Abstract
It is becoming increasingly apparent that beta cell dysfunction resulting in abnormal insulin secretion is the essential element in the progression of patients from a state of impaired glucose tolerance to frank type 2 diabetes (Del Prato, 2003; Del Prato and Tiengo, 2001). Although extensive studies have examined the molecular, cellular and physiologic mechanisms of insulin granule biogenesis, sorting, and exocytosis the precise mechanisms controlling these processes and their dysregulation in the developed of diabetes remains an area of important investigation. We now know that insulin biogenesis initiates with the synthesis of preproinsulin in rough endoplastic reticulum and conversion of preproinsulin to proinsulin. Proinsulin begins to be packaged in the Trans-Golgi Network and is sorting into immature secretory granules. These immature granules become acidic via ATP-dependent proton pump and proinsulin undergoes proteolytic cleavage resulting the formation of insulin and C-peptide. During the granule maturation process, insulin is crystallized with zinc and calcium in the form of dense-core granules and unwanted cargo and membrane proteins undergo selective retrograde trafficking to either the constitutive trafficking pathway for secretion or to degradative pathways. The newly formed mature dense-core insulin granules populate two different intracellular pools, the readily releasable pools (RRP) and the reserved pool. These two distinct populations are thought to be responsible for the biphasic nature of insulin release in which the RRP granules are associated with the plasma membrane and undergo an acute calcium-dependent release accounting for first phase insulin secretion. In contrast, second phase insulin secretion requires the trafficking of the reserved granule pool to the plasma membrane.
The initial trigger for insulin granule fusion with the plasma membrane is a rise in intracellular calcium and in the case of glucose stimulation results from increased production of ATP, closure of the ATP-sensitive potassium channel and cellular depolarization. In turn, this opens voltage-dependent calcium channels allowing increased influx of extracellular calcium. Calcium is thought to bind to members of the fusion regulatory proteins synaptogamin that functionally repressors the fusion inhibitory protein complexin. Both complexin and synaptogamin interact as well as several other regulatory proteins interact with the core fusion machinery composed of the Q- or t-SNARE proteins syntaxin 1 and SNAP25 in the plasmamembrane that assembles with the R- or v-SNARE protein VAMP2 in insulin granules. In this chapter we will review the current progress of insulin granule biogenesis, sorting, trafficking, exocytosis and signaling pathways that comprise the molecular basis of glucose-dependent insulin secretion.
I. Introduction
In the postprandial state, a variety of nutritional factors in circulation including amino acids, fatty acids and glucose serve as insulin secretagogues resulting in the release of insulin that initiates signaling cascades responsible for the suppression hepatic glucose output, increased macromolecular synthesis (glycogen and triglycerides) and stimulation of peripheral tissue (skeletal muscle and adipose tissue) uptake of glucose. In addition, signals in the gastrointestinal track stimulate the release of gut hormones (incretins) in particular glucagon-like peptide-1 (GLP-1) that markedly potentiates glucose-stimulated insulin secretion (Holst, 2007). Defects in the actions of insulin result in a physiologic state of insulin resistance in which relatively higher concentrations of insulin are required to maintain normal glucose homeostasis. However, type II diabetes only ensures when cell insulin secretory properties becomes abnormal and/or the levels of secreted insulin are insufficient to compensate for the increase demand. The dysfunction of insulin secretion and associated hyperglycemia ultimately leads to microand macrovascular damage, causing long-term complications including neuropathy, nephropathy, retinopathy and cardiovascular disease that significantly affects quality of life and reduces life expectancy.
As insulin secretion is a unique property of pancreatic beta cells in the Islets of Langerhans, considerable effort has been applied to understand the biology of this cell type. The pancreas is composed of both an exocrine component (responsible for the release of digestive enzymes into the gastrointestinal lumen), and a much smaller endocrine component composed of the islets that are responsible for the regulated release of a variety of hormones into the circulation. Central component of the islet are the insulin secreting beta cells surrounded by a smaller amount of alpha cells (glucagon), delta cells (somatastatin), PP cells (pancreatic polypeptide) (Weir and Bonner-Weir, 1990) and perhaps Ghrelin secreting cells (Volante et al., 2002; Wierup et al., 2002). These cells release these hormones into the portal circulation and following first pass through the liver enter into the systemic circulation. Of all these cell types, the insulin secreting beta cells have been the primary focus of research effort, in part due to the severity of Type 1 diabetes in which cellular autoimmunity results in the destruction of beta cells and the loss of insulin secretion. In addition, Type 2 diabetes typically initiates with peripheral insulin resistance that continues to increase in severity but only progresses to the diabetic state when beta cells are no longer able to compensate for the worsening insulin resistance.
Cumulative studies on insulin secretion have defined several of the basic mechanisms responsible for insulin biogenesis and processing, dense-core granule formation, intracellular sorting and signaling pathways mediating the trafficking and fusion of insulin granules with the plasma membrane. Although this framework has provided significant insight into these processes, there are numerous areas of these signals and molecular pathways that remain to be studied. More importantly, many of the pathophysiological alterations in the coupling between different signaling pathways mediating insulin granule release remain at the forefront of our understanding of beta cell dysfunction that is leading cause of beta cell failure and hence Type II diabetes. In this chapter, we will attempt to review the current progress and our understanding of insulin granule biogenesis, sorting, trafficking and fusion with the plasma membrane resulting in insulin secretion and the mechanisms by which these events are controlled through intracellular signaling and metabolic pathways.
II. Section I
A. Insulin granule biogenesis (Fig. 16.1)
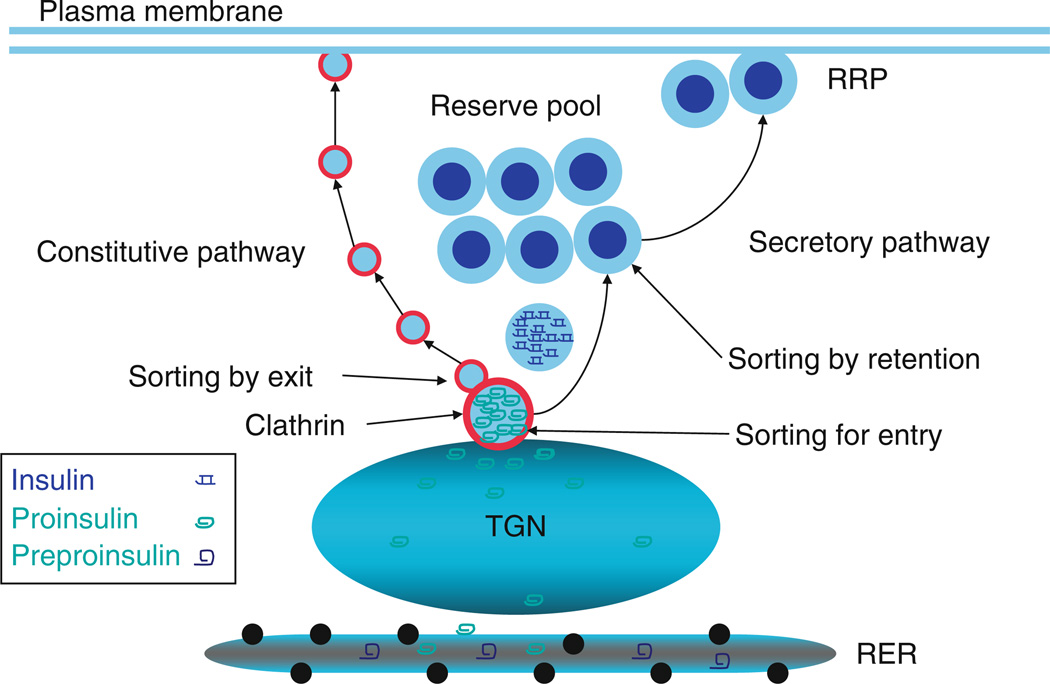
Figure 16.1.
Insulin biogenesis: Preproinsulin is synthesized in rough endoplasmic reticulum (RER) and is converted to proinsulin. Proinsulin is later transported to Trans-Golgi Network (TGN) and packed into immature granule via sorting for entry. The immature granule loses the clathrin coating and other unwanted components through sorting by exit. In parallel, proinsulin is converted to insulin and condensation/crystallization (sorting by retention) occurs. These processes account for the maturation of the immature insulin granules into a mature insulin secretory granule following TGN exit.
Insulin is initially synthesized as preproinsulin on the rough endoplasmic reticulum (RER) and during co-translational insertion into the lumen is converted to proinsulin by removal of the amino terminal signal sequence (Dodson and Steiner, 1998). The initiation of specific proinsulin sorting probably occurs in RER (Balch et al., 1994; Tooze et al., 1989) as many cargo proteins are concentrated during export from the endoplasmic reticulum (Balch et al., 1994). In addition, zinc and calcium may become packaged with proinsulin in RER lumen en route to the Golgi (Howell et al., 1978). However, the major sorting and packaging events appear to occur in Trans-Golgi Network (TGN).
B. Proinsulin TGN sorting
In the TGN cargo molecules destined for constitutive (unregulated secretion) are packaged into small transport vesicles that either directly traffic to and fuse with the plasma membrane or alternatively enters the constitutively recycling endosome system and thereby traffic to the plasma membrane. These constitutive endomembrane trafficking pathways allow for rapid movement of membrane phospholipids, integral membrane and secreted proteins from TGN to plasma membrane (Farquhar and Palade, 1981; Kelly, 1985; Palade, 1975). For example, the plasma form of alkaline phosphatase (SEAP) is release in a constitutive manner, and is restricted away from the regulated secretory pathway in beta cells (Molinete et al., 2000b).
