Abstract
Acute and chronic pain (post-herpetic neuralgia or PHN) are encountered in patients with herpes zoster that is caused by reactivation of varicella-zoster virus (VZV) from a state of neuronal latency. PHN is often refractory to current treatments, and additional strategies for pain relief are needed. Here we exploited a rat footpad model of PHN to show that herpes simplex virus (HSV) vector-mediated gene delivery of human preproenkephalin (vHPPE) effectively reduced chronic VZV-induced nocifensive indicators of pain. VZV inoculated at the footpad induced prolonged mechanical allodynia and thermal hyperalgesia that did not develop in controls or with ultraviolet light-inactivated VZV. Subsequent footpad administration of vHPPE relieved VZV-induced pain behaviors in a dose-dependent manner for extended periods, and prophylactic vector administration prevented VZV-induced pain from developing. Short-term pain relief following low-dose vHPPE administration could be effectively prolonged by vector re-administration. HPPE transcripts were increased three- to fivefold in ipsilateral ganglia, but not in the contralateral dorsal root ganglia. VZV hypersensitivity and its relief by vHPPE were not affected by peripheral delivery of opioid receptor agonist or antagonist, suggesting that the efficacy was mediated at the ganglion and/or spinal cord level. These results support further development of ganglionic expression of enkephalin as a novel treatment for the pain associated with Zoster.
INTRODUCTION
Varicella-zoster virus (VZV), a ubiquitous human herpesvirus, causes herpes zoster (‘shingles’) following its reactivation from a neuronal latent state that was established during the primary disease, varicella (‘chickenpox’). Herpes zoster is associated with considerable morbidity as a result of debilitating acute and chronic pain, with incidence increasing with rising age and/or declining immune status. Zoster will eventually occur in approximately one-fifth to one-third of the population, usually occurring in those over age 60.1,2 Although vaccines for both varicella and zoster are available,3 the zoster vaccine is only partially effective in preventing the occurrence of zoster and pain associated with it.2
Pain may occur before, during and/or after the skin disease of zoster and even occurs in its absence.4 Up to 90% of zoster patients experience acute pain,5 which may be alleviated by timely antiviral administration to limit viral replication. However, one-third of patients develop chronic, more difficult to treat pain states known as post-herpetic neuralgia (PHN) that usually fail to respond to antiviral treatments.6 The most common and debilitating pain experienced by PHN patients is moderate to severe mechanical allodynia (MA) and/or thermal hypersensitivity. These may become so severe that they lead to disparate secondary consequences such as depression, withdrawal from society and loss in the quality of life.7,8 Current treatment strategies for PHN include tricyclic antidepressants, topical lidocaine or capsaicin patch treatments, opioids and gabapentinoids, but these are often ineffective and are associated with problematic side effects, poor patient compliance or abuse.6 PHN remains a significant public health concern in urgent need for improved treatment strategies.9
Although there is no small animal model of VZV latency, reactivation, zoster-like disease and subsequent pain, a rat model of VZV-induced pain has been described.10–13 Animals inoculated at the footpad with VZV-infected cells develop long-term chronic nocifensive behaviors similar to those exhibited by PHN patients, including MA, thermal hyperalgesia (TH) and anxious-like behaviors.12 VZV-infected animals show a viral dose-dependent increase in sensitivity with the expression of some VZV proteins in neurons colocalizing with peripherin, neurofilament 200 and neuropeptide y in ipsilateral but not contralateral ganglia.11 It has been established that pain behaviors developing in the VZV-inoculated rat model do not respond to acyclovir blockade of viral replication, which mirrors the observations that pain in the majority of human PHN patients is not alleviated by antiviral therapy.6,9,12–14 Although the pain indices that develop in the rat differ from human PHN in that it follows an acute primary infection rather than a reactivation from latency, the rat model has proved highly useful for preclinical assessment of many current and novel drug treatment strategies,11–13 and many treatments in the rat echo the response of some PHN patients. Animals treated with gabapentin, morphine, sodium channel blockers (mexiletine and lamotrigine) or tricyclic antidepressant (amitriptiline) showed significant reduction in hypersensitivity. However, many drug treatments show only short-term relief, and some of the treatment strategies evaluated in the rat requires administration routes that are impractical for PHN patients.
Here, we show that nocifensive behaviors developing in VZV footpad-inoculated Sprague–Dawley rats are effectively treated and prevented with herpes simplex virus (HSV) vector-delivered expression of human preproenkephalin (vHPPE). PPE gives rise to natural opioids that modulate pain perception and can be found in interneurons that synapse onto primary and second-order neurons in the dorsal horn of the spinal cord.15 Release of vesicle-stored Met- and Leu-enkephalin opioids bind and activate ∂-, and to a lesser extent, μ-opioid receptors on both primary and second-order neurons. This results in the lowering of cyclic AMP production and a hyperpolarization of the neuronal membrane, thereby activating inwardly rectifying K+ channels with concurrent inhibition of voltage-sensitive Ca++ channels.16 The vHPPE is based on replication-defective HSV type 1 (HSV-1), a neurotrophic herpes virus that establishes latency (and expresses) in sensory neurons but is not associated with chronic pain. The effective long-term relief of VZV-induced pain in the rat induced by this vector promotes the exciting potential for long-acting treatment of the PHN that follows herpes zoster.
