Abstract
Purpose
Identify an orotopical vehicle to deliver an α-adrenergic vasoconstrictor to submucosal vasculature that is readily palatable to cancer/bone marrow transplant patients that suppresses chemo-radiotherapy-associated oral mucositis.
Methods
A [3H] norepinephrine ligand binding assay was developed to quantify receptor binding in hamster oral mucosa. Vehicle components (alcohols, polyols, cellulose, PVP) were tested versus [3H] norepinephrine binding. Vehicle refinement was also done to mask phenylephrine bitter taste and achieve human subject acceptance. The optimized vehicle was tested with α-adrenergic active agents to suppress radiation-induced oral mucositis in mice.
Results
The ligand binding assay quantified dose- and time-dependent, saturable binding of [3H] norepinephrine. An ethanol:glycerol:propylene glycol:water (6:6:8:80) vehicle provided the best delivery and binding. Further vehicle modification (flavoring and sucralose) yielded a vehicle with excellent taste scores in humans. Addition of phenylephrine, norepinephrine or epinephrine to the optimized vehicle and painting into mouse mouths 20 min before 19 Gy irradiation conferred significant suppression of the weight loss (P < 0.001) observed in mice who received oral vehicle.
Conclusion
We identified a highly efficient vehicle for the topical delivery of phenylephrine to the oral mucosa of both hamster and human subjects. This will enable its testing to suppress oral mucositis in an upcoming human clinical trial.
Keywords: alpha adrenergic vasoconstrictor, epinephrine, norepinephrine, phenylephrine, topical formulation
INTRODUCTION
The clinical impact of radiotherapy- and chemotherapy-induced oral mucositis includes pain, side effects from prolonged opioid usage, dehydration and weight loss, and local and systemic infection among others (1–3). Strategies to suppress or manage oral mucositis have taken a range of approaches from palliation with mucosal barriers, antibiotics, analgesics and oral hygiene to systemic growth factors to hasten reepithelialization of the mucosal barrier (4–6).
A strategy involving topical vasoconstrictor application for preventing radiotherapy and chemotherapy toxicities to skin and its organelles has emerged and has been proven effective in a preclinical study demonstrating suppression of radiation-or chemotherapy-induced alopecia (7) as well as in both preclinical and clinical studies showing up to 100% prevention of radiation dermatitis (8,9). A recent preclinical study has also shown efficacy of topical vasoconstrictor in suppression of oral mucositis in two separate animal models (10).
Clinical testing of topically applied vasoconstrictor to the oropharyngeal cavity prior to radiotherapy or chemotherapy as a means to suppress oral mucositis will require a delivery vehicle which provides: i) the liquid physical characteristic of a reduced surface tension that enable its sprayed or swished application with adhesion and coating of each type of oropharyngeal epithelial surface, ii) sustained solubility of the small molecule α-adrenergic agonist to enable both its delivery and chemically stable, long term storage, iii) efficient delivery of the α-adrenergic agonist across the mucosa to submucosal vasculature that lies ~1 mm beneath the mucosal surface, iv) a flavoring agent and sweetener to mask the bitter adrenergic agonist taste, and v) a coloring agent that enables both patient and nursing staff to see that the protective topical formulation has been applied to all of the at-risk oropharyngeal surfaces.
Topical application to the oral mucosa of a small molecule anesthetic like benzocaine for delivery to submucosal pain receptors can provide direction for the general design of a suitable adrenergic agonist mucosal delivery vehicle (11). But, issues specific to the individual α-adrenergic agonist, including: salt form and formula weight, solubility in co-solvents that enable mucosal coating, taste, α-adrenergic receptor affinity and thus required concentration to achieve receptor occupancy, and concentration of α-agonist catabolic enzymes in the oral mucosa environs, all impact the choice of the agonist, and thus, the topical vehicle constituents.
Topical, sublingual delivery has also been used to achieve systemic delivery of thin film formulated small molecule drugs like nitroglycerin, misoprostol and clonazepam that provide insight on transmucosal delivery (12).