In contrast, the regulated secretory pathway involves the concentration and packaging of proinsulin into immature secretory granules, a process termed sorting for entry. The sorting and packaging of proinsulin into immature granule is very dynamic and highly efficient process with the vast majority (over 99%) of proinsulin molecules being correctly sorted to compartments that will directly generate dense-core glucose-stimulated secretory granules (Rhodes and Halban, 1987). Although the exact mechanism of proinsulin sorting in TGN is far from clear, it is thought to occur through interactions between elements of TGN membrane and specific features of the proinsulin molecule. Early studies suggested the presence of a TGN sorting receptor that bound to specific subtypes of cargo proteins allowing for a selective TGN membrane domain that would develop a clatherin coat (Halban and Irminger, 1994; Orci, 1982). The first identified “sorting receptor” was a 25kD Golgi protein but subsequent studies demonstrated that this was not a specific cargo recognizing receptor (Chung et al., 1989; Irminger et al., 1997; Thiele et al., 1997). More recently, carboxypeptidase E (CPE), a prohormone converting enzyme, was suggested to have dual function, both as processing enzyme as well as the sorting receptor in all endocrine cells (Cawley et al., 2003; Dhanvantari et al., 2003). However, CPE loss of function failed to impair proinsulin targeting to the regulated pathway (Natori and Huttner, 1996) and is now thought to serve as a chaperone in the sorting of several prohormones (Kim et al., 2001). Since no specific TGN sorting receptor has been identified, several interesting alternative models have emerged, such as the self-organization of TGN luminal and membrane lipids. In this model, proteins in the lumen of the TGN aggregate in a mildly acidic, high Ca2+ concentration environment, and the aggregated proteins directly interact with lipid membrane cholesterol, which in turn leads to reorganization of cholesetrol-rich microdomains. The subsequent budding of these cholesterol-rich micro-domains provides the nascent characteristic of immature secretory granules that are destined to enter the regulated secretory pathway.
At present the hunt for specific sorting receptor/mechanisms remains an ongoing effort, however significant progress has been made in the identification of specific features of prohormones that are responsible for secretory pathway trafficking. For example, expression of mutants of the proinsulin at residues 16 (Leu) and 17 (Glu) of A-chain and 13 (Glu) and 17 (Leu) of B-chain results in proinsulin default to the constitutive pathway in AtT20 or Neuro2a cells (Molinete et al., 2000a). This result suggests that these regions may serve as “sorting domains.” Other studies suggested that the sorting domains are located in the insulin B-chain of proinsulin via comparison of various prohormone sequences and molecular modeling techniques (Gorr and Darling, 1995; Kizer and Tropsha, 1991). However, most of these studies did not employ primary β cell or cultured β cell lines, and thus may not necessarily represent the sorting process of proinsulin from TGN to immature granules in β cells in vivo. Nevertheless, the current consensus is that proinsulin and other secretory granule proteins have specific sorting information for entry into the secretory granules in TGN.
C. Granule maturation
Although the TGN plays an important role in defining the targeting of proinsulin away from the large amount of bulk flow proteins thereby increasing proinsulin trafficking efficiency (sorting for entry), the immature secretory granule itself probably serves as a critical secondary decision point through the mechanisms termed sorting for exit and sorting by retention (Arvan and Castle, 1992, 1998; Arvan and Halban, 2004). As described above, proinsulin is initially packaged in the immature granules emanating from the TGN. Subsequent granule maturation can be divided into three steps: (i) acidification of the granule lumen, (ii) conversion of proinsulin to insulin and C-peptide through proteolysis by endoproteases PC1/3 and PC2, followed by trimming of the carboxyl termini carboxypeptidase E, and (iii) loss of the coat protein clatherin (Orci, 1982; Orci et al., 1985, 1986). Several studies have shown that mildly acidic immature granule become more acidic as the granule mature (Orci, 1985; Orci et al., 1986). The granule luminal ATP-dependent proton pump serves to produce the acidification of the granule milieu that facilitates the conversion of proinsulin to insulin since both convert enzymes (endoproteases PC1/3 and PC2) display an acidic pH optimum (Davidson et al., 1988). The requirement for initial granule acidification was demonstrated by the pharmacological inhibition of the luminal ATP-dependent pump resulting in an elevation of intra-granular pH and inhibition of proinsulin conversion to insulin (Rhodes et al., 1987). These data are also consistent with proinsulin being initially packaged into immature granule where the conversion to insulin occurs and that these processing events do not occur in the TGN (Huang and Arvan, 1994; Rhodes and Halban, 1987).
Insulin secretory granules exist as large dense-core structures that are easily discernible by electron microscopic as an electron dense interior surrounded by a clear region in a 300–350nm intracellular membrane delineated compartment (Greider et al., 1969; Lange, 1974). The insulin dense-core structures are generated by a calcium and zinc-dependent condensation process (Hill et al., 1991). During the secretory granule maturation, proinsulin converts to insulin by excision of the C-peptide through proteolysis by endoproteases PC1/3 and PC2, followed by trimming C-terminal by carboxypeptidase E. The conversion of (Zn2+)2(Ca2+)(Proinsulin)6 to (Zn2+)2(Ca2+)(Insulin)6 significantly decreases the solubility of the hexamer leading to crystallization within the lumen of the granule and formation of the dense-core granule (Dunn, 2005).
Several investigators initially suggested that the concentration of insulin initiates during exit from the endoplasmic reticulum and continues as part of the TGN sorting into immature secretory granules (Bauerfeind and Huttner, 1993; Chanat and Huttner, 1991; Sossin et al., 1990; Tooze and Huttner, 1990). More recently, significant evidence has accumulated that the condensation reaction occurs within the secretory granule, rather than in TGN (for review, see Arvan and Castle (1992)). This was based both upon the presence of the prohormone convertase, Zinc transporter and ATP pump being present in insulin secretory granules (Arias et al., 2000; Chimienti et al., 2006; Rhodes et al., 1987). Moreover, proinsulin has very poor avidity to form hexamers and a mutation of proinsulin that is defective for hexamerization tends to associate with constitutively secretory pathway (Carroll et al., 1988; Gross et al., 1989; Kuliawat et al., 2000). On the other hand, other investigators observed that the same mutant was efficiently sorted to secretory granules and was retained in this compartment similar to that of fully processed insulin (Halban and Irminger, 2003). In this regard, guinea-pig insulin does not crystallize but these animals display relatively normal insulin secretion with an appropriate number and concentration of beta cell insulin granules (Arvan and Halban, 2004). Whether or not insulin condensation per se is a necessary sorting reaction, insulin granule biogenesis appears to require signals for both sorting by entry at the TGN and subsequent sorting for exit and sorting by retention in the post-TGN immature granules.
This latter process has been demonstrated for several immature granule proteins but is most clearly exemplified by proinsulin packaging into clathrin coated compartments during TGN exit, which is regulated via the AP-1 complex, and the ADP-ribosylation factor Arf-1 (Dittie et al., 1996). Arf-1 is a small GTP binding protein that undergoes successive rounds of GTP binding and GTP hydrolysis to GDP. In the GTP bound state, Arf-1 is in an active conformation and interacts with effectors. However, in the GDP bound state the affinity of Arf-1 for effectors is markedly reduced resulting a dissociation of these interactions (Randazzo and Kahn, 1994).
As indicated above, the immature insulin granules function as a sorting compartment that during the maturation process in which proinsulin is converted to insulin there is a concomitant decrease in clathrin association (Orci et al., 1985, 1986, 1987; Steiner et al., 1987). Insulin and other insoluble granules components are retained within the granule while other non-selective soluble proteins and granule membrane components bud from the immature granule as clathrin-coated transport vesicles. This “sorting for exit” process removes the undesirable components from secretory granules thereby resulting in the maturation of the immature granules to mature granules (Feng and Arvan, 2003). It should be noted that although this post-granular constitutive-like retrieval pathway is generally accepted for insulin granule maturation, it remains controversial in other systems.
III. Section II
A. Insulin granule trafficking
It is generally accepted that there are at least two populations of insulin secretory granules, the readily releasable pool (RRP) that is responsible for the initial (first phase) insulin secretion and a second reserve pool that is responsible for a more prolonged (second phase) insulin secretion (Bratanova-Tochkova et al., 2002; Rorsman and Renstrom, 2003; Rutter, 2001; Straub and Sharp, 2002). The readily releasable granule pool is apparently pre-docked at the cell surface membrane in a complex with SNARE and calcium-regulated proteins that allow for the rapid calcium-dependent fusion of primed insulin granules with the plasma membrane (Daniel et al., 1999). Following this initial rapid first phase of calcium-dependent insulin secretion (1–5 min), a more prolonged (5–60 min) second phase insulin secretion results from the recruitment of reserve granule populations to the same release sites. Although the signaling events and fusion mechanisms have been intensively studied, there remain several unanswered questions with regard to the exact regulatory proteins and specific fusion reactions that define first and second phase secretion.