RESULTS
Nocifensive behaviors induced in Sprague–Dawley rats inoculated with VZV
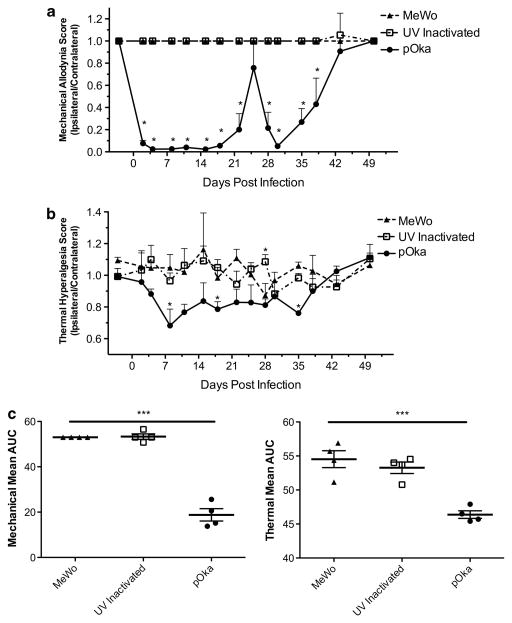
We first established the model of VZV-induced hypersensitivity in Sprague–Dawley rats that have been used extensively in many inflammatory and neuropathic pain models.17 Previous reports on VZV-induced pain used Wistar rats.10–14 Nocifensive behaviors were induced by VZV parent Oka (pOka), a wild-type varicella isolate of Japanese origin that was the basis for subsequent attenuation for use in the current VZV vaccines.18,19 We used this viral strain as it has been used in most genetically manipulatable systems for VZV. Animals were inoculated with live cell-associated VZV, because VZV infectivity remains highly cell associated and cell-free virus cannot be obtained at the titers required to induce pain. All animals receiving VZV in multiple studies developed markedly different behavioral responses to mechanical and thermal stimulation compared with animals receiving uninfected cell equivalents (Figures 1a and b) or sham-inoculated animals (data not shown). No difference was observed between control cell-inoculated animals and sham-inoculated animals. Chronic hypersensitivity to mechanical stimuli developed only in the VZV-inoculated paw, resulting in a biased ipsilateral/contralateral ratio lasting several weeks. The contralateral paw of animals receiving VZV, and the ipsilateral and contralateral paws of animals receiving uninfected cells did not respond to most von Frey filament stimulations. Significant hypersensitivity to thermal stimuli (Figure 1b) also only developed in VZV-injected footpads as compared with the uninoculated contralateral paw or uninfected cell-inoculated paws. VZV-induced nocifensive behaviors persisted in this study for ~ 5 weeks post infection (w.p.i.), whereupon hypersensitivity responses reduced, and by 6–8 w.p.i. these were similar to untreated animals. Timing of spontaneous recovery showed consistency between groups of animals within the same experiment, although rats in other studies showed variation in the time at which spontaneous resolution occurred. We also evaluated MA and TH responses in rats receiving VZV preparations that were ultraviolet light (UV)-inactivated just before inoculation. These showed no significant mechanical or thermal hypersensitivity (Figure 1). This indicates that the VZV-infected cell inoculum used in these studies did not contain pre-existing factors inducing the hypersensitivity, and that live virus with de novo transcription and gene expression are required for the induction of pain.
Figure 1.
Mechanical and thermal hypersensitivity induced by VZV in Sprague–Dawley rat requires de novo transcription. Animals were pretested 2 days before infection for baseline MA and TH responses, and then inoculated (n =4 per group) at day 0 with 2 × 105 PFU of pOka, UV inactivated, or uninfected cell equivalents. Animals were evaluated for (a) MA scores using von Frey filaments; and for (b) TH using a Hargreaves apparatus, as detailed in the Methods section with the graphs depicting the ratio of ipsilateral to contralateral responses. Mean+s.e.m. is plotted. This study is representative of three similar studies. Statistics used one-way analysis of variance (ANOVA) between groups and Dunnett’s post test comparing groups to MeWo. Mean AUC is plotted for each animal; line represents the mean, and bars are s.e.m. (c) Mean AUC with one-way ANOVA between groups and Tukey post test, with *P<0.05, ***P<0.001 indicating significance.
HSV vector PPE expression modulates VZV-induced nocifensive behaviors
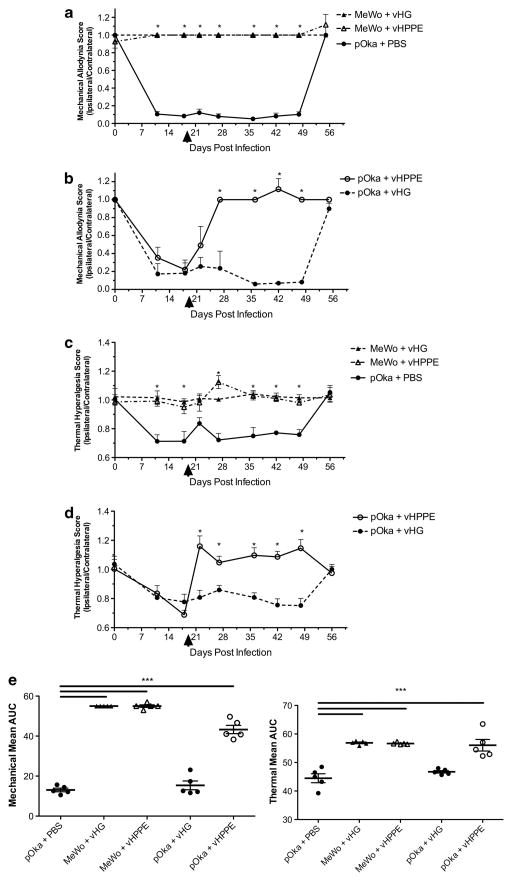
Although novel drug treatments have been evaluated in the rat PHN model, most provide only a short-lived relief of pain and often require administration routes that would be impractical for the treatment of human PHN. Given that human PHN can be prolonged, a long-term treatment strategy would be desirable. Therefore, we evaluated a treatment using a replication-defective HSV-1 vector expressing HPPE.20 Rats inoculated with VZV and showing significant mechanical and thermal hypersensitivity by 19 days post infection (d.p.i) (Figure 2) were then inoculated at the same footpad with PBS or 108 infectious units of HSV vector (vHPPE or vHG control). Animals receiving PBS or vHG continued to show significantly biased MA and TH ipsilateral/contralateral responses after vector administration (Figure 2). Remarkably, VZV-induced MA and TH nocifensive responses showed prolonged relief following a single inoculation of 108 vHPPE (Figures 2b and d). Neither of the HSV vectors induced changes in behavioral responses of uninfected cell-inoculated rats (Figures 2a and c). These results strongly suggest that HSV-1 vector-mediated expression of HPPE could effectively alleviate VZV-induced nocifensive responses in the rat model for extended periods.
Figure 2.
Administration of vHPPE provides prolonged relief of VZV-induced hypersensitivity. Animals were injected on day 0 with 2 × 105 PFU of pOka or equivalent number of uninfected MeWo cells (n =5 per group) and assessed for MA and TH at times shown. At 19 d.p.i., animals were injected with 108 PFU of vHPPE, vHG or equivalent volume of PBS and subsequently evaluated for (a, b) MA using von Frey filaments and (c, d) TH using a Hargreaves apparatus. All results are presented as a ratio of the score of the ipsilateral to contralateral sides. Mean+s.e.m. is plotted. Arrowheads indicate when HSV vectors were administered. The study shown is representative of two studies with similar results. Statistics used one-way analysis of variance (ANOVA) between groups and Dunnett’s posttest comparing groups to pOka+PBS. Mean AUC is plotted for each animal; line represents the mean, and bars are s.e.m. (e) Mean AUC with one-way ANOVA between groups and Tukey post test, with *P<0.05, ***P<0.001 indicating significance.