Our goals in this study were to i) develop a [3H] α-adrenergic agonist ligand binding assay using a mucosal tissue target, here hamster oral mucosa, ii) use the assay to demonstrate saturable ligand binding, which was both time and ligand concentration-dependent, iii) systematically screen potential delivery vehicle components to quantify the contribution of each to efficient delivery of the α-adrenergic ligand to the tissue adrenergic receptor, iv) demonstrate that the most efficient topical delivery vehicle was fully functional in delivering phenylephrine and suppressing oral mucositis in a functional mouse assay of radiation-induced oral mucositis, and v) in a step to enable a clinical trial of this strategy, demonstrate both functional ability of the delivery vehicle to coat oral mucosa and to garner patient acceptance of its taste characteristics.
MATERIALS AND METHODS
Materials
(±)-epinephrine HCl, (L)-norepinephrine bitartrate, (R)-phenylephrine HCl, sodium pentobarbital, Nile Red dye and solvents for drug delivery were all obtained from Sigma (St. Louis, MO). [3H] norepinephrine (13.9 Ci/mmol) and Ultima Gold™ liquid scintillation cocktail were obtained from Perkin Elmer (Boston MA). Proteinase K (# 03 115887001) was from Roche. Female Golden Syrian hamsters (100 g) and ICR female mice (20–25 g) were purchased from Harlan Laboratories (Indianapolis, IN). Mice and hamsters were maintained on a 12 h light/dark cycle and provided water and Harlan 5305 (mice) or Harlan 2018 (hamsters) lab chow ad libitum. Animal procedures were approved by University of Wisconsin IACUC (Protocol # M0476).
[3H] Norepinephrine Hamster Cheekpouch Binding Assay
Vehicle formulations containing [3H] norepinephrine (13.9 Ci/mmol) were first prepared. A 1.10 ml aliquot of vehicle formulation (0.50 ml/cheekpouch × 2 cheekpouches/hamster) was prepared in an eppendorf tube. 5.5 μl aliquots of [3H] norepinephrine [5.50 μCi, 0.395 nmol norepinephrine] were added to the 1.10 ml formulations composed as shown in Table I. “Water” vehicle formulations contained 30 mM potassium phosphate buffer at pH 7.0. Duplicate 2.00 μl aliquots of each formulation were counted in a Beckman scintillation counter.
Table I.
Screening of Orotopical Delivery Vehicle Formulations for the Highest [3H] Norepinephrine Binding in Hamster Cheek Pouch Tissue
| Vehicle | Solvent Components (vol):(vol):(vol):(vol) | Solvent Percentages | [3H] Norepinephrine cpm/500 mg Cheek Pouch Mean±SD (n≥4) | P value Versus Group 13 | Percentage of Group 13 Binding |
|---|---|---|---|---|---|
| 1 | Water | 100 | 16783±1760 | .004 | 57 |
| 2 | PEG:water | 80:20 | 8549±1221 | .004 | 29 |
| 3 | HPMC:water | 1:99 | 19614±1681 | .016 | 66 |
| 4 | ETOH:PEG:water | 20:30:50 | 10716±1966 | .028 | 36 |
| 5 | “ | 15:30:55 | 17692±2613 | .028 | 60 |
| 6 | “ | 10:30:60 | 10654±2890 | .047 | 36 |
| 7 | “ | 5:30:65 | 13799±3934 | .028 | 47 |
| 8 | “ | 20:25:55 | 16356±1833 | .026 | 55 |
| 9 | “ | 20:20:60 | 12328±3313 | .029 | 42 |
| 10 | “ | 20:15:65 | 18459±6151 | .020 | 62 |
| 11 | “ | 10:15:75 | 16714±1839 | .028 | 56 |
| 12 | ETOH:glycerol:PG:water | 3:6:8:83 | 33481±5422 | .685 | 113 |
| 13 | “ | 6:6:8:80 | 29458±524 | - | 100 |
| 14 | “ | 9:6:8:77 | 38770±15785 | .342 | 131 |
| 15 | “ | 12:6:8:74 | 21551±4088 | .028 | 73 |
| 16 | “ | 6:3:8:83 | 26548±1395 | .114 | 90 |
| 17 | “ | 6:9:8:77 | 25926±6462 | .685 | 88 |
| 18 | “ | 6:12:8:72 | 23294±8030 | .342 | 79 |
| 19 | “ | 6:6:5:83 | 28455±1575 | .685 | 96 |
| 20 | “ | 6:6:11:77 | 22387±2330 | .028 | 75 |
| 21 | “ | 6:6:14:74 | 30403±9425 | .342 | 103 |
| 22 | ETOH:glycerol:PG:water:PVP | 6:6:8:80:0 | 29458±5758 | - | 100 |
| 23 | “ | 6:6:8:78:2 | 12297±5126 | .021 | 41 |
| 24 | “ | 6:6:8:76:4 | 14143±6334 | .028 | 48 |
| 25 | “ | 6:6:8:74:6 | 7813±2320 | .031 | 26 |
| 26 | “ | 6:6:8:72:8 | 20001±8915 | .114 | 67 |
| 27 | “ | 6:6:8:70:10 | 6046±256 | .032 | 21 |