1. Metabolic signaling pathway leads to insulin secretion (Fig. 16.2)
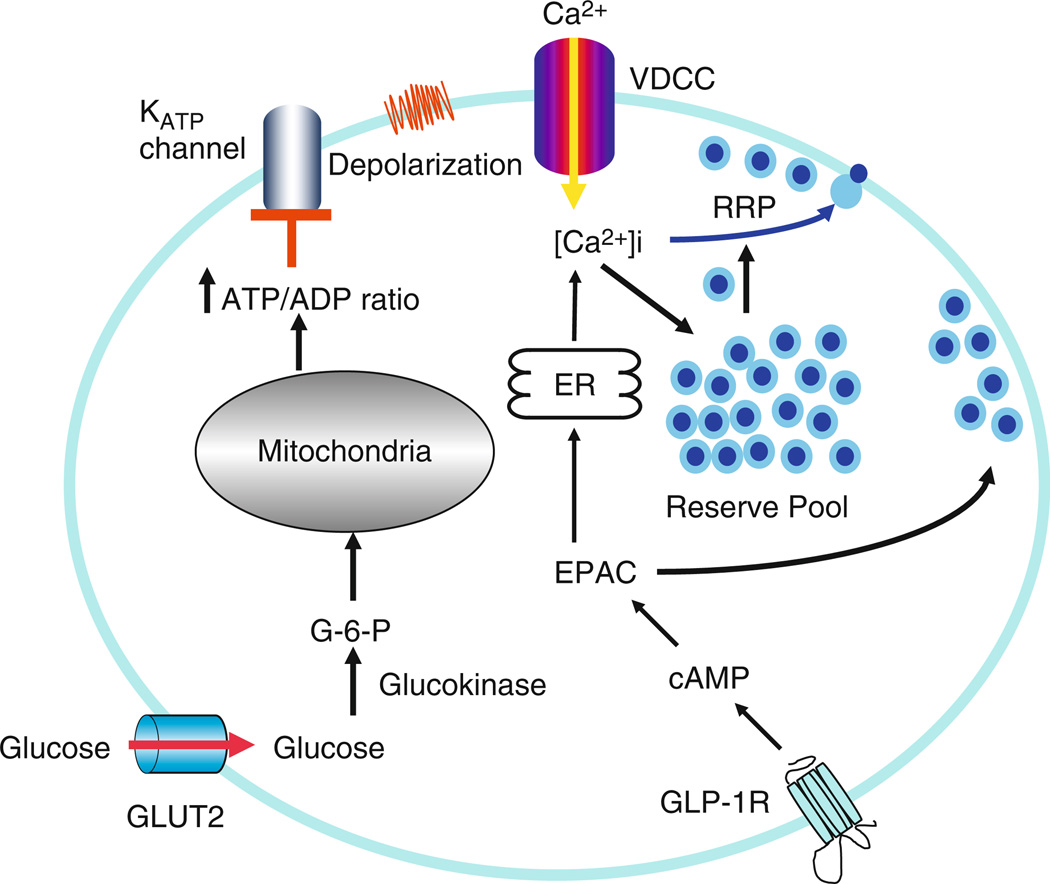
Figure 16.2.
Signaling pathway: Glucose, the major stimulant, via glycolysis and mitochondrial ATP energy production increases the ATP/ADP ratio that leads to the closure of the ATP-sensitive K+ (KATP) channels. The subsequent cellular depolarization activates voltage dependent calcium channels resulting in extracellular Ca2+ influx and fusion of insulin granules with the plasma membrane. The incretin hormone GLP-1 acts on its receptor at β cell plasma membrane to activate adenylyl cyclase and increase intracellular cAMP levels. In turn, cAMP binds and activates protein kinase A and EPAC. EPAC then functions to increase intracellular calcium level from intracellular calcium stores in the endoplasmic reticulum and to increase the number of readily releasable pool of insulin granules at the plasma membrane. The combination of these two processes results in a potentiation of insulin secretion.
The β cells coupled insulin secretion to nutrient availability through various pathways leading to calcium mobilization and activation of cAMP-dependent signaling cascades. Although beta cells are highly sensitive to fatty acid, amino acids and muscarinic agonists, glucose has been the most intensively studied metabolite trigger as dysregulation in plasma glucose levels is the hallmark of impaired glucose tolerance (insulin resistance) and the subsequent development of Type 2 Diabetes Mellitus.
The postprandial rise in plasma glucose levels results in increase flux of glucose into β cells via high capacity, low affinity glucose transporter 2 (GLUT2) isoform present in the plasma membrane (Thorens, 1992). The kinetic properties of GLUT2 are such that rate of glucose uptake is proportional to the physiologic rise in glucose concentration (Newgard and McGarry, 1995). The essential nature of GLUT2 was demonstrated in GLUT2 null mice that fail to sense ambient glucose concentration but also display defects in pancreatic β cell development (Guillam et al., 1997). Following plasma membrane transport, the cytosolic glucose is converted to glucose-6-phophate by the high KM glucokinase (hexokinase IV), which is the rate-limiting step in glycolysis (De Vos et al., 1995; Iynedjian, 1993; Newgard and McGarry, 1995). The importance of glucokinase as the “glucose sensor” (Matschinsky, 1990) in insulin secretion has been documented by the finding that the patients with a variety of mutations in glucokinase gene result in impaired insulin secretion and develop a genetic syndrome termed MODY for maturity onset diabetes of the young (Fajans et al., 2001). The production of glucose-6-phosphate drives an increase in energy production via glycolysis and carbohydrate oxidation most notably through an increase in the cytosolic ATP/ADP ratio (Rorsman et al., 2000). In the pancreatic β cells, ATP-sensitive K+ (KATP) channels set the membrane potential and closure of these channels results in depolarization that in turn opens voltage-dependent Ca2+ channels (VDCCs) resulting in Ca2+ influx. Insulin secretion is directly triggered through Ca2+ dependent insulin granule fusion with the plasma membrane as discussed below. However, the subsequent of opening of voltage-dependent K+ channels limits Ca2+ influx and therefore terminates insulin secretion.
In 1995 the β cell KATP channel along with its regulatory sulphonylurea receptor (SUR) subunits were first cloned from insulinoma cells (Aguilar-Bryan et al., 1995; Inagaki et al., 1992; Sakura et al., 1995). There are four pore forming inward-rectifier K+ channel subunits (Kir6.2 in β cell) and four regulatory SUR subunits (Aguilar-Bryan and Bryan, 1999; Ashfield et al., 1999). In the basal state, KATP has a high tendency to open and cause K+ efflux that maintains the membrane potential close to –70mV (Atwater et al., 1979; Dukes and Philipson, 1996). Several modulators are thought to regulate KATP channels and in particular the Kir6.2 subunit is inhibited by ATP (John et al., 1998; Mikhailov et al., 1998; Tucker et al., 1997) and mutation of the Kir6.2 subunit reverse the effect of ATP on KATP channel closure. Anti-diabetic sulphonylurea drugs have the similar property to induce the closure of the KATP channel but this class of drugs interact with SUR1 regulatory subunit (a member of the ATP-binding cassette super-family) to induce cellular depolarization (Aguilar-Bryan et al., 1995; Ashfield et al., 1999; Philipson and Steiner, 1995). Interestingly, the SUR1 subunit can bind to both ATP and MgADP, and binding of MgADP may stabilize the KATP channel in the open state. Since MgADP can be displaced when the ratio of ATP to ADP increase this allows the Kir6.2 subunit to display ATP inhibition (Reimann et al., 2000). In addition to adenine nucleotides, other metabolic products may also modulate KATP channels independent of ATP (Ainscow et al., 2002). For example, lipid-derived products such as long chain-coenzyme A derivatives activate mouse and human KATP channels and hyperpolarize β cell thereby reducing insulin secretion (Branstrom et al., 1997, 2004).
In any case, the closure of ATP-sensitive K+ channels results in cellular depolarization that activates L-type VDCCs resulting the influx of extracellular Ca+2 (Horvath et al., 1998; Keahey et al., 1989; Ligon et al., 1998). The requirement for extracellular Ca+2 influx for insulin secretion has been well documented by numerous investigators and includes the use of Ca+2 ionophores to induce secretion, blockade of secretion by removal of extracellular Ca+2 (Ashby and Speake, 1975; Eddlestone et al., 1995) and directed monitor of cytosolic free Ca2+ (Prentki and Wollheim, 1984). Moreover, membrane depolarization by electrophysiologic methods and KCl induced depolarization simulates whereas inhibition of L-type VDCC function inhibits β cell insulin secretion (Giugliano et al., 1980). These data demonstrate L-type Ca2+ channels are required effectors for glucose-stimulated insulin secretion. In addition, influx of extracellular Ca2+ as well as other secretagogues acetylcholine and cholecystokinin can potentiate insulin secretion through the release of intracellular Ca2+ stores and activation of protein kinase C (Graves and Hinkle, 2003; Zawalich et al., 1989).
Although the established linked between ATP/ADP ratio as the initiating event resulting in β cell depolarization and Ca2+ mediated insulin secretion has been well documented, the metabolic pathway that generates the ATP responsible for this process has remained unresolved. The first step in glucose metabolism is the generation of glucose-6-phosphate that consumes one mole ATP and the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate consumes another. However, ATP is generated at other steps during the glycolytic process that generates pyruvate, which is transported into the mitochondria to drive the tricarboxylic acid (TCA) cycle. Recent studies using stable isotope (13C NMR) flux analysis, the recycling of pyruvate across the mitochondrial inner membrane was found to directly correlate with glucose-stimulated insulin secretion (Lu et al., 2002). However, previous studies showed that inhibition of pyruvate transport into the mitochondria or reduction in TCA cycling did not prevent glucose-stimulated insulin secretion (Hildyard et al., 2005; MacDonald et al., 2005; Mertz et al., 1996; Zawalich and Zawalich, 1997). Taking together, these data suggest that ATP levels generated by the TCA cycle probably does not contribute to glucose-dependent insulin secretion.
Alternatively, NADH enters the mitochondria via two shuttles (glycerol–phosphate and malate–aspartate shuttles) to produce ATP (Eto et al., 1999; Hedeskov et al., 1987; Malaisse et al., 1979; Pralong et al., 1990). Compared to most other mammalian cells, in β cells display high activities of the glycerol–phosphate shuttle and mitochondrial glycerol–phosphate dehydrogenase (mGPDH) suggesting an important role for NADH in β cell function (MacDonald et al., 1990). However, inhibition of either shuttle independently fails to inhibit insulin-secretion whereas genetic ablation of both shuttles, completely abolished insulin secretion (Eto et al., 1999). In addition, NADPH is another high energy intermediate in glucose metabolism and high ratios of NADPH to NADH appears to follow secretagogue-stimulated secretion (Ashcroft and Christie, 1979; Hedeskov et al., 1987). Moreover, inhibition of NADPH production was found to decrease insulin secretion (Ammon and Steinke, 1972a,b; MacDonald et al., 1974). Since mitochondrial oxidative metabolism generates nearly 98% of β cell ATP, it is likely that NADH and NADPH generated mitochondrial-derived ATP provides the critical regulation of β cell KATP channels (Dukes et al., 1994; Jensen et al., 2006; Ohta et al., 1992).