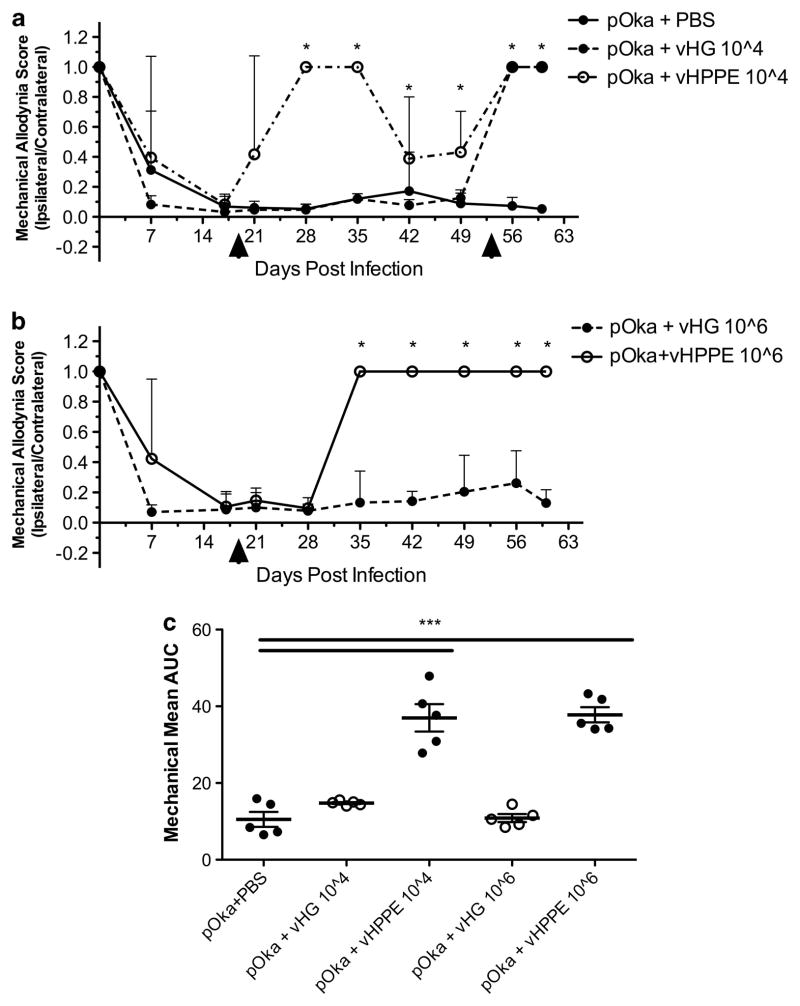
To evaluate whether alleviation of nocifensive behaviors by HSV-delivered PPE was dose dependent, incremental lower doses of the HSV vectors were examined. All rats injected with VZV-infected cells developed nocifensive behaviors. On day 19, VZV-infected and control animals were inoculated with either PBS or 104 or 106 plaque-forming units (PFUs) per footpad of vHPPE or vHG control. Rats receiving PBS treatment or HSV vHG at 104 or 106 PFU continued to show a biased MA footpad response to the VZV-inoculated paw (Figures 3a and b). In contrast, animals with a VZV-induced hypersensitivity that received vHPPE at 104 or 106 PFU developed obvious relief from MA within 14 days of administration. However, the MA response in rats receiving the lower dose (104 PFU per footpad) showed only short-term alleviation, and biased MA responses returned in the VZV-infected paw at 42 d.p.i. of VZV. This result indicated that duration relief of the MA responses by vHPPE was dose dependent. Interestingly, the short-term relief by vHPPE could be re-initiated by re-administration of 108 PFU of vHPPE at 53 d.p.i. (Figure 3a). Such animals showed an immediate relief from VZV-induced nocifensive behaviors that then persisted for the length of the study. Thermal responses were concurrently evaluated, and while they showed a similar trend statistical difference could not be established between the groups (data not shown). We conclude that vHPPE can modulate VZV-induced nocifensive responses in a dose-dependent manner that affects duration of analgesia and does not preclude potential for re-administration for extension of relief.
Figure 3.
VZV-induced nocifensive behaviors respond to reduced vHPPE dosing and vector re-administration. Animals were evaluated for MA at day 0 and then injected with 2 × 105 PFU of pOka or equivalent number of uninfected MeWo cells (n =5 per group), followed by MA evaluation at days 7 and 16. At 19 d.p.i., animals were injected with either (a) 104 or (b) 106 PFU of vHPPE, vHG or PBS. Animals were evaluated for sensitivity by MA using von Frey filaments. All results presented as a ratio of the score of the ipsilateral to contralateral sides. All animal experiments were repeated with similar results. Animals that were originally dosed with (a) 104 PFU of vHPPE or vHG were given a later dose of 108 PFU of vHPPE at 53 d.p.i. Mean+s.e.m. plotted. Arrowheads indicate when HSV vectors were administered. Statistics used included one-way analysis of variance (ANOVA) between groups and Dunnett’s post test comparing groups to pOka+PBS. Mean AUC is plotted for each animal; line represents the mean, and bars are s.e.m. (c) Mean AUC was analyzed by one-way ANOVA between groups and Tukey post test, with *P<0.05, ***P<0.001 indicating significance.
Prophylactic vHPPE administration prevented VZV-induced hypersensitivity
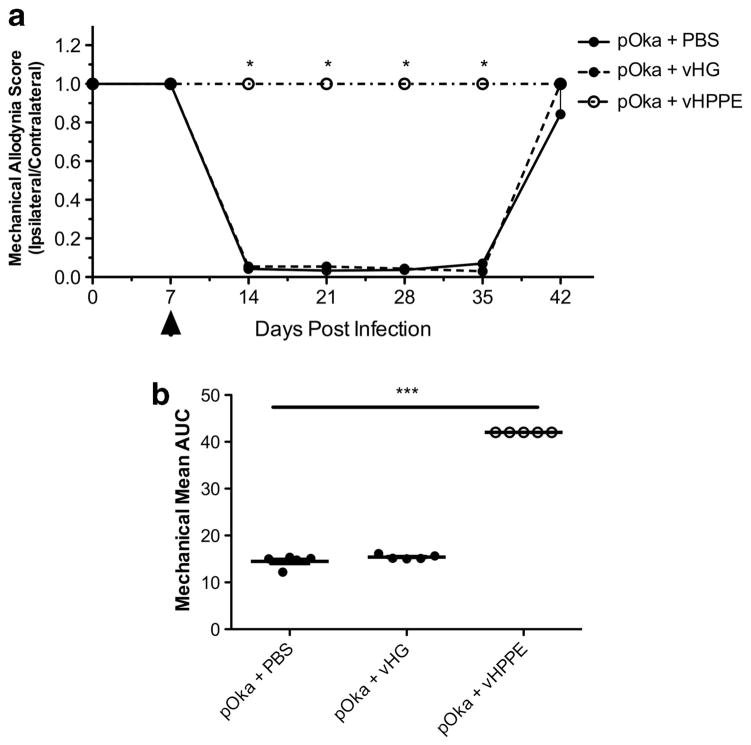
Zoster is often associated with prodromal signs that predict the development of disease,21,22 so it is feasible that prophylactic treatment could prevent chronic VZV-induced pain. To evaluate this experimentally, animals were first inoculated with PBS or 108 PFU of control vHG or vHPPE, followed by VZV inoculation at the same site. Nocifensive behaviors were not induced by the HSV vectors alone (data not shown), mirroring previously reported absence of pain from replication-competent HSV23–30 or replication-defective HSV,20,31–38 even when expressing reporter genes or other proteins. However, VZV-induced MA nocifensive behaviors only developed in animals pre-inoculated with PBS or vHG vector, and did not develop in vHPPE-treated animals (Figure 4). As such, we conclude that prophylactic administration of vector vHPPE can block the development of VZV-induced pain.