PEG polyethylene glycol, ETOH ethanol, PG propylene glycol, PVP polyvinylpyrrolidone, HPMC hydroxypropylmethylcellulose
Hamsters were anesthetized under 5% isoflurane, and cheekpouches were everted with blunt forceps, rinsed with water, blotted, and repositioned in cheeks. A 0.5 ml aliquot of [3H] norepinephrine vehicle formulation was delivered from a 1.0 ml disposable syringe into each cheekpouch. A blunt forceps was used to spread open the cheekpouch while filling it with the 0.5 ml volume that nearly filled the cheekpouch. Hamsters were placed on their backs in a chamber charged with 2% isoflurane. After 1.0 or 4.0 min of incubation, hamsters were placed on an absorbant pad and were given an immediately lethal 0.2 ml injection of Nembutal to the brain administered through the eye orbital cavity using a 23 g needle. Cheekpouches were quickly everted, and with a scissors, clipped free of their attachments to the cheek, clipped completely open with scissors, drained of their radioactive contents onto the absorbant pad, and dropped into a glass scintillation vial containing 10 ml of phosphate-buffered saline. Both pouches from the same hamster were “washed” concurrently but separately with 7 × 10 ml exchanges (vortex 10 s) of phosphate-buffered saline. No additional [3H] cpms were released after the seventh wash. Pouches were blotted to dryness and weighed, and transferred to 20 ml glass scintillation vials.
Pouches were digested in buffer containing Proteinase K at 50°C overnight, shaking, until the solution was clear. One ml of digestion buffer (10 mM Tris HCl, pH 7.5, 25 mM EDTA, 100 mM NaCl, 0.5% SDS, 100 μg/ml Proteinase K) was added per 80 mg of cheekpouch weight. One ml aliquots of the digest were added to 10 ml Ultima Gold scintillation cocktail and counted. After sample counting, 10,000 dpm of a tritium counting standard was added to some of the previously counted vials, and they were recounted to establish counting efficiency. Raw cpms were normalized for counting efficiency and for a standard amount of cheekpouch weight (500 mg) before plotting the “adjusted cpms.”
Mouse Oral Mucositis Assay
Assays were done as previously described (10). Briefly, following a single IP injection of pentobarbital (55 μg/g BW), the oral cavity, tongue and lips were painted for 10 s with topical vehicle (+/− phenylephrine, epinephrine or norepinephrine at specified concentrations) using a small artist’s paintbrush. Twenty minutes later, the opened snouts of the sleeping mice were irradiated in a J.L. Shepherd Cs137 irradiator. Thermoluminescent dosimeters on the mouse mouths indicated a dose rate of 2.0 Gy/min. Mice were subsequently observed and weighed daily for the next 14–20 days; representative mice from some treatment groups were photographed and some mice were euthanized and tissue samples were taken for histology.
Additional Procedures
To determine the efficiency with which a topical delivery vehicle could deliver an organic molecule similar to phenylephrine HCl (FW: 204) to oral mucosa and submucosal vasculature, Nile Red (FW: 318) was applied topically to hamster cheekpouch mucosa in several vehicles, and mucosa tissue samples were excised 30 min later for fluorescence microscopy analysis. Unfixed mucosal tissue samples were embedded in OCT (Tissue-Tek™, Fisher) and sectioned.
To assess the practicality of orotopical spray delivery in humans as well as the coating and retention of delivery vehicle on the human oral cavity surface, the chosen delivery vehicle, alone, with no active vasoconstrictor added, was sprayed into mouths using a Madomizer™ (Moore Medical, Farmington, CT) spray applicator. Each applicator pulsed spray delivered 0.10 ml of liquid. The delivery vehicle used in the experiment contained: ethanol:glycerol:propylene glycol:water (6:6:8:80) with 0.10 mg/ml erioglaucine, 2.0 μl/ml cherry flavor (#D19, LorAnn Oils) and 15 mg/ml Splenda sweetener.