2. The role of incretin hormones on insulin secretion
In addition to direct activators of insulin secretion, over the past several years a family of gut-derived regulatory hormones has been shown to function as regulators of insulin secretion. After absorption of nutrients, particularly glucose, the intestine endocrine L cells secrete gluco-incretin hormones. GLP-1 and glucose-dependent insulinotropic peptide (GIP, also known as gastric inhibitory polypeptide) are the two main incretin hormones that have been intensively investigated (Drucker et al., 1987; Meier et al., 2002a,b; Mojsov et al., 1987). GLP-1 is produced and secreted in the intestinal L cells by the prohormone convertase PC1/3 mediated posttranslational procession of proglucagon (Dhanvantari et al., 2001). In human, the major biological form of GLP-1 is the GLP-1 (7–36) amide form, another bioactive GLP-1 (7–37) form is also present but at much lower levels. GLP-1 is coupled to a specific G-protein coupled receptor and when activated increases adenylyl cyclase activity and subsequent activation of the cAMP-dependent second messengers pathways such as PKA and EPAC. The acute effect of GLP-1 is to enhance glucose-stimulated insulin secretion and accounts for increase in insulin secretion observed between an oral glucose tolerance test and an intraperitoneal glucose tolerance test. However, GLP-1 also displays long-term effects to promote insulin synthesis, β cell proliferation and neogenesis (Stoffers, 2004). Naturally occurring GLP-1 receptor agonist, exendin-4, now being used to treat type 2 diabetes and significantly improves glucose tolerance, first and second phase insulin secretion along with weight loss (Doyle and Egan, 2007). Physiologically, GLP-1 has a very short half-life in the plasma being inactivated by dipepti-dylpeptidase-IV (DDP-IV, also known as CD26) proteolytic cleavage (Hansen et al., 1999). This enzyme presents in blood stream and cell membrane and cleaves GLP-1 (7–36) to the inactive form GLP-1(9–36) (Hansen et al., 1999).
IV. Section III
A. Granule fusion
1. SNARE-dependent fusion
Many models have been proposed to elucidate the mechanism of regulated secretory granule exocytosis. It is now well established that SNARE proteins are the minimal machinery required for in vivo membrane fusion (Leabu, 2006). SNARE proteins belong to a superfamily consisting of over 35 proteins that share a common structural SNARE domain (Weimbs et al., 1997). Based upon structural considerations required for the formation of the fusogenic SNAREpin complex, the SNARE proteins have been further classified into R-SNAREs and Q-SNAREs depending on whether the central amino acid in the SNARE domain is an arginine (R-SNARE) or a glutamine (Q-SNARE) residue, respectively (Bock et al., 2001). The subset of SNARE proteins required for plasma membrane fusion in a variety of cell types is restricted to the Q-SNAREs, syntaxin 1–4, SNAP25, SNAP23, and the R-SNAREs (VAMP1–3). Specific interaction between a vesicle-SNARE with the cognate target-SNARE leads to the formation of a SNAREpin complex in which four SNARE motifs assemble into a twisted parallel four-helical bundle (Hong, 2005). It is this helical structure that catalyzes the fusion of vesicles with their target compartments.
In the case of insulin release, several studies have established that the t-SNARE proteins syntaxin 1 (Stx1) and SNAP25 are plasma membrane localized whereas the v-SNARE protein VAMP2 is associated with insulin secretory granules. The functional role for this core SNARE complex is essential for insulin secretion based upon the use of various proteolytic neurotoxins and expression of dominant-interfering and protease resistant mutants (Boyd et al., 1995; Gonelle-Gispert et al., 1999, 2000; Huang et al., 1998; Regazzi et al., 1995, 1996; Sadoul et al., 1995, 1997; Wheeler et al., 1996). However, although the SNARE proteins forms the catalytic entity required for membrane fusion they are not sufficient to account for rapid Ca2+ dependent exocytosis in most secretory cells. Thus, in addition to this core SNARE complex, several accessory proteins have also been implicated in the regulation of the granule docking/priming/fusion process. Synaptotagmin is currently the most favorite candidate Ca2+-sensor in synaptic vesicle fusion (Chapman, 2002; Shin et al., 2002; Sugita et al., 2002). Synaptotagmin also comprise a very large group of proteins with most of the family members containing two Ca2+ binding sites, termed C2A and C2B. Synaptotagmin I and II are essential for synaptic vesicle exocytosis but do not appear responsible for releasing of large dense-core vesicles (Sugita et al., 2002). In β cells, the Ca2+/phospholipid binding proteins synaptotagmin III, IV, V, VII and VIII and syncollin are insulin granule membrane proteins that have been suggested to be responsible for mediating calcium-dependent secretion. Syncollin has been shown to interact with syntaxin 1 and to dissociate in the presence of physiologic concentrations of calcium that induce membrane fusion (Edwardson et al., 1997). Over expression of syncollin in isolated islets inhibits glucose-stimulated insulin secretion, however, there are no direct data demonstrating a secretagogue-dependent dissociation of syncollin from Stx1 or an increase in VAMP2 association in β cells (Hays et al., 2005; Li et al., 2005). On the other hand, over expression of synaptotagmin III and/or VII increases calcium-mediated insulin secretion whereas blocking antibodies to both synaptotagmin I and II also inhibit calcium-stimulated exocytosis (Gao et al., 2000; Lang et al., 1997). Synaptotagmin peptide inhibitory experiments have suggested that its isoforms, V, VII and VIII may mediate calcium-regulation of exocytosis whereas siRNA mediated gene silencing indicate that V and IX are responsible (Gut et al., 2001; Iezzi et al., 2004). In addition, synaptotagmin I deficient mice display a prolonged delay and slowed calcium-dependent fusion of large dense-core granules in chromaffin cells (Voets et al., 2001a). Thus, it remains unclear whether a single specific synaptotagmin isoforms is required or that these calcium sensors function in combinatorial manner.
Biochemical and cell biological studies have developed a model for calcium stimulated membrane fusion of primed vesicles assembled at the plasma membrane. Recent studies have indicated that the Q- and R-SNAREs are partially zippered but are prevented from fully assembling into the four-α-helical bundle due to the presence of another protein, termed complexin (Pobbati et al., 2004). Thus complexin appears to function as a repressor of fusion by pausing complete SNAREpin formation. Calcium binding to synaptotagmin blocks the inhibitory function of complexin and thereby allows fusion to rapidly proceed (Tang et al., 2006). Consistent with this model, complexin I and II are both expressed in β cells and siRNA-mediated gene silencing of complexin has been found to inhibit depolarization-stimulated (calcium) mediated insulin secretion (Abderrahmani et al., 2004).
In addition to the potential roles of syncollin, synaptotagmin and complexin as part of the calcium fusion sensor, the voltage-sensitive N- and L-subtype calcium channels directly bind several SNARE proteins. For example, the N-type channels associates with SNAP25 and syntaxin 1 and expression of the interacting domain of the channel inhibited calcium-dependent secretion (Wiser et al., 1999b). Similarly, in pancreatic β cells the L-subtype calcium channel (primarily Ca(V)1.2) can physically associate with syntaxin, synaptotagmin and SNAP25 (Bokvist et al., 1995; Trus et al., 2001; Wiser et al., 1999b; Yang et al., 1999). Interestingly, syntaxin binding appears to modulate the channel kinetics and injection of the channel peptide responsible for SNARE interaction inhibits insulin secretion in response to channel opening but does not alter calcium current properties or the release of insulin in response to photolysis of caged calcium. These data suggest that the calcium channel is part of the fusion machinery directly coupling spatially localized calcium influx to the rapidly releasable granule pool. Similarly the voltage sensitive potassium channel Kv2.1 can also interact with syntaxin 1 (Leung et al., 2003, 2005).
Although not directly part of the calcium regulated secretory process, the syntaxin binding protein Munc18a (n-sec1) plays a critical modulatory role (both positive and negative) in the granule docking/fusion process. In multiple systems, over expression of Munc18 proteins has a marked inhibitory effects to reduce vesicle exocytosis (Houng et al., 2003; Khan et al., 2001; Pevsner et al., 1994b; Schulze et al., 1994; Thurmond et al., 1998, 2000; Verhage et al., 2000; Yang et al., 2000; Zhang et al., 2000). These data suggest that Munc18 is a negative regulator of syntaxin function and is consistent with the ability of Munc18 to compete for SNAP25 and VAMP2 binding to syntaxin (Araki et al., 1997; Dulubova et al., 2003; Perez-Branguli et al., 2002; Pevsner et al., 1994a; Tamori et al., 1998; Tellam et al., 1997). In addition, structural analysis has revealed that Munc18a maintains syntaxin 1 in a closed conformational state that is inaccessible for SNAP25 and VAMP binding (Bracher and Weissenhorn, 2001; Dulubova et al., 1999; Misura et al., 2000; Yang et al., 2000). Thus, it has been postulated that Munc18a must dissociate and/or undergo a conformational change to allow for the conversion of syntaxin to the open state required for SNAP25 and subsequently VAMP2 binding. Although several studies have suggested that the Munc18 proteins undergo serine/threonine phosphorylation by PKC and Cdk5 these modifications occur very slowly and kinetically cannot account for the initial steps of granule release (Barclay et al., 2003; de Vries et al., 2002; Lilja et al., 2004). Moreover, neither a dissociation nor conformational state change of Munc18a has yet to be detected within the time frame necessary to promote exocytosis.