Figure 4.
Prophylactic administration of vHPPE blocks development of VZV-induced mechanical hypersensitivity. (a) Animals were injected 7 days before VZV infection with either 108 PFU of vHPPE, vHG or PBS. On day 0, animals were inoculated with 2 × 105 PFU of VZV pOka or uninfected MeWo cell equivalents (n =5 per group). MA scores were determined at the indicated times using von Frey filaments and are presented as a ratio of ipsilateral to contralateral paw responses. All animal experiments were repeated with similar results. Mean+s.e.m. is plotted. Statistics used one-way analysis of variance (ANOVA) between groups and Dunnett’s post test comparing groups to pOka+PBS, and mean AUC is plotted for each animal; line represents the mean, and bars are s.e.m. (b) Mean AUC was analyzed for significance using one-way ANOVA between groups and Tukey post test, with *P<0.05, ***P<0.001 indicating significance.
VZV-induced hypersensitivity and its relief by PPE do not act at the periphery
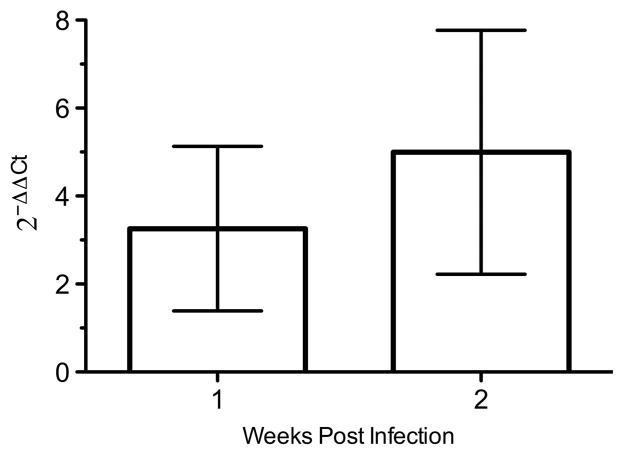
VZV-induced hypersensitivity and its relief by peripheral administration of vHPPE were suspected to act at the ganglia, but it is possible that vHPPE-expressed enkephalin acts on sensory nociceptor termini at the periphery. Direct quantification of enkephalin expression was not sufficiently sensitive to reveal differences between treated and untreated controls, as seen previously.20 Global quantification of innervating ganglionic transcripts of HPPE mRNA revealed a three- to fivefold 2−ΔΔCt increase in levels over that detected in control animals (Figure 5).
Figure 5.
Ganglionic expression of HPPE in animals injected with vHPPE. Animals were inoculated with 108 PFU of vHPPE or vHG and tissue harvested at 1 and 2 weeks post inoculation (n =6 per group). The L4-6 DRG were removed and the extracted RNA was converted to cDNA. Transcripts for HPPE were quantified by Taqman reverse transcription-PCR and are presented using the 2−ΔΔCt compared with rat GAPDH as a surrogate for normalized gene expression against control animals. Mean ±s.e.m. is plotted.
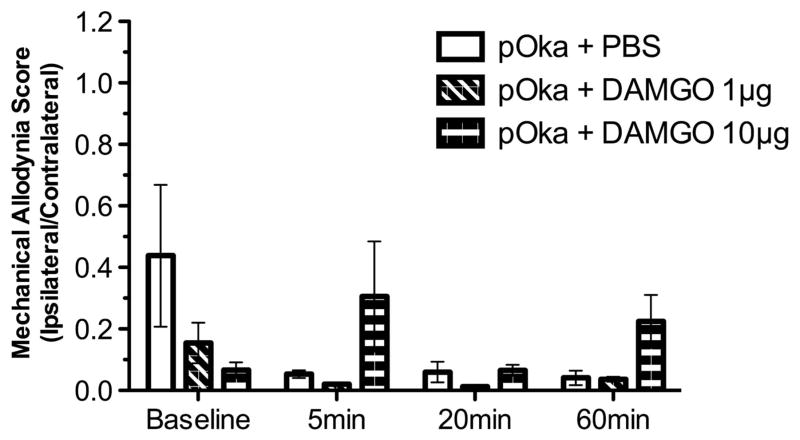
To further probe for effects at the periphery, we analyzed whether peripheral administration of [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) or naloxone affected VZV-induced pain or its relief by vHPPE administration. Animals showing VZV-induced nocifensive MA behaviors at 3 w.p.i. were subcutaneously injected at the footpad with 1 or 10 μg of DAMGO, a synthetic μ-opioid receptor agonist with high specificity for the μ-opioid receptor. We reasoned that the peripheral administration of DAMGO would alter VZV-induced pain responses if a mechanism of vHPPE relief acted at the periphery (Figure 6). However, VZV-induced biased MA responses showed no detectable relief immediately following DAMGO administration into the footpad. Higher dosing with 10 μg DAMGO did not lead to any relief of nocifensive behaviors over a period of 1 h. We then assessed whether peripheral administration of naloxone to the footpad would negate relief of VZV-induced MA responses by 108 vHPPE. Peripherally delivered naloxone, a competitive antagonist of the opioid receptors, would be expected to block enkephalin interaction with any peripherally located opioid receptors, but not with dorsal root ganglia (DRG) axons terminating within the dorsal horn of the spinal cord. VZV-infected animals showing nocifensive behaviors that were then relieved by 108 HSV vHPPE showed no biased MA responses, whereas control animals continued to show a strong ipsilateral to contralateral bias (Figure 7). Animals receiving either 5 or 50 μg of naloxone in the same paw showed no change in MA responses, either short term (up to 60 min) or after 24 h post administration (data not shown). Furthermore, peripheral nalox-one administration did not affect vHPPE relief of VZV-induced nocifensive behaviors, suggesting that HSV vector-mediated expression of enkephalin within the DRG acts centrally within the spinal cord. The latter has been strongly implied from prior studies using similar pain-relieving vectors, in which blockade of the effects of enkephalin were obtained following intrathecal administration of antagonists.32,39–42 We conclude from this data that it is unlikely that VZV-induced pain and its relief induced by vHPPE vector were a consequence of effects mediated at the periphery.