To determine our ability to mask the bitter taste of phenylephrine to achieve patient acceptance of an orotopical phenylephrine formulation, the solutions indicated in Table II were prepared and sterile-filtered, and 2.0 ml aliquots were then taste-tested by a panel of five normal human subjects and scored for desirability of taste on a 0–10 scale.
Table II.
Phenylephrine Vehicle Formulation Taste Scores
| Treatment Group | Oral Formulation | Taste Desirability Score 0=Undesirable 10=Very Desirable |
|---|---|---|
| 1 | Control 5% sodium chloride 5% sodium bicarbonate 90% water |
0.5±0.3 |
| 2 | 0.25% (12.3 mM) Phenylephrine 99% water |
2.5±0.5a |
| 3 | 0.25% Phenylephrine 0.25% cherry flavor 16% sucrose 84% water |
7.8±0.5 |
Some subjects indicated that sample “tasted bitter” or “tasted like medicine”
Graphpad Prism software, typically using Student t test, was used for analysis of differences between treatment groups.
RESULTS
Topical Mucosal Delivery and Fluorescent Dye Distribution
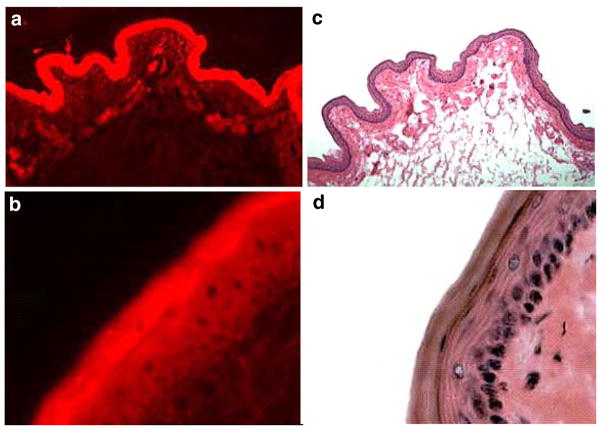
Histologic analysis of the hamster cheekpouch (Fig. 1d) shows standard oral mucosa architecture with progenitor cells in the stratum basale which differentiate through the stratum granulosum into the cornified squames of the thin surface stratum corneum. Circular blood vessels can be seen in the lamina propria and connective tissue that underlie the mucosa (Fig. 1c). Figure 1a and b illustrate that the Nile Red small molecule topically applied in an ethanol:water (50:50) delivery vehicle clearly permeates all aspects of the mucosal and submucosal structures, including the lamina propria, thus accessing the vasculature to which the vasoconstrictor must bind to achieve constriction and secondary, transient hypoxia of (and/or reduced systemic chemotherapy delivery to) the overlying tissue that includes the at-risk mucosal progenitor cells. Because the Nile Red formula weight (318) is greater than that of phenylephrine HCl (204) and they have similar hydrophobicities, we concluded that a topically applied adrenergic vasoconstrictor in an alcohol:water vehicle would be delivered in a like manner throughout the oral mucosa.
Fig. 1.
Fluorescence microscopy cross section ((a), 10×; (b), 50× magnification) of hamster cheek pouch mucosa to which Nile Red dye was applied in an ethanol:water (50:50) topical delivery vehicle. Histology cross section ((c); 10×, (d): 50× magnification) of hamster cheek pouch mucosa stained with hematoxylin and eosin.
[3H] a-Adrenergic Agonist Ligand Binding Assay
In initial experiments with a simple delivery vehicle of 30 mM potassium phosphate pH 7.0, topical delivery of the [3H] norepinephrine ligand to intact hamster cheekpouches achieved nearly saturated binding by 16 min (Fig. 2a). A linear, time-dependent increase in ligand binding was observed over the first 4 min following topical application of [3H] norepinephrine to the cheekpouch. Because we were interested in studying, and maximizing, the degree of ligand binding that was accomplishable in a 1 min topical application to patients in a clinical setting, we limited further observations and measurement of ligand binding to timepoints in the first 1–4 min following topical application.
Fig. 2.