On the other hand, there is also strong evidence that Munc18a plays a necessary positive role in vesicle membrane exocytosis. For example, using in vitro fusion assays, Munc18 was recently shown to accelerate the rate of SNARE-dependent membrane fusion suggesting a positive fusogenic role (Shen et al., 2007; Tareste et al., 2008). Loss of function of Munc18 homologues in yeast, flies and mice completely abrogates the vesicle secretory process (Harrison et al., 1994; Novick et al., 1980; Verhage et al., 2000; Voets et al., 2001b). Although homozygotic Munc18a knockout mice have apparently normal neural development they die at birth and are nearly completely devoid of synaptic transmission (Verhage et al., 2000). Similarly, disruption of the Munc18 interaction with syntaxin through the use of blocking peptides also prevents vesicle fusion events (Dresbach et al., 1998; Thurmond et al., 2000). These data suggest that the Munc18a protein has multiple functions displaying very complex but distinct properties probably in different stages of docking, priming and fusion process. In this regard, studies in Munc18a null chromaffin cells have observed the expected inhibition of calcium-dependent exocytosis with a reduction in the number of docked vesicles. Surprisingly however, the kinetics of the residual release as well as single fusion events was not different from control cells (Voets et al., 2001b). One interpretation of these data is that the relative level of expression of Munc18a is responsible for the docking of secretory vesicles and conversion from the docked to the primed (readily releasable state) but is not involved in the fusion process per se. Thus, Munc18a probably is required for the docking of secretory vesicles (positive role) and in its absence the number of docked vesicles is decreased, hence there is decreased secretion. In contrast, over expression would increase the number of docked vesicles but would prevent vesicle priming (formation of the high affinity four helix bundle between syntaxin/SNAP25 and VAMP2), hence reduced secretion. Consistent with this interpretation, Munc18a is expressed in islet cells and also appears to serve as a negative regulator of insulin secretion when over expressed (Zhang et al., 2000). Moreover, Munc18 is decreased along with SNAP25 in islets with impaired insulin secretion from GK rats, a non-obese model of NIDDM (Zhang et al., 2002).
2. Fusion pore opening
There are three general fusion processes that have been proposed. The first is a complete fusion of the granule membrane with the plasma membrane that results in the emptying of the granule contents and complete mixing of the granule membrane contents (membrane proteins and lipids) with the plasma membrane. The second is a kiss and run type mechanisms in which transient pores open between the granule membrane and the plasma membrane allowing for the partial or full release of the granule contents followed by a closure of the membrane fusion pore. A subset of the kiss and run model is a process termed cavicapture in which only selective component of the granule membrane and perhaps granule content undergo exocytosis before the membrane fusion pore closes.
Several approaches have been developed to examine granule fusion and insulin release from pancreatic β cells. Early studies took advantage of cell surface membrane capacitance measurements to examine granule fusion events. Membrane capacitance is proportional to the surface area and therefore an increase in membrane capacitance reflects and increases in the insertion of a granule membrane (Neher, 1998; Neher and Marty, 1982). Since insulin granules are significantly larger (~300–400nm diameter) compared to small transport vesicles (~50 nm diameter) changes in membrane capacitance is sufficiently sensitive to detect single fusion events. These types of analyses demonstrated increases in steady state in β cell membrane capacitance that occurred in a step wise fashion (quantal) suggesting each insulin granule undergoes a full fusion with the plasma membrane (Ma et al., 2004; Orci et al., 1973; Takahashi et al., 2002). Although membrane capacitance is a very powerful and informative tool, it does not distinguish the dynamic interaction between exocytosis and endocytosis but only the change in the steady state membrane surface area. Furthermore it does not discriminate between which membranes are undergoing fusion/ endocytosis with the plasma membrane or whether there is an actual release of cargo contents.
To address this latter issue, carbon fiber amperometry provides a direct way to monitor the exocytosis of granule contents following membrane fusion. This technique delivers high voltage that causes oxidation of released substance that generates a current spike and has been successfully used for numerous types of exocytosis events including insulin (Huang et al., 1995). Carbon fiber amperometry studies have indicated that a single secretory granule in a human β cell contains 1.7amol of insulin, equal to 118mmol/l of intra-granular insulin. These studies also demonstrated that pH gradient between the granule lumen and extracelluar fluid is required for insulin release and the dissociation of the Zn-insulin complex limits the rate of hormone release (Aspinwall et al., 1997). As with membrane capacitance measurements, carbon fiber amperometry is a very sensitive measurement of quantal release events. However, this approach only measures content release and does provide information with regard to the mechanism of membrane fusion itself.
More recent advances in fluorescent microscopy have allowed use of various fluorescently tagged reporter proteins to examine the dynamics of membrane fusion. In particular, confocal fluorescent microscopy and total internal reflectance fluorescent (TIRF) microscopy has markedly increased the sensitivity and spatial resolution so that time-lapse visualization can be routinely performed (Ohara-Imaizumi et al., 2002; Steyer et al., 1997). These techniques have primarily utilized chimeric reporter proteins that are fused with various colored fluorescent protein tags, such as green fluorescent protein (GFP), red fluorescent protein (RFP), cyan fluorescent protein (CFP). Although these approaches allow for dynamic visualization, the spatial resolution remains limited to the wavelength of visible light (~220nm) and the introduction of the relatively large fluorescent reporter protein may in of itself alter the properties of the trafficking and fusion events observed.
Several studies have reported that individual insulin granules independently form a plasma membrane fusion and release their intralumenal cargo followed by the flattening and mixing of the membrane components with the plasma membrane (Takahashi et al., 2002, 2004). In this regard, others have suggested that the release of the insulin-containing dense-core granule occurs en mass consistent with the formation of a plasma membrane pore that fully expands to encompass the granule membrane proteins and lipids (Ma et al., 2004; Orci et al., 1973). However, in addition to individual insulin granule fusion events, it has also been suggested that insulin granules secretion may occur through a compound exocytosis mechanism whereby already fused granules undergo additional fusion events with other granules, termed sequential exocytosis (Bokvist et al., 2000; Leung et al., 2002; Orci and Malaisse, 1980). These classic models of exocytosis involves complete fusion of the vesicular and plasma membranes, followed by retrieval of membrane at a different site (Ohara-Imaizumi et al., 2002).
However, other studies have suggested that a transient fusion pore can occur that results in either non-selective (kiss and run) or selective (cavicapture) release of granule contents (Tsuboi and Rutter, 2003; Tsuboi et al., 2004). In particular, it was reported that the insulin granule containing membrane protein phogrin (phosphatase-like protein on the granules of insulin containing cells) is not transferred to the plasma membrane following granule secretion (Tsuboi et al., 2004). Furthermore, using TIRF microscopy, dynamin was observed to frequently accumulate at the sites of β cell granule release whereas typical markers of clathrin-mediated endocytosis (clathrin or epsin) were generally absent from these release domains. This study also examined the trafficking/release of single granule events that appeared to be unaffected.
Further studies revealed that a neuropeptide Y-Venus fusion protein, a highly fluorescent and pH-insensitive variant of GFP, could also be released from vesicles without full membrane fusion (Nagai et al., 2002). TIRF microscopy demonstrated that the granule membrane remained intact at cell surface while the release of cargo proteins from the same pool of granules frequently occurred (Rutter and Tsuboi, 2004). In another cell type, PC12 (a pheochromocytoma cel line), Taraaska et al. observed the partial release of the cargo protein plasminogen activator (tPA), whereas phogrin again remained on the membrane of the same vesicle. Taking together, these findings proposed a unique mechanism, namely, kiss and run fusion model to elucidate insulin granule exocytosis events. The granule content is released via the fusion pore that transiently and reversibly opens during the exocytosis. In this model, the fusion pore can close even before the release of granule content and without mixing of the granule membrane with the plasma membrane (Galli and Haucke, 2001; Jarousse and Kelly, 2001). In fact, it was estimated that 90% of the release events occurs via a selective kiss and run mechanism with only 10% accounted for by complete fusion (Obermuller et al., 2005).
3. Membrane retrieval
At present it is difficult to reconcile the apparent different results obtained by capacitance measurements versus that observed by fluorescent microscopy. The increase in capacitance must necessarily occur as a result of a steady-state increase in membrane surface area. In contrast, a kiss and run mechanism necessarily results in transient fusion pore openings that will not contribute to a stable increase in membrane capacitance. If a kiss and run mechanism was predominantly responsible for insulin secretion there would not be a requirement for membrane retrieval (endocytosis) as there will be little contribution to an increase in membrane surface area. However, if complete fusion occurs the subsequent increase in plasma membrane mass must be balanced by an equivalent amount of membrane retrieval.
Capacitance measurement in chromaffin cells demonstrated that two kinetically distinct endocytosis events occur after exocytosis, one with a rapid rate (time constant 10s), which requires dynamin 1 binding to the endocytotic vesicles that is calcium dependent but independent of clathrin coats (Eliasson et al., 1996). The second is a slow endocytosis process (time constant 100s) that involves both clathrin and dyanmin 2 but does not require any change in calcium concentration (Gopel et al., 1999). The rapid endocytosis process appears to be more important following weak stimulation, whereas the slow type is responsible for intense stimulation that resulted in large exocytotic increases in membrane surface area. Tsuboi et al. using a DsRed-VAMP2 chimera and endocytosis with dynamin 1 EGFP showed that only 20% of exocytotic events appear to involve in endocytosis in PC12 cells (Tsuboi et al., 2002). However, these earlier studies did not investigate the recruitment of classic endocytotic pathway proteins such as clathrin and other proteins including the adaptor protein Epsin, which binds to adaptor protein-2 and results in formation of clathrin coats around vesicles (Barg et al., 2001). The interaction between the Epsin N-terminal domain and phosphatidylinositol-4,5-bisphosphate initiates the binding of Epsin to the plasma membrane that generates a signal for endocytosis (Wiser et al., 1999a). The insertion of Epsin into the membrane appears to cause membrane curvature (Curry et al., 1968), which in turn facilitates vesicle formation and recapture.