Figure 6.
Effect of peripheral delivery of DAMGO on VZV-induced MA hypersensitivity. Animals inoculated with 2 × 105 PFU of pOka at the footpad were allowed to develop MA hypersensitivity. Animals were evaluated to determine baseline responses and then injected with 1 or 10 μg per 20 μl DAMGO or equivalent volume of PBS at 9 d. p.i. (n =4). Animals were subsequently tested for MA by von Frey filaments and presented as ipsilateral to contralateral ratio. MA was assessed at 10 min before injection for baseline determination and 5, 20 and 60 min post injection. Mean ±s.e.m. is plotted.
Figure 7.
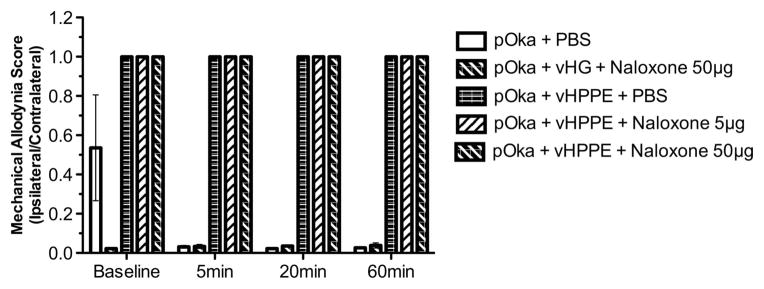
Effect of peripheral delivery of naloxone on VZV-induced MA hypersensitivity treated with vHPPE or vHG vector. Animals were inoculated with 2 × 105 PFU of pOka, and then received at 9 d.p.i. either 108 PFU of vHPPE, vHG or PBS. Animals received a third footpad injection at 23 d.p.i. with 5 or 50 μg per 20 μl naloxone or the equivalent volume of PBS (n =4). Animals were tested for MA by von Frey filaments, 10 min before injection and 5, 20 and 60 min post injection. The ratio of ipsilateral to contralateral paw responses is shown. Mean ±s.e.m. is plotted.
DISCUSSION
The goal of this study was to further develop a rat model of VZV-induced pain to evaluate the therapeutic and prophylactic administration of human enkephalin using an HSV vector ganglionic delivery system. The efficacy of this strategy strongly promotes the potential for use of enkephalin gene delivery as a long-acting treatment for the pain and PHN associated with zoster. The model appears robust and reproducible, particularly with respect to induced mechanical hypersensitivity, but it is not yet clear exactly how VZV induces nocifensive behaviors in rats. The length of hypersensitivity induced by VZV in rats was found to vary somewhat between different experiments. We postulate that this could be the consequence of the outbred strain and subtle differences in the genetics of these animals. It is also possible that subtle variations in the experimental procedures and even subtle changes in housing conditions could contribute to this variation, although every step was taken to minimize these experimental differences. We note that the duration of chronic nocifensive behaviors were also variable in previously reported studies using the Wistar rat model.11,12,14 However, the variation reflects the duration of extended pain in PHN patients, which may be short or last for years. Differences may also be the consequence of virus strain used. Each lab has evaluated different strains in the rat model that differ from the virus we used here, pOka. However, we point out that the pOka strain is the standard virus most used in the field of VZV genetics and should be useful for future genetic studies to evaluate pain-inducing VZV genes in the rat model. Most rodents are not permissive for VZV replication although some early phases of infection may occur. Two separate studies have reported that the development of VZV-induced pain behaviors in rats is not prevented by the viral DNA replication inhibitor, acyclovir.12,14 However, given that some viral gene products can be detected in neurons of the innervating ganglia, but not in contralateral ganglia (including VZV immediate-early (IE) protein, IE62 (refs 9,11,12)), it seems likely that an abortive type of infection with some viral gene expression is required for pain development. Our data showing that rats receiving UV-inactivated VZV failed to develop hypersensitivity behaviors are consistent with this hypothesis, as it indicates infectivity and/or de novo VZV gene transcription are required. We suspect that transcription of VZV genes within virus-infected neurons may themselves lead to the chronic pain state either through induction of inflammatory mechanisms or through expression of proteins, such as transcriptional regulators, that may alter host cell expression patterns.11,43,44 Studies are ongoing to determine whether viral transactivators, IE62 and IE63, are required for the induction of the chronic pain state.
We show that VZV-induced pain behavioral indicators can be effectively reversed and even prevented with sustained effect by HSV vector-mediated delivery of PPE. We exploited replication-defective HSV-1 vectors that are engineered to enter a latent-like state in sensory neurons. In contrast to VZV, human HSV recurrent infections are not associated with more than slight acute pain. Indeed, Wistar rats inoculated with high titers of replicating HSV-1 show only momentary behavioral pain responses.14 Consistent with this, we found no pain indicators developing in Sprague–Dawley rats inoculated with either vHG control vector or vHPPE vector (data not shown) as has been previously described in replication-competent HSV23–30 or replication-defective HSV20,31–38 vectors expressing genes other than enkephalin in various pain models. This supports the fact that while HSV and VZV are genetically related, they have very different consequences on pain induction. In addition, this is consistent with a variety of HSV vectors that do not induce pain in preclinical animal models and human clinical trials.23,45 This supports the safety for the potential use of such vectors in humans. Our study extends the application of this vector–gene delivery combination to a model of a common and significant clinical problem, PHN. HSV-1 vectors expressing HPPE have been used as a treatment approach in various other pain animal models, including pain associated with pancreatitis,25,36 formalin injection,32 spinal nerve ligation,35 complete Freund’s adjuvant-induced arthritis,24,28,30 chronic constriction injury (CCI),29,38 bone cancer pain,31 pertussis toxin-induced pain26 and in a bladder nociception model.20,33,34 In these studies, HSV vector-mediated enkephalin produced abrogation of nocifensive behaviors to varying extents. The long-term effects of such vectors and their peripheral administration are particularly attractive for application to PHN, which may last weeks to years. Evaluation of morphine, amitriptyline, gabapentin, ibuprofen and the Win55212-2 2 compound in the rat PHN model12 all provide only temporary relief lasting 4–6 days or less, and some human treatments such as morphine are associated with tolerance and abuse issues. Oral administration of gabapentin or the sodium channel blockers, mexiletine and lamotrigine, were also found to last for hours or less in the rat model.11 Short-term effects on VZV pain in the rats by agents such as astrocyte toxin, iNOS inhibitors, NO scavengers, IL1 receptor antagonist, cytokine inhibitor, NMDA receptor antagonists and non-competitive NMDA receptor antagonist have also been reported, but these require intraperitoneal or intrathecal administration, routes not particularly suited to human treatment.11–13 The single peripheral administration of vHPPE abrogated VZV-induced nocifensive behaviors completely at high doses (Figures 2b and d). We argue that this is the first strategy to show a prolonged effect on VZV-induced pain behaviors with single dosing and relatively simple administration. The potential for vHPPE re-administration (Figure 3) was also demonstrated. A similar replication-defective HPPE expressing HSV vector has been evaluated in a phase-I trial for patients with intense chronic cancer-related pain and reduced pain scores.45 We believe our data establish a basis for evaluating the efficacy of this vector in human PHN trials.