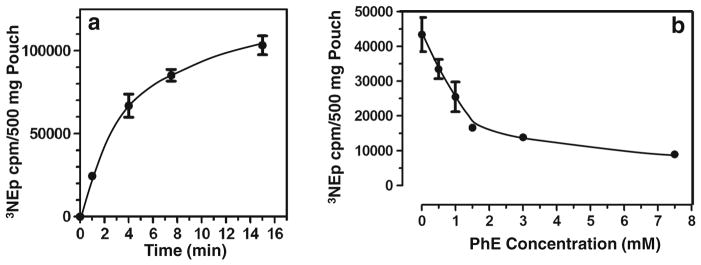
Quantification of [3H] norepinephrine binding and adrenergic agonist competitive displacement from hamster mucosa. [3H] norepinephrine (2.25 μCi, 0.198 nmol norepinephrine) dissolved in 0.5 ml of topical vehicle formulation, here 30 mM potassium phosphate buffer at pH 7.0, was delivered to the hamster cheekpouch (see Materials and Methods), and after the indicated times (a) or 4 min (b), hamsters were euthanized, cheekpouches were removed, minced and washed in phosphate-buffered saline. Following Proteinase K digestion to clarity, samples were counted in a scintillation counter. Unlabeled phenylephrine HCl was added to the [3H] norepinephrine formulations at the indicated concentrations (b) and each formulation was then tested in at least 4 hamster cheekpouches.
The majority (34,000/44,000) of the bound [3H] norepinephrine cpms in the cheekpouch mucosa were displaced by a large molar excess (4 mM phenylephrine/0.4 μM [3H] nor-epinephrine) of cold phenylephrine (Fig. 2b), indicating that 7 × 10 s washes in phosphate-buffered saline of the minced cheekpouch tissue after recovery from the hamster were sufficient to remove the majority of nonspecifically bound [3H] norepinephrine and thus provide an assay with a valid representation of how changes in topical vehicle composition affected delivery of an α-adrenergic agonist to specific binding sites within the cheekpouch mucosal tissue.
With a validated assay in hand to quantify specific mucosal binding of a topically delivered α-adrenergic agonist, we undertook a systematic analysis of topical vehicle components with the goal of identifying the vehicle formulation that would confer the highest level of [3H] norepinephrine binding during a 1 min incubation in the intact hamster cheekpouch (Table I). As seen in Table I, a wide variety of alcohols, polyols and known permeation enhancers were tested in various combinations.
The second-lowest binding level was seen with a vehicle that was 80% polyethylene glycol and 20% water (Group 2). The mucosal tissue looked “fixed” and somewhat rubbery after the incubation. The addition of cellulose to water (Group 3) provided a viscous vehicle with excellent mucosa coating capability, but it provided little improvement over water in ligand binding. The addition of increasing concentrations of a “penetration enhancer,” polyvinylpyrrolidone, conferred a decreasing improvement in ligand binding, and at 10% PVP added to the otherwise best vehicle (Group 27) we saw the lowest overall binding. Vehicles using the addition of polyethylene glycol and ethanol to water (Groups 4–11) gave no improvement over water alone.
The combined addition of ethanol, glycerol and propylene glycol, in aggregate about 20% of the total volume, to water by far gave the most efficient topical delivery vehicles (Groups 12–21). Though some percentages other than the 6:6:8:80 (ethanol:glycerol:propylene glycol:water) formulation we chose as the best (Group 13) gave values greater than “100%,” none of the values were statistically greater, and at total alcohol concentrations much greater than 20% it became somewhat uncomfortable to hold the material in one’s mouth for 1 min after orotopical application.
Orotopical Vehicle Application to Humans
To determine: i) how well the optimum vehicle chosen above (Group 13) would spread and adhere to human oral mucosa, and ii) what Group 13 vehicle volume would be required to create a film that covered a defined mucosal surface, the Group 13 vehicle was initially sprayed into a human subject’s oral cavity. To visually assess the degree of surface coating and to monitor the volume required to do so, a food coloring (Blue-4/erioglaucine) was added to the vehicle at 0.1%, and a commercially available spray applicator (LMA MADomizer™) was adopted because it delivered liquid at 0.10 ml/spray, making it easy to record the volume applied to a surface to achieve appropriate coverage and retained coating. A single 0.10 ml spray of the dye-containing Group 13 vehicle to the human buccal wall gave a thin blue coating to the surface (Fig. 3b). Ten 0.10 ml sprays to the buccal wall area gave a denser blue coating with modest pooling at the base of the buccal wall (Fig. 3c, +insert image) as evidenced by some vehicle/dye uptake by a cotton roll placed at the base of the buccal wall. Fifteen sprays gave no denser blue coating on the buccal wall, but substantial runoff and pooling at the base of the buccal wall was observed (Fig. 3c, +insert). With 5–7 sprays to each buccal wall, and with spraying at the same “rate” to other surfaces in the oropharyngeal cavity, we observed uniform blue coverage by the blue Group 13 vehicle of the entire adult oropharyngeal surface using 30 × 0.10 ml sprays. Per the study by Kerr et al. (13), which measured a total surface area of 175 cm2 within an adult human oral cavity, 3.00 ml vehicle/175 cm2 area equals a spray application rate of 17 μl liquid/cm2 of oral surface area.