Whether or not full fusion or a kiss and run type mechanism occurs, recent studies have suggested a functional coupling between insulin secretion (exocytosis) and endocytosis. As indicated above, dynamin is a GTPase protein that is required for the severing of the plasma membrane invagination that are typically clathrin coated (coated pits) that will form the endocytotic vesicle (Hill et al., 2001). Inhibition of dynamin function results in an accumulation of coated pits with elongated necks that are unable to undergo scission effectively blocking endocytosis (Damke et al., 1994). Expression of a dominant-interfering dynamin mutant or siRNA knockdown of dynamin 2 in β cells was reported to inhibit depolarization stimulated insulin secretion (Min et al., 2007). Interestingly, the very initial secretion was relatively normal whereas the remaining first and second phase secretion was nearly completely inhibited. Similar findings using a blocking dynamin antibody have also been observed catecholamine release in chromaffin cells (Elhamdani et al., 2001). These data suggest that secretory cells have a mechanism that couples the rate and/or extent of membrane exocytosis with that of endocytosis.
V. Section IV
A. Biphasic insulin secretion
The majority of studies previously described have focused on the initial insulin granule fusion and release process. However, there are at least two populations of insulin secretory granules, the RRP that is responsible for the initial (first phase) insulin secretion and a second reserve pool that is responsible for a more prolonged (second phase) insulin secretion (Bratanova-Tochkova et al., 2002; Rorsman and Renstrom, 2003; Rutter, 2001; Straub and Sharp, 2002). The readily releasable granule pool is apparently pre-docked at the cell surface complex with syntaxin 1 (Stx1), SNAP25 and VAMP2 together with one of the calcium-regulated protein, synaptotagmin (Daniel et al., 1999). Following the initial rapid first phase calcium-dependent insulin secretion, second phase secretion results from the recruitment of the reserve granules to the same release sites that are also dependent on t- and v-SNARE interaction. Interestingly, a recent study has also implicated Stx4 in facilitating biphasic insulin secretion (Spurlin and Thurmond, 2006). The exact mechanism involved, however, is not well characterized and specific details of the fusion process responsible for first and second phase insulin secretion is far from clear.
1. Characteristic feature of insulin granule biphasic insulin secretion
The RRP has been estimated to account for 1–5% of the granules, that is 20–100 granules depending on the conditions of the experiments (Gromada et al., 1999; Renstrom et al., 1997), which can undergo exocytosis following stimulation without any further modification. It is estimated that about 40 granules per beta cell that undergo exocytosis during the first-phase insulin secretion (Rorsman et al., 2000). Thus, the majority of the first-phase insulin secretion could be attributable to exocytosis of RRP granules (Rorsman et al., 2000). Since the first-phase insulin secretion is only transient and once RRP has been depleted, the reserve pool granules start to process exocytosis at a much slower rate to supply new granules for the second-phase insulin secretion. However, recruiting insulin granules from reserve pool that consists of 95–99% of total insulin granules requires a series of ATP, Ca2+, time, and temperature dependent reactions. This process is also referred to as mobilization or priming in order to obtain release competence. Moreover, the capacitance measurements demonstrate exocytosis of RRP granules does not require ATP hydrolysis, in contrast refilling the RRP pool is highly ATP dependent (Eliasson et al., 1997). Interestingly, activation by cAMP elevation and the incretin hormone GLP-1 facilitate granule mobilization and increase the size of RRP in both mouse β cells and mouse islets (Eddlestone et al., 1985; Renstrom et al., 1997). The mobilization involves the interaction between t-SNARE and v-SNARE, the formation of SNARE complexes (Rettig and Neher, 2002; Xu et al., 1999) and additional maturation reactions leading to an increased probability of release.
As mentioned above, the SNARE proteins play very important role in the granule membrane fusion. More importantly SNARE proteins are the security to ensure Ca2+ is restricted to the plasma membrane area in close contact with the secretory granules. The L-type Ca2+ channel (L-loop) binds to syntaxin, SNAP 25 and synaptotagmin, this in turn will secure the Ca2+ channel to the secretory granule. This unique arrangement will allow RRP granules expose to high level of Ca2+ to ensure high efficient exocytosis (Rorsman et al., 2000). Since the first detailed ultrastructural analysis of pancreatic β cells were published (Dean, 1973), electron microscopy become a very powerful tool to study the functional and ultrastructural correlates of insulin secretion. Using electron microscope, Olofesson et al. reported that mouse beta cells contain 10,000 granules per cell (Olofsson et al., 2002), with approximately 5% of the granules pre-docked close to membrane and another 2000 granules located less than one granule distance from the plasma membrane. This study used very high extracellular K+ (depolarization) to stimulate first-phase insulin secretion in the absence of glucose. This increased insulin secretion is associated with 30% reduction of pre-docked insulin granules. Under these conditions, a second treatment with K+ failed to further increase insulin secretion. In contrast, following K+ depolarization glucose was still able to enhance insulin secretion (Olofsson et al., 2002). Data from electron microscopy revealed that the depletion of docked granule pool is much more than the defined RRP by capacitance measurements. One possibility for this discrepancy is the presence of another pool of granules named “nearly RRP” and these types of granules can maintain the competence of RRP that is responsible for repopulating the RRP in an energy (ATP) dependent process (Henquin et al., 2002). Thus, a current model is that following an initial burst by the RRP the nearly RRP granules account for the total amount quantity of insulin release during first phase secretion. Subsequently, second phase secretion requires the mobilization of the reserve pool to replenish the RRP, which requires energy (ATP) and calcium (Rorsman and Renstrom, 2003).
As previously indicated, the reserve pool represents more than 95% of the secretory insulin granules that are mobilize to and replete the RRP to maintain sustained (second phase) insulin release in respond to secretagogue stimulation. (Rorsman et al., 2000). Cyclic AMP stimulated and Ca2+ dependent insulin secretion was associated with the enhancement of insulin release from RRP and the acceleration of refilling of this pool from reserve pool (Renstrom et al., 1997). Several studies have also suggested the existence of two granule population including fast-moving granules and random but restrict movement granules in reserve pool (Ivarsson et al., 2004; Pouli et al., 1998; Varadi et al., 2003). Recent image analysis data examining the tracking of individual granule motions support this model and further indicate that the fasting-moving granules accounting for less than 10% granules are required to replete the RRP (Hao et al., 2005). More recently, the small GTPase RalA has been shown to be involved in the regulation of the RRP mobilization from the reserve pool. Depletion of RalA in islet and cultured INS beta cells blocked insulin secretion accompanied with the reduction in the subsequent mobilization and exocytosis of the reserve pool of granules (Lopez et al., 2008).
In summary, we discussed the insulin biogenesis, insulin granule sorting, maturation, distribution, signaling pathway and exocytosis. Sorting for entry, sorting by exit and sorting by retention are currently proposed models to elucidate insulin granule sorting. During the sorting process, proinsulin is converted to insulin followed by condensation/crystallization while immature proinsulin granule becomesmature secretory granule and is distributed in two different pools, namely, readily release pool and reserve pool. The existence of two pools of insulin secretory granule appears to account for the biphasic secretion pattern. It has been well documented that elevation of intracellular Ca2+ play critical role in insulin secretion, facilitating the SNARE complex regulated membrane fusion and enhancing mobilization of the granule from reserve pool to replete RRP. Glucose, the major stimulant, via glycolysis increases the ATP/ADP ratio that lead to close the ATP-sensitive K+ (KATP) channels and closure of these channels results in depolarization and subsequent Ca2+ influx. The research on the incretin hormones has provided a unique way to treat Type 2 Diabetes Mellitus. Exenatide, an analog of human GLP1 and incretin mimetics, has been successfully used clinically. GLP-1 binds its receptor on β cell plasma membrane via cyclic AMP signal pathway to promote insulin synthesis, β cell proliferation and neogenesis. Exocytosis occurs when the granule membrane fuses with plasma membrane that lead release of the granule components. The role of SNARE complex in insulin exocytosis has been well established and regulator proteins such as syncollin, synaptotagmin and complexin have been proposed as brakes between the t-SNARE and v-SNARE complex. These proteins act as calcium sensors that trigger the fusion event when bind with calcium currently, there are several working models full fusion, Kiss and run and cavicapture that can account for insulin granule fusion. Further studies are still necessary to determine whether one of these models, a combination or an alternative model can fully describe the dynamics of beta cell insulin secretion.
