohort study. Int J Dermatol. 2013 doi: 10.1111/ijd.12243. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venning VA, Wojnarowska F. Lack of predictive factors for the clinical course of bullous pemphigoid. J Am Acad Dermatol. 1992;26:585–9. doi: 10.1016/0190-9622(92)70085-t. [DOI] [PubMed] [Google Scholar]
- 39.Cortés B, Marazza G, Naldi L, et al. Autoimmune Bullous Disease Swiss Study Group . Mortality of bullous pemphigoid in Switzerland: a prospective study. Br J Dermatol. 2011;165:368–74. doi: 10.1111/j.1365-2133.2011.10413.x. [DOI] [PubMed] [Google Scholar]
- 40.Cortés B, Khelifa E, Clivaz L, et al. Mortality rate in bullous pemphigoid: a retrospective monocentric cohort study. Dermatology. 2012;225:320–5. doi: 10.1159/000345625. Epub 2012 Dec 21. [DOI] [PubMed] [Google Scholar]
- 41.Gual A, Mascaro JM, Jr, Rojas-Farreras S, et al. Mortality of bullous pemphigoid in the first year after diagnosis: a retrospective study in a Spanish medical centre. J Eur Acad Dermatol Venereol. doi: 10.1111/jdv.12065. advance online publication, 22 Dec 2012. [DOI] [PubMed] [Google Scholar]
- 42.Zhang LM, Wu J, Xiao T, et al. Treatment and mortality rate of bullous pemphigoid in China: a hospital-based study. Eur J Dermatol. 2013;23:94–8. doi: 10.1684/ejd.2012.1906. [DOI] [PubMed] [Google Scholar]
- 43.Li J, Zuo YG, Zheng HY. Mortality of bullous pemphigoid in China. JAMA Dermatol. 2013;149:106–8. doi: 10.1001/archdermatol.2012.2994. [DOI] [PubMed] [Google Scholar]
- 44.Fivenson DP, Breneman DL, Rosen GB, et al. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol. 1994;130:753–8. [PubMed] [Google Scholar]
- 45.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 46.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 47.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US Census Bureau. US Department of Commerce. Available from: http://www.census.gov/
- 49.Therneau T, Sicks J, Bergstralh E, et al. Technical report series no. 52, expected survival based on hazard rates. Rochester (MN): Department of Health Sciences Research; 1994. [Google Scholar]