Fig. 3.
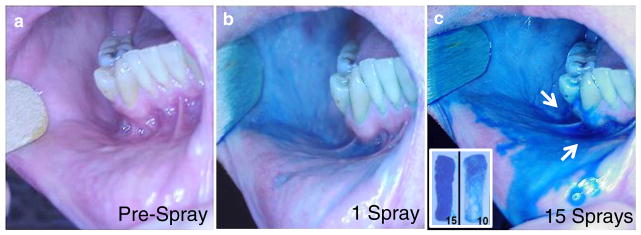
Sprayed application of optimized orotopical delivery vehicle to assess surface coating and retention properties. Oral cavity (a) before, (b) following 1× 0.10 ml sprayed application from a Madomizer applicator and (c) following 15×0.10 ml sprayed applications. (c) Excess topical vehicle from the 15 sprays can be seen pooled (arrows) in the buccal-gingival trough. In a parallel experiment, dental cotton rolls were placed in the buccal-gingival trough just before either 10 or 15×0.10 ml sprayed applications to the buccal wall. Cotton rolls were then retrieved (C inset) to assess amount of excess topical formulation that had not been retained on the buccal wall.
Flavoring in Vehicle to Suppress Phenylephrine Bitterness
To determine whether phenylephrine would be tolerated in an orotopically applied drug to suppress oral mucositis, 0.25% (12.3 mM) phenylephrine as a 2.0 ml taste sample in water alone was given to five healthy humans who swished it in their mouths for 5 s and then spit the sample out. Subjects were then asked to score the taste desirability on a 0 to 10 scale of this phenylephrine sample versus two other samples, the contents of which were all blinded to the subjects. Table II shows that subjects disliked the taste of phenylephrine in water; some subjects indicated that it “tasted bitter,” or “it tasted like medicine.” It was clear with Treatment Group 3 (in Table II) that addition of both 0.25% of a commercial cherry flavor along with 16% sucrose as a sweetener markedly improved subject acceptance of the phenylephrine once it contained appropriate taste masking materials.
Optimum Vehicle With Phenylephrine Suppresses Oral Mucositis in Mice
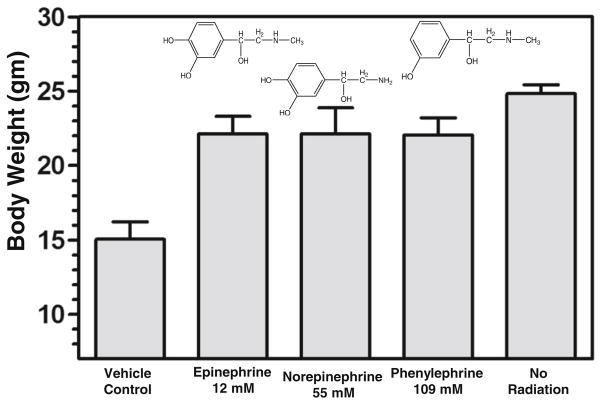
Using the radiation-induced mouse oral mucositis assay developed in our laboratory (10), the Group 13 topical delivery vehicle, now optimized to also contain 0.1% erioglaucine dye, 0.25% cherry flavor and 1.5% sucralose (Splenda™) sweetener, was tested for its efficacy as a vehicle to enable phenylephrine, norepinephrine or epinephrine suppression of oral mucositis. Figure 4 shows that each of the three α-adrenergic vasoconstrictors, topically applied (painted on) to the mouse oral cavities 20 min before irradiation, suppressed greater than 75% of the weight loss (P<0.001) seen in mice who received only topical vehicle to their oral cavities before irradiation with 19 Gy to their snouts.