REFERENCES
- Abderrahmani A, Niederhauser G, Plaisance V, Roehrich ME, Lenain V, Coppola T, Regazzi R, Waeber G. Complexin I regulates glucose-induced secretion in pancreatic beta-cells. J. Cell Sci. 2004;117:2239–2247. doi: 10.1242/jcs.01041. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphatesensitive potassium channels. Endocr. Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JPt, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: Evidence for ATP-independent control of ATP-sensitive K(+) channels. J. Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon HP, Steinke J. 6-Amnionicotinamide (6-AN) as a diabetogenic agent. In vitro and in vivo studies in the rat. Diabetes. 1972a;21:143–148. doi: 10.2337/diab.21.3.143. [DOI] [PubMed] [Google Scholar]
- Ammon HP, Steinke J. Effect of 6-aminonicotinamide on insulin release and C-14 glucose oxidation by isolated pancreatic rat islets: Difference between glucose, tolbutamide and aminophylline. Endocrinology. 1972b;91:33–38. doi: 10.1210/endo-91-1-33. [DOI] [PubMed] [Google Scholar]
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, Niki T, Okazawa H, Kubota T, Kasuga M. Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c. Biochem. Biophys. Res. Commun. 1997;234:257–262. doi: 10.1006/bbrc.1997.6560. [DOI] [PubMed] [Google Scholar]
- Arias AE, Velez-Granell CS, Mayer G, Bendayan M. Colocalization of chaperone Cpn60, proinsulin and convertase PC1 within immature secretory granules of insulin-secreting cells suggests a role for Cpn60 in insulin processing. J. Cell Sci. 2000;113(Pt 11):2075–2083. doi: 10.1242/jcs.113.11.2075. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: Looking backward and looking forward. Biochem. J. 1998;332(Pt 3):593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Ashby JP, Speake RN. Insulin and glucagon secretion from isolated islets of Langerhans. The effects of calcium ionophores. Biochem. J. 1975;150:89–96. [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJ, Christie MR. Effects of glucose on the cytosolic ration of reduced/oxidized nicotinamide-adenine dinucleotide phosphate in rat islets of Langerhans. Biochem. J. 1979;184:697–700. doi: 10.1042/bj1840697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- Aspinwall CA, Brooks SA, Kennedy RT, Lakey JR. Effects of intravesicular H+ and extracellular H+ and Zn2+ on insulin secretion in pancreatic beta cells. J. Biol. Chem. 1997;272:31308–31314. doi: 10.1074/jbc.272.50.31308. [DOI] [PubMed] [Google Scholar]
- Atwater I, Dawson CM, Ribalet B, Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J. Physiol. 1979;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J. Biol. Chem. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- Barg S, Ma X, Eliasson L, Galvanovskis J, Gopel SO, Obermuller S, Platzer J, Renstrom E, Trus M, Atlas D, Striessnig J, Rorsman P. Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys. J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr. Opin. Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Bokvist K, Eliasson L, Ammala C, Renstrom E, Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokvist K, Holmqvist M, Gromada J, Rorsman P. Compound exocytosis in voltage-clamped mouse pancreatic beta-cells revealed by carbon fibre amperometry. Pflugers Arch. 2000;439:634–645. doi: 10.1007/s004249900211. [DOI] [PubMed] [Google Scholar]
- Boyd RS, Duggan MJ, Shone CC, Foster KA. The effect of botulinum neurotoxins on the release of insulin from the insulinoma cell lines HIT-15 and RINm5F. J. Biol. Chem. 1995;270:18216–18218. doi: 10.1074/jbc.270.31.18216. [DOI] [PubMed] [Google Scholar]
- Bracher A, Weissenhorn W. Crystal structures of neuronal squid Sec1 implicate inter-domain hinge movement in the release of t-SNAREs. J. Mol. Biol. 2001;306:7–13. doi: 10.1006/jmbi.2000.4347. [DOI] [PubMed] [Google Scholar]
- Branstrom R, Corkey BE, Berggren PO, Larsson O. Evidence for a unique long chain acyl-CoA ester binding site on the ATP-regulated potassium channel in mouse pancreatic beta cells. J. Biol. Chem. 1997;272:17390–17394. doi: 10.1074/jbc.272.28.17390. [DOI] [PubMed] [Google Scholar]
- Branstrom R, Aspinwall CA, Valimaki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, Larsson O. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: Potential role in Type 2 diabetes. Diabetologia. 2004;47:277–283. doi: 10.1007/s00125-003-1299-x. [DOI] [PubMed] [Google Scholar]
- Bratanova-Tochkova TK, Cheng H, Daniel S, Gunawardana S, Liu YJ, Mulvaney-Musa J, Schermerhorn T, Straub SG, Yajima H, Sharp GW. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes. 2002;51:S83–S90. doi: 10.2337/diabetes.51.2007.s83. [DOI] [PubMed] [Google Scholar]
- Carroll RJ, Hammer RE, Chan SJ, Swift HH, Rubenstein AH, Steiner DF. A mutant human proinsulin is secreted from islets of Langerhans in increased amounts via an unregulated pathway. Proc. Natl. Acad. Sci. USA. 1988;85:8943–8947. doi: 10.1073/pnas.85.23.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Rodriguez YM, Maldonado A, Loh YP. Trafficking of mutant carboxypeptidase E to secretory granules in a beta-cell line derived from Cpe (fat)/Cpe(fat) mice. Endocrinology. 2003;144:292–298. doi: 10.1210/en.2002-220588. [DOI] [PubMed] [Google Scholar]
- Chanat E, Huttner WB. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J. Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: A Ca(2+) sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van Lommel L, Grunwald D, Favier A, Seve M. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- Chung KN, Walter P, Aponte GW, Moore HP. Molecular sorting in the secretory pathway. Science. 1989;243:192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Noda M, Straub SG, Sharp GW. Identification of the docked granule pool responsible for the first phase of glucose-stimulated insulin secretion. Diabetes. 1999;48:1686–1690. doi: 10.2337/diabetes.48.9.1686. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Rhodes CJ, Hutton JC. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988;333:93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Dean PM. Ultrastructural morphometry of the pancreatic-cell. Diabetologia. 1973;9:115–119. doi: 10.1007/BF01230690. [DOI] [PubMed] [Google Scholar]
- Del Prato S. Loss of early insulin secretion leads to postprandial hyperglycaemia. Diabetologia. 2003;46(Suppl 1):M2–M8. doi: 10.1007/s00125-002-0930-6. [DOI] [PubMed] [Google Scholar]
- Del Prato S, Tiengo A. The importance of first-phase insulin secretion: Implications for the therapy of type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J. Clin. Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries KJ, Geijtenbeek A, Brian EC, de Graan PN, Ghijsen WE, Verhage M. Dynamics of munc18-1 phosphorylation/dephosphorylation in rat brain nerve terminals. Eur. J. Neurosci. 2002;12:385–390. doi: 10.1046/j.1460-9568.2000.00931.x. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Izzo A, Jansen E, Brubaker PL. Coregulation of glucagon-like peptide-1 synthesis with proglucagon and prohormone convertase 1 gene expression in enteroendocrine GLUTag cells. Endocrinology. 2001;142:37–42. doi: 10.1210/endo.142.1.7870. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Shen FS, Adams T, Snell CR, Zhang C, Mackin RB, Morris SJ, Loh YP. Disruption of a receptor-mediated mechanism for intracellular sorting of proinsulin in familial hyperproinsulinemia. Mol. Endocrinol. 2003;17:1856–1867. doi: 10.1210/me.2002-0380. [DOI] [PubMed] [Google Scholar]
- Dittie AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J. Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr. Opin. Struct. Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresbach T, Burns ME, O’Connor V, DeBello WM, Betz H, Augustine GJ. A neuronal Sec1 homolog regulates neurotransmitter release at the squid giant synapse. J. Neurosci. 1998;18:2923–2932. doi: 10.1523/JNEUROSCI.18-08-02923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. USA. 1987;84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes ID, Philipson LH. K+ channels: generating excitement in pancreatic beta-cells. Diabetes. 1996;45:845–853. doi: 10.2337/diab.45.7.845. [DOI] [PubMed] [Google Scholar]
- Dukes ID, McIntyre MS, Mertz RJ, Philipson LH, Roe MW, Spencer B, Worley JF. Dependence on NADH produced during glycolysis for beta-cell glucose signaling. J. Biol. Chem. 1994;269:10979–10982. [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Arac D, Li H, Huryeva I, Min SW, Rizo J, Sudhof TC. Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. Proc. Natl. Acad. Sci. USA. 2003;100:32–37. doi: 10.1073/pnas.232701299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer - A review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- Eddlestone GT, Oldham SB, Lipson LG, Premdas FH, Beigelman PM. Electrical activity, cAMP concentration, and insulin release in mouse islets of Langerhans. Am. J. Physiol. 1985;248:C145–C153. doi: 10.1152/ajpcell.1985.248.1.C145. [DOI] [PubMed] [Google Scholar]
- Eddlestone GT, Komatsu M, Shen L, Sharp GW. Mastoparan increases the intracellular free calcium concentration in two insulin-secreting cell lines by inhibition of ATP-sensitive potassium channels. Mol. Pharmacol. 1995;47:787–797. [PubMed] [Google Scholar]
- Edwardson JM, An S, Jahn R. The secretory granule protein syncollin binds to syntaxin in a Ca2(+)-sensitive manner. Cell. 1997;90:325–333. doi: 10.1016/s0092-8674(00)80340-2. [DOI] [PubMed] [Google Scholar]