The presumed mechanism(s) of action in the enkephalin treatment strategy is that following HSV infection of sensory nerves, ganglionic expression of enkephalin probably results in Leu– and Met–enkephalin incorporation into secretory vesicles and release at synapses of sensory nerve axons terminating within the dorsal horn of spinal cord, where binding to opioid receptors prevents synaptic transmission of pain signals. Our studies with the peripheral administration of enkephalin agonists and antagonists suggest that enkephalin is acting centrally. Previous reports31,32 indicate that intrathecal administration of naltrexone, an opioid receptor antagonist, reversed vHPPE vector-mediated relief, suggesting a central acting role for enkephalin in those models. In our VZV PHN model, peripheral injection of DAMGO or naloxone had no influence on hypersensitivity or effect on vHPPE-mediated relief, respectively. All time points had similar effects, suggesting local delivery of opioid receptor agonist or antagonist does not alleviate hypersensitivity or block analgesia. This further supports the hypothesis that HSV vector-mediated enkephalin acts centrally.
Taken together, we have established that VZV induces a robust mechanical and quantifiable thermal hypersensitivity in Sprague–Dawley rats, which is dependent upon de novo VZV gene expression. These pain behaviors can be effectively relieved for prolonged periods by peripheral administration of an HSV vector expressing human PPE, and can be prevented from developing by prophylactic vector administration. These studies suggest further development of these or similar vectors as prolonged treatment strategies for pain associated with herpes zoster.
MATERIALS AND METHODS
Cells and VZV
VZV pOka is a low-passage clinical varicella isolate used at <12 passes following receipt as a kind gift from Dr M Takahashi (Osaka University, Japan). VZV was grown in the human melanoma cell line MeWo (American Type Culture Collection, Manassas, VA, USA) using modified Eagle’s media (Life Technologies Inc., Grand Island, NY, USA) supplemented with 10% fetal bovine serum and antibiotic and antimycotics mixture (Sigma, St Louis, MO, USA). High-titer VZV was prepared on fresh monolayers of MeWo cells infected at ~ 0.1 PFUs per cell and harvested at 48–72 h post infection by trypsin digestion when cells showed ~ 70–80% cytopathic effect. Analyses of the stock virus by immunostaining for VZV surface proteins revealed that >90% of cells were infected. VZV viability and infectious titer were maintained by slow-freezing-infected cells as aliquots under conditions to maintain cell viability (modified Eagle’s media containing 20% fetal bovine serum and 10% dimethyl sulfoxide (Sigma), and were stored under liquid nitrogen. Uninfected cell equivalents used as controls were treated similarly. VZV titers were determined by plaque assay on MeWo cells.46
Animal inoculations used rapid thawed VZV-infected or equivalent uninfected cells that were washed twice in ice-cold PBS, suspended at 2 × 106 PFU per ml (or cell equivalents for uninfected cell controls) and used within 1–2 h. UV light inactivation of VZV infectivity was carried out in 1 ml aliquots of virus spread over a 35-mm diameter well of a six-well plate and irradiated using parameters determined empirically from UV inactivation kill curves. The light source (Spectronics, Westbury, NY, USA) was a 2 × 15 W unfiltered source emitting ultraviolet light in the C wavelength (280–100 nm) with intensity of 1550 J at 10 inches. Exposures (<10 min) were timed to result in a reduction of virus titer by >100 fold. Cells were then washed and treated for rat inoculation. Behavioral responses of animals inoculated with UV-inactivated VZV were directly compared to virus that had been identically treated but UV-shielded.
HSV vectors
HSV-1 vectors used here (vHPPE and vHG), detailed previously,20 are based on HSV-1 strain KOS 321 and rendered replication incompetent by deletion of the essential ICP27 gene and both gene copies of the essential ICP4 protein. The vector also contains alterations of the promoters of the IE ICP22 and ICP47 genes, so that they are expressed as early or β-genes. Virus was prepared and titrated in Vero 7b-complementing cells expressing the ICP4 and ICP27 genes in trans.47 Control HSV virus vHG contains two copies of a cytomegalovirus IE promoter-driven enhanced green fluorescent protein (EGFP) gene, followed by a bovine growth hormone polyadenylation signal at each ICP4 locus (Figure 8). HSV vector vHPPE was generated from vHG by standard co-transfection homologous recombination methods so that the EGFP genes were replaced with cDNA for HPPE (Figure 8).48 HSV vectors were prepared using Vero 7b cells grown in 5-layer Cell Factories (Corning, Lowell, MA, USA) infected at an multiplicity of infection of 0.001. Virus was grown for 24 h at 37 °C followed by subsequent growth at 33 °C until ~ 80% cytopathic effect. Virus was released from the surface of cells by adjusting the entire harvest to 0.45 M NaCl for 3 h at room temperature. Cellular debris was removed by low-speed (3000 r.p.m. for 5 min) centrifugation and the supernatant filtered using 0.8-μm filtration units (Thermo Fisher, Pittsburgh, PA, USA). Virus in the filtrate was concentrated by high-speed centrifugation (19 000 r.p.m. for 45 min) and resuspended in 1x PBS (Sigma) containing 10% glycerol, aliquoted and stored at −80 °C. All HSV vector stocks were verified to lack replication-competent virus by titration on Vero cells.
Figure 8.
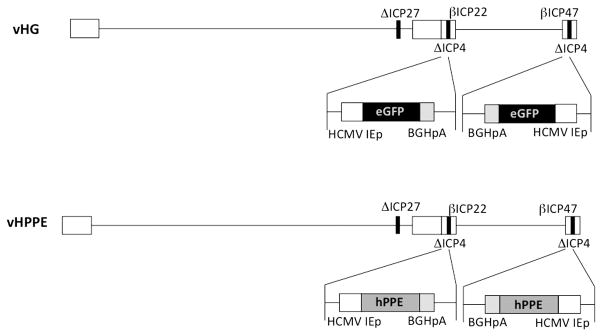
Schematic of HSV vectors used in this study. The top line represents the vHG control vector, which has the essential IE gene ICP27 and both copies of essential IE ICP4 gene deleted (bold black lines). The virus is also altered to render the ICP22 and ICP47 gene promoters to be expressed as early (or β-) genes. The minimal human cytomegalovirus IE gene promoter (HCMV IEp) EGFP expression cassette (black box) is inserted into both copies of the ICP4 locus. The lower line represents the vHPPE vector, derived from the vHG control vector, which contains the insertion of the cDNA for HPPE (gray box), driven by the HCMV IEp, followed by the bovine growth hormone polyadenylation signal (BGHpA), inserted in place of EGFP.