Fig. 4.
Significant suppression of radiation-induced weight loss in mice treated with topically applied α-adrenergic vasoconstrictors to the oral cavity surface before irradiation. Adrenergic agonists at the indicated concentrations were dissolved in an optimized orotopical delivery vehicle comprising ethanol:glycerol:propylene glycol:water (6:6:8:80) containing blue dye, cherry flavor and Splenda sweetener (see Fig. 3) and then painted onto orotopical surfaces of mice 20 min before irradiation. Following 19 Gy irradiation of the snout, mice were weighed and observed daily for 20 days post-irradiation.
DISCUSSION
The occurrence of oral mucositis during bone marrow transplant, radiotherapy and chemotherapy is common. Clinical management of the pain and narcotic side effects, dehydration, dysphagia and weight loss, oral and septic infections, and substantial costs to HMOs are significant problems. The U.S. Cancer Pain Relief Committee concluded that “effective treatments to reduce the pain and functional impairment of oral mucositis are needed in this patient population (14).”
Our goal in these experiments was to identify an orotopical vehicle that could efficiently deliver an α-adrenergic vasoconstrictor to submucosal blood vessels and that could be made readily palatable to enable patient acceptance of repeated applications to their mouths, such as during the conditioning regimen prior to bone marrow transplant or daily radiotherapy sessions for a head and neck tumor.
The delivery vehicle with a 20% content of alcohols that included ethanol and the polyols glycerol and propylene glycol (Group 13) worked very well (P=0.004 versus water vehicle) to efficiently deliver an α-adrenergic agonist, [3H] norepinephrine, to specific oral mucosa binding sites, which could be competed off by our target α-adrenergic agonist, phenylephrine. Phenylephrine doesn’t bind to β1 or β2 adrenergic receptors so it lacks the cardiac side effects associated with β agonist function (15); this makes it a safer active agent for this orotopical application.
Minor modifications in each of the alcohol or polyol contents of Group 13 (Table I) provided improved, but not significant, differences in vehicle delivery performance. Human testing (not shown) also indicated that combined alcohol contents greater than 20% began to feel uncomfortable (e.g., stinging) when held in the mouth for 1 min after application. Other delivery vehicle components that were tested, including i) hydroxypropylmethylcellulose (16), polyethylene glycol (17), and polyvinylpyrrolidone (18), which have been used widely in other delivery vehicles including the oral vehicles cited here, provided no improvement in delivery of the small molecule adrenergic agonist. These aggregate observations, most of which are non-intuitive, directed us to adopt Group 13 as the vehicle for future human clinical trial use.
The physical characteristics of the Group 13 vehicle were also excellent for both spray delivery from the commercial LAM MADomizer™ spray applicator as well as oral mucosal coating properties. By providing a uniform, adherent film on the buccal and other oral surfaces, it provides time for transmucosal delivery of phenylephrine to submucosal vasculature. The MADomizer applicators are commercially available and can be used to dispense precise volumes of the oral formulation (19).
Significant for the clinical use of this OM-suppression strategy, we found that an unacceptable, poorly tolerated taste of phenylephrine could be masked by addition of a flavoring agent and sweetener (Table II and ref. 8). A score of 7.8 on a 0–10 Scale indicates ready human acceptance and will enable its use in the clinical setting.
The efficacy of the Group 13 formulation to enable highly significant suppression of OM in the mouse model (P<0.001) is very encouraging because this vehicle is now ready to be used in a planned Phase I/IIa clinical trial to determine both the safety and efficacy of orotopical phenylephrine in suppressing the Grade 3 oral mucositis that is observed in >95% of bone marrow transplant patients who receive the cyclophosphamide + total body irradiation conditioning regimen.
In conclusion, we have identified a highly efficient vehicle for the topical delivery of phenylephrine to the oral mucosa of both hamster and human subjects, and with further modification of taste, this technically efficient vehicle also became a well-tolerated vehicle that masked unfavorable phenylephrine taste to enable its use and testing to suppress oral mucositis.
Acknowledgments
This work was supported by grants from the Wisconsin Alumni Research Foundation (WARF, University of Wisconsin-Madison) and ProCertus BioPharm, Inc. (Madison, WI).
ABBREVIATIONS
- OM
Oral mucositis
Contributor Information
Cheryl M. Soref, McArdle Laboratory for Cancer Research, University of Wisconsin Carbone Cancer Center, Madison, Wisconsin, USA. ProCertus BioPharm, Inc., Madison, Wisconsin, USA
William E. Fahl, Email: fahl@oncology.wisc.edu, McArdle Laboratory for Cancer Research, University of Wisconsin Carbone Cancer Center, Madison, Wisconsin, USA. ProCertus BioPharm, Inc., Madison, Wisconsin, USA. 1400 University Avenue, Madison, Wisconsin 53706, USA
References
- 1.Scully C, Sonis S, Diz PD. Mucosal diseases series: oral mucositis. Oral Dis. 2006;12:229–42. doi: 10.1111/j.1601-0825.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JB, Beaumont JL, Gwede CK. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer; a patient-reported outcomes questionnaire. Cancer. 2007;109:1914–22. doi: 10.1002/cncr.22620. [DOI] [PubMed] [Google Scholar]
- 3.Trotti A, Bellm LA, Epstein JB. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal DI, Trotti A. Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol. 2009;19:29–34. doi: 10.1016/j.semradonc.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Okuno SH, Foote RL, Loprinzi CL. A randomized trial of a nonabsorbable antibiotic lozenge given to alleviate radiation-induced mucositis. Cancer. 1997;79:2193–9. doi: 10.1002/(sici)1097-0142(19970601)79:11<2193::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Nicolatou-Galitis O, Sarri T, Bowen J. Systematic review of amifostine for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21:357–64. doi: 10.1007/s00520-012-1613-6. [DOI] [PubMed] [Google Scholar]
- 7.Soref CM, Fahl WE. A new strategy to prevent chemotherapy and radiotherapy-induced alopecia using topically applied vasoconstrictor. Int J Cancer. doi: 10.1002/ijc.28961. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahl WE, Ruoho AE, Mehta MP. Topical vasoconstrictor preparations and methods for protecting cells during cancer chemotherapy and radiotherapy. 8,114,914. US patent. ( http://patft.uspto.gov)
- 9.Cleary JF, Anderson BM, Cannon G, Fahl WE. Exploratory study of topical norepinephrine for the prevention of radiodermatitis in post-surgical breast cancer patients. Int J Rad Oncol Biol Phys. (submitted) [Google Scholar]
- 10.Soref CM, Fahl WE. A new topical vasoconstrictor-based strategy for prevention of oral mucositis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:454–61. doi: 10.1016/j.oooo.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Carr MP, Horton JE. Evaluation of a transoral delivery system for topical anesthesia. J Am Dent Assoc. 2001;132:1714–9. doi: 10.14219/jada.archive.2001.0127. [DOI] [PubMed] [Google Scholar]
- 12.Kathpalia H, Gupte A. An introduction to fast dissolving oral thin film drug delivery systems: a review. Curr Drug Deliv. 2013;10:667–84. doi: 10.2174/156720181006131125150249. [DOI] [PubMed] [Google Scholar]
- 13.Kerr WJ, Kelly J, Geddes DA. The areas of various surfaces in the human mouth from nine years to adulthood. J Dent Res. 1991;70:1528–30. doi: 10.1177/00220345910700121001. [DOI] [PubMed] [Google Scholar]
- 14.Scully C, Sonis S, Diz PD. Mucosal diseases series: oral mucositis. Oral Dis. 2006;12:229–41. doi: 10.1111/j.1601-0825.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- 15.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s the pharmacologic basis of therapeutics. 12. New York: McGraw Hill; 2011. pp. 277–334. [Google Scholar]
- 16.Peh KK, Wong CF. Polymeric films as vehicle for buccal delivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharm Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 17.Ong CM, Heard CM. Permeation of quinine across sublingual mucosa, in vitro. Int J Pharm. 2009;366:58–64. doi: 10.1016/j.ijpharm.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Rao PR, Chalasani KB, Chauhan AS, Jain AK, Diwan PV, Ram MK. Controlled systemic delivery of indomethacin using membrane-moderated, cream formulation-based transdermal devices. Drug Deliv. 2006;13:207–13. doi: 10.1080/10717540500309172. [DOI] [PubMed] [Google Scholar]
- 19.http://www.lmana.com/pwpcontrol.php?pwpID=6549