Animal inoculation
Animals were housed and experimental manipulations on animals performed in ABSL-2 facilities approved by the Association for the Accreditation of Laboratory Animal Care. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male 200–250 g Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were acclimatized to housing for 1 week, and then assessed for baseline MA and TH responses, as detailed below, to identify and exclude pre-existing atypical responses. Following isoflurane-induced anesthesia, 2 × 105 PFU of cell-associated VZV (or uninfected cell equivalents) were injected subcutaneously beneath the plantar surface of the right hind footpad. Rats were monitored for recovery and return to normal activity. Administration of VZV-infected cells, uninfected cells or the maximal dose of HSV vector did not induce changes in grooming, self-maintenance and feeding habits or weight loss owing to treatments. After 24 h, we were unable to observe any significant indication of swelling, inflammation or general redness that was in the ipsilateral-injected footpad as compared with the contralateral non-injected hindpaw.
Behavioral testing
MA was determined using one set of calibrated von Frey filaments (Stoetling, Wood Dale, IL, USA) and the ‘up down’ method established previously.49,50 In brief, animals were allowed to equilibrate on a wire grid-based platform apparatus (IITC Life Sciences, Woodland Hills, CA, USA) for 15 min. Starting with a von Frey filament of 10 g weight of force, each filament was applied for 6 s with sufficient force to cause bending against the paw when applied to one foot at a time. A positive response was defined as rapid withdrawal and/or licking of the paw upon application of the stimulus, and was followed by application of the next finer von Frey filament. A negative response was followed by testing with the next higher weight von Frey filament. A total of six measurements were recorded for both left contralateral (uninoculated) and right ipsilateral (inoculated) footpads. As some animal to animal variation of sensitivity was occasionally found, MA sensitivity variations from animal to animal were minimized by representing responses as a ratio of the calculated mean ipsilateral/contralateral response for each animal. MA testing preceded TH measurement on the same animal. TH was determined using a Hargreaves apparatus (IITC) according to established methodology.51 In brief, rats were allowed to equilibrate on a glass stage heated to 34 °C for at least 15 min. A calibrated focused radiant heat source was placed at a set distance under one paw at a time, starting with the contralateral paws, and the time recorded for animals to remove their paws in response to the heat source was recorded as the latency withdrawal period with a cutoff of 25 s. Paw withdrawals because of locomotion or weight shifting were not counted, and the trials were repeated. Animals were given 1–2 min rest between measurements and were tested at least three times per paw.
RNA extraction
Animals were euthanized with CO2 and subsequent cardiac puncture. DRG L4-6 were removed and snap-frozen in a dry ice/ethanol bath until processing. Tissues were then mechanically homogenized in TRIzol reagent (Life Technologies Inc., Carlsbad, CA, USA) using a Kinematica PT1200E homogenizer (Kinematica, Bohemia, NY, USA) for 7 s at 70% power. RNA was extracted using TRIzol reagent per manufacturer’s protocol, quantified using a NanoDrop spectrophotometer (Thermo Fisher), and then converted to cDNA using a High Capacity RNA/cDNA Kit (Life Technologies Inc.). Nucleic acids were quantified using standard quantitative PCR methods and TaqMan primer probe sets to rat GAPDH (Applied Biosystems, Rn01775763_gI, Life Technologies Inc.) and HPPE (Hs00175049_m1, Applied Biosystems). Data are plotted as 2−ΔΔCt comparing relative levels of human enkephalin to rat GAPDH.
Drug treatment
DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) and naloxone hydrochloride were purchased from Sigma. Rats were first assessed for VZV-induced behavioral indicators of pain by MA. Animals then received 20 μl injections containing DAMGO administered subcutaneously into the footpad at 1 and 10 μg per 20 μl. Animals were assessed for MA measurements at 5, 20 and 60 min post-drug administration using von Frey filaments. Naloxone treatment studies used animals showing a VZV-induced pain response that had been alleviated by HSV vHPPE. These rats were subjected to footpad administration of 5 and 50 μg total per 20 μl of naloxone, followed by similar MA behavioral testing at 5, 20, 60 min and at 24 h post administration. In both studies, control animal groups received each compound without prior VZV inoculation, or received PBS into the ipsilateral or contralateral footpad following VZV inoculation. All animals underwent identical treatments and handling.
Statistical analysis
All statistical tests were performed on Prism 5 (GraphPad, LaJolla, CA, USA) software. All curves were plotted and the area under the curve (AUC) calculated for each animal. Mean AUC was then compared between groups using a one-way analysis of variance with Tukey post test comparing all groups to each other. Individual time points were compared using one-way analysis of variance with Dunnett post test to the specified control group.
Acknowledgments
This work was supported by National Institutes of Health Public Health Service grant NS064822 (to PRK), NS064988-02 (to JCG and WFG); a CORE grant for Vision Research (EY08098), training grants T32AI049820 and T32NS073548 (J-MGG); funds from the Eye and Ear Institute of Pittsburgh and the Research to Prevent Blindness Inc. We would also like to thank Drs Gerald Gebhart, Kathryn Albers, Fred Homa and James Goss for their insightful comments.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–340. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 2.Harpaz R, Ortega-Sanchez IR, Seward JF Advisory Committee on Immunization Practices Centers for Disease, C. & Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. MMWR. 2008;57:1–30. [PubMed] [Google Scholar]
- 3.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 4.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haanpaa M, Laippala P, Nurmikko T. Pain and somatosensory dysfunction in acute herpes zoster. Clin J Pain. 1999;15:78–84. doi: 10.1097/00002508-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kim SR, Khan F, Tyring SK. Varicella zoster: an update on current treatment options and future perspectives. Exp Opin Pharmacother. 2014;15:61–71. doi: 10.1517/14656566.2014.860443. [DOI] [PubMed] [Google Scholar]
- 7.Schmader K. Postherpetic neuralgia in immunocompetent elderly people. Vaccine. 1998;16:1768–1770. doi: 10.1016/s0264-410x(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J Neurovirol. 2011;17:590–599. doi: 10.1007/s13365-011-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, et al. Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol. 1999;80 (Pt 9):2433–2436. doi: 10.1099/0022-1317-80-9-2433. [DOI] [PubMed] [Google Scholar]
- 11.Garry EM, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang GH, Lv MM, Wang S, Chen L, Qian NS, Tang Y, et al. Spinal astrocytic activation is involved in a virally-induced rat model of neuropathic pain. PLoS One. 2011;6:e23059. doi: 10.1371/journal.pone.0023059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalziel RG, Bingham S, Sutton D, Grant D, Champion JM, Dennis SA, et al. Allodynia in rats infected with varicella zoster virus--a small animal model for post-herpetic neuralgia. Brain Res Brain Res Rev. 2004;46:234–242. doi: 10.1016/j.brainresrev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann K. In: Science of Pain. 1. Basbaum AI, Bushnell C, editors. Academic Press; 2009. p. 1088. Reviewed by Katherine Zimmermann Eur J Pain 2011, 15, 731. [Google Scholar]
- 16.Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997;41:94–111. doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- 17.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi M, Okuno Y, Otsuka T, Osame J, Takamizawa A. Development of a live attenuated varicella vaccine. Biken J. 1975;18:25–33. [PubMed] [Google Scholar]
- 19.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama H, Oguchi T, Goins WF, Goss JR, Nishizawa O, de Groat WC, et al. Effects of herpes simplex virus vector-mediated enkephalin gene therapy on bladder overactivity and nociception. Hum Gene Therapy. 2013;24:170–180. doi: 10.1089/hum.2011.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagel MA, Gilden D. Complications of varicella zoster virus reactivation. Curr Treat Options Neurol. 2013;15:439–453. doi: 10.1007/s11940-013-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilden D, Nagel MA, Cohrs RJ, Mahalingam R. The variegate neurological manifestations of varicella zoster virus infection. Curr Neurol Neurosci Rep. 2013;13:374. doi: 10.1007/s11910-013-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC, et al. Anti-hyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci USA. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y, McNearney TA, Lin W, Wilson SP, Yeomans DC, Westlund KN. Treatment of inflamed pancreas with enkephalin encoding HSV-1 recombinant vector reduces inflammatory damage and behavioral sequelae. Mol Ther. 2007;15:1812–1819. doi: 10.1038/sj.mt.6300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeomans DC, Jones T, Laurito CE, Lu Y, Wilson SP. Reversal of ongoing thermal hyperalgesia in mice by a recombinant herpesvirus that encodes human preproenkephalin. Mol Ther. 2004;9:24–29. doi: 10.1016/j.ymthe.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Yeomans DC, Lu Y, Laurito CE, Peters MC, Vota-Vellis G, Wilson SP, et al. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;13:589–597. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, McNearney TA, Wilson SP, Yeomans DC, Westlund KN. Joint capsule treatment with enkephalin-encoding HSV-1 recombinant vector reduces inflammatory damage and behavioural sequelae in rat CFA monoarthritis. Eur J Neurosci. 2008;27:1153–1165. doi: 10.1111/j.1460-9568.2008.06076.x. [DOI] [PubMed] [Google Scholar]
- 29.Meunier A, Latrémolière A, Mauborgne A, Bourgoin S, Kayser V, Cesselin F, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Pinto M, Castro AR, Tshudy F, Wilson SP, Lima D, Tavares I. Opioids modulate pain facilitation from the dorsal reticular nucleus. Mol Cell Neurosci. 2008;39:508–518. doi: 10.1016/j.mcn.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X, et al. Herpes vector-mediated expression of proenkephalin reduces bone cancer pain. Ann Neurol. 2002;52:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 32.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ, et al. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Therapy. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama H, Sasaki K, Franks ME, Goins WF, Goss JR, de Groat WC, et al. Gene therapy for bladder overactivity and nociception with herpes simplex virus vectors expressing preproenkephalin. Hum Gene Ther. 2009;20:63–71. doi: 10.1089/hum.2008.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura N, Franks ME, Sasaki K, Goins WF, Goss J, Yokoyama T, et al. Gene therapy of bladder pain with herpes simplex virus (HSV) vectors expressing preproenkephalin (PPE) Urology. 2001;57:116. doi: 10.1016/s0090-4295(01)01060-3. [DOI] [PubMed] [Google Scholar]
- 35.Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect in neuropathic pain. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, McNearney TA, Chu R, Lu Y, Ren Y, Yeomans DC, et al. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol Pain. 2008;4:8. doi: 10.1186/1744-8069-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao S, Wolfe D, Glorioso JC, Mata M, Fink DJ. Effects of transgene-mediated endomorphin-2 in inflammatory pain. Eur J Pain. 2009;13:380–386. doi: 10.1016/j.ejpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou W, Guo Q, Chen C, Yang Y, Wang E. Intrathecal herpes simplex virus type 1 amplicon vector-mediated human proenkephalin reduces chronic constriction injury-induced neuropathic pain in rats. Mol Med Rep. 2011;4:529–533. doi: 10.3892/mmr.2011.445. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe D, Hao S, Hu J, Srinivasan R, Goss J, Mata M, et al. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain. 2007;133:29–38. doi: 10.1016/j.pain.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Sun J, Liu S, Mata M, Fink DJ, Hao S. Transgene-mediated expression of tumor necrosis factor soluble receptor attenuates morphine tolerance in rats. Gene Therapy. 2012;19:101–108. doi: 10.1038/gt.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W, Zheng W, Liu S, Zeng W, Levitt RC, Candiotti KA, et al. HSV-mediated p55TNFSR reduces neuropathic pain induced by HIV gp120 in rats through CXCR4 activity. Gene Therapy. 2014;21:328–336. doi: 10.1038/gt.2013.90. [DOI] [PubMed] [Google Scholar]
- 43.Hamza MA, Higgins DM, Ruyechan WT. Two alphaherpesvirus latency-associated gene products influence calcitonin gene-related peptide levels in rat trigeminal neurons. Neurobiol Dis. 2007;25:553–560. doi: 10.1016/j.nbd.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy PG, Montague P, Scott F, Grinfeld E, Ashrafi GH, Breuer J, et al. Varicella-zoster viruses associated with post-herpetic neuralgia induce sodium current density increases in the ND7-23 Nav-1. 8 neuroblastoma cell line. PLoS One. 2013;8:e51570. doi: 10.1371/journal.pone.0051570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, et al. Gene therapy for pain: results of a phase I clinical trial. Ann Neurol. 2011;70:207–212. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erazo A, Yee MB, Osterrieder N, Kinchington PR. Varicella-zoster virus open reading frame 66 protein kinase is required for efficient viral growth in primary human corneal stromal fibroblast cells. J Virol. 2008;82:7653–7665. doi: 10.1128/JVI.00311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marconi P, Krisky D, Oligino T, Poliani PL, Ramakrishnan R, Goins WF, et al. Replication-defective herpes simplex virus vectors for gene transfer in vivo. Proc Natl Acad Sci USA. 1996;93:11319–11320. doi: 10.1073/pnas.93.21.11319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan R, Huang S, Chaudhry S, Sculptoreanu A, Krisky D, Cascio M, et al. An HSV vector system for selection of ligand-gated ion channel modulators. Nat Methods. 2007;4:733–739. doi: 10.1038/nmeth1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 50.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 51.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]