- Elhamdani A, Palfrey HC, Artalejo CR. Quantal size is dependent on stimulation frequency and calcium entry in calf chromaffin cells. Neuron. 2001;31:819–830. doi: 10.1016/s0896-6273(01)00418-4. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Proks P, Ammala C, Ashcroft FM, Bokvist K, Renstrom E, Rorsman P, Smith PA. Endocytosis of secretory granules in mouse pancreatic beta-cells evoked by transient elevation of cytosolic calcium. J. Physiol. 1996;493(Pt 3):755–767. doi: 10.1113/jphysiol.1996.sp021420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Renstrom E, Ding WG, Proks P, Rorsman P. Rapid ATP-dependent priming of secretory granules precedes Ca(2+)-induced exocytosis in mouse pancreatic B-cells. J. Physiol. 1997;503(Pt 2):399–412. doi: 10.1111/j.1469-7793.1997.399bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Tsubamoto Y, Terauchi Y, Sugiyama T, Kishimoto T, Takahashi N, Yamauchi N, Kubota N, Murayama S, Aizawa T, Akanuma Y, Aizawa S, et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N. Engl. J. Med. 2001;345:971–980. doi: 10.1056/NEJMra002168. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J. Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Arvan P. The trafficking of alpha 1-antitrypsin, a post-Golgi secretory pathway marker, in INS-1 pancreatic beta cells. J. Biol. Chem. 2003;278:31486–31494. doi: 10.1074/jbc.M305690200. [DOI] [PubMed] [Google Scholar]
- Galli T, Haucke V. Cycling of synaptic vesicles: How far? How fast. Sci. STKE. 2001 doi: 10.1126/stke.2001.88.re1. 2001 RE1. [DOI] [PubMed] [Google Scholar]
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet beta -cells. J. Biol. Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Torella R, Cacciapuoti F, Gentile S, Verza M, Varricchio M. Impairment of insulin secretion in man by nifedipine. Eur. J. Clin. Pharmacol. 1980;18:395–398. doi: 10.1007/BF00636791. [DOI] [PubMed] [Google Scholar]
- Gonelle-Gispert C, Halban PA, Niemann H, Palmer M, Catsicas S, Sadoul K. SNAP-25a and -25b isoforms are both expressed in insulin-secreting cells and can function in insulin secretion. Biochem. J. 1999;339:159–165. [PMC free article] [PubMed] [Google Scholar]
- Gonelle-Gispert C, Molinete M, Halban PA, Sadoul K. Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells. J. Cell. Sci. 2000;113:3197–3205. doi: 10.1242/jcs.113.18.3197. [DOI] [PubMed] [Google Scholar]
- Gopel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J. Physiol. 1999;521(Pt 3):717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr SU, Darling DS. An N-terminal hydrophobic peak is the sorting signal of regulated secretory proteins. FEBS. Lett. 1995;361:8–12. doi: 10.1016/0014-5793(95)00142-v. [DOI] [PubMed] [Google Scholar]
- Graves TK, Hinkle PM. Ca(2+)-induced Ca(2+) release in the pancreatic beta-cell: Direct evidence of endoplasmic reticulum Ca(2+) release. Endocrinology. 2003;144:3565–3574. doi: 10.1210/en.2002-0104. [DOI] [PubMed] [Google Scholar]
- Greider MH, Howell SL, Lacy PE. Isolation and properties of secretory granules from rat islets of Langerhans. II. Ultrastructure of the beta granule. J. Cell. Biol. 1969;41:162–166. doi: 10.1083/jcb.41.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Hoy M, Renstrom E, Bokvist K, Eliasson L, Gopel S, Rorsman P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J. Physiol. 1999;518(Pt 3):745–759. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DJ, Halban PA, Kahn CR, Weir GC, Villa-Komaroff L. Partial diversion of a mutant proinsulin (B10 aspartic acid) from the regulated to the constitutive secretory pathway in transfected AtT-20 cells. Proc. Natl. Acad. Sci. USA. 1989;86:4107–4111. doi: 10.1073/pnas.86.11.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Deriaz N, Thorens B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat. Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- Gut A, Kiraly CE, Fukuda M, Mikoshiba K, Wollheim CB, Lang J. Expression and localisation of synaptotagmin isoforms in endocrine beta-cells: Their function in insulin exocytosis. J. Cell Sci. 2001;114:1709–1716. doi: 10.1242/jcs.114.9.1709. [DOI] [PubMed] [Google Scholar]
- Halban PA, Irminger JC. Sorting and processing of secretory proteins. Biochem. J. 1994;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Irminger JC. Mutant proinsulin that cannot be converted is secreted efficiently from primary rat beta-cells via the regulated pathway. Mol. Biol. Cell. 2003;14:1195–1203. doi: 10.1091/mbc.E02-05-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36) amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- Hao M, Li X, Rizzo MA, Rocheleau JV, Dawant BM, Piston DW. Regulation of two insulin granule populations within the reserve pool by distinct calcium sources. J. Cell Sci. 2005;118:5873–5884. doi: 10.1242/jcs.02684. [DOI] [PubMed] [Google Scholar]
- Harrison SD, Broadie K, van de Goor J, Rubin GM. Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron. 1994;13:555–566. doi: 10.1016/0896-6273(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Hays LB, Wicksteed B, Wang Y, McCuaig JF, Philipson LH, Edwardson JM, Rhodes CJ. Intragranular targeting of syncollin, but not a syncollin GFP chimera, inhibits regulated insulin exocytosis in pancreatic beta-cells. J. Endocrinol. 2005;185:57–67. doi: 10.1677/joe.1.05934. [DOI] [PubMed] [Google Scholar]
- Hedeskov CJ, Capito K, Thams P. Cytosolic ratios of free [NADPH]/ [NADP+] and [NADH]/[NAD+] in mouse pancreatic islets, and nutrient-induced insulin secretion. Biochem. J. 1987;241:161–167. doi: 10.1042/bj2410161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC. Signals and pools underlying biphasic insulin secretion. Diabetes. 2002;51(Suppl 1):S60–S67. doi: 10.2337/diabetes.51.2007.s60. [DOI] [PubMed] [Google Scholar]
- Hildyard JC, Ammala C, Dukes ID, Thomson SA, Halestrap AP. Identification and characterisation of a new class of highly specific and potent inhibitors of the mitochondrial pyruvate carrier. Biochim. Biophys. Acta. 2005;1707:221–230. doi: 10.1016/j.bbabio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hill CP, Dauter Z, Dodson EJ, Dodson GG, Dunn MF. X-ray structure of an unusual Ca2+ site and the roles of Zn2+ and Ca2+ in the assembly, stability, and storage of the insulin hexamer. Biochemistry. 1991;30:917–924. doi: 10.1021/bi00218a006. [DOI] [PubMed] [Google Scholar]
- Hill E, van Der Kaay J, Downes CP, Smythe E. The role of dynamin and its binding partners in coated pit invagination and scission. J. Cell Biol. 2001;152:309–323. doi: 10.1083/jcb.152.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Horvath A, Szabadkai G, Varnai P, Aranyi T, Wollheim CB, Spat A, Enyedi P. Voltage dependent calcium channels in adrenal glomerulosa cells and in insulin producing cells. Cell Calcium. 1998;23:33–42. doi: 10.1016/s0143-4160(98)90072-0. [DOI] [PubMed] [Google Scholar]
- Houng A, Polgar J, Reed GL. Munc18-syntaxin complexes and exocytosis in human platelets. J. Biol. Chem. 2003;278:19627–19633. doi: 10.1074/jbc.M212465200. [DOI] [PubMed] [Google Scholar]
- Howell SL, Tyhurst M, Duvefelt H, Andersson A, Hellerstrom C. Role of zinc and calcium in the formation and storage of insulin in the pancreatic beta-cell. Cell Tissue Res. 1978;188:107–118. doi: 10.1007/BF00220518. [DOI] [PubMed] [Google Scholar]
- Huang XF, Arvan P. Formation of the insulin-containing secretory granule core occurs within immature beta-granules. J. Biol. Chem. 1994;269:20838–20844. [PubMed] [Google Scholar]
- Huang L, Shen H, Atkinson MA, Kennedy RT. Detection of exocytosis at individual pancreatic beta cells by amperometry at a chemically modified microelectrode. Proc. Natl. Acad. Sci. USA. 1995;92:9608–9612. doi: 10.1073/pnas.92.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Wheeler MB, Kang YH, Sheu L, Lukacs GL, Trimble WS, Gaisano HY. Truncated SNAP-25 (1–197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998;12:1060–1070. doi: 10.1210/mend.12.7.0130. [DOI] [PubMed] [Google Scholar]
- Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+ -dependent insulin exocytosis. J. Cell. Sci. 2004;117:3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yasuda K, Inoue G, Okamoto Y, Yano H, Someya Y, Ohmoto Y, Deguchi K, Imagawa K, Imura H, et al. Glucose as regulator of glucose transport activity and glucose-transporter mRNA in hamster beta-cell line. Diabetes. 1992;41:592–597. doi: 10.2337/diab.41.5.592. [DOI] [PubMed] [Google Scholar]
- Irminger JC, Verchere CB, Meyer K, Halban PA. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J. Biol. Chem. 1997;272:27532–27534. doi: 10.1074/jbc.272.44.27532. [DOI] [PubMed] [Google Scholar]
- Ivarsson R, Obermuller S, Rutter GA, Galvanovskis J, Renstrom E. Temperature-sensitive random insulin granule diffusion is a prerequisite for recruiting granules for release. Traffic. 2004;5:750–762. doi: 10.1111/j.1600-0854.2004.00216.x. [DOI] [PubMed] [Google Scholar]
- Iynedjian PB. Mammalian glucokinase and its gene. Biochem. J. 1993;293(Pt 1):1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarousse N, Kelly RB. Endocytotic mechanisms in synapses. Curr. Opin. Cell Biol. 2001;13:461–469. doi: 10.1016/s0955-0674(00)00237-4. [DOI] [PubMed] [Google Scholar]
- Jensen MV, Joseph JW, Ilkayeva O, Burgess S, Lu D, Ronnebaum SM, Odegaard M, Becker TC, Sherry AD, Newgard CB. Compensatory responses to pyruvate carboxylase suppression in islet beta-cells. Preservation of glucose-stimulated insulin secretion. J. Biol. Chem. 2006;281:22342–22351. doi: 10.1074/jbc.M604350200. [DOI] [PubMed] [Google Scholar]
- John SA, Monck JR, Weiss JN, Ribalet B. The sulphonylurea receptor SUR1 regulates ATP-sensitive mouse Kir6.2 K+ channels linked to the green fluorescent protein in human embryonic kidney cells (HEK 293) J. Physiol. 1998;510(Pt 2):333–345. doi: 10.1111/j.1469-7793.1998.333bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keahey HH, Rajan AS, Boyd AE, Kunze DL. Characterization of voltage-dependent Ca2+ channels in beta-cell line. Diabetes. 1989;38:188–193. doi: 10.2337/diab.38.2.188. [DOI] [PubMed] [Google Scholar]
- Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Khan AH, Thurmond DC, Yang C, Ceresa BP, Sigmund CD, Pessin JE. Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J. Biol. Chem. 2001;276:4063–4069. doi: 10.1074/jbc.M007419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/ off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- Kizer JS, Tropsha A. A motif found in propeptides and prohormones that may target them to secretory vesicles. Biochem. Biophys. Res. Commun. 1991;174:586–592. doi: 10.1016/0006-291x(91)91457-n. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Prabakaran D, Arvan P. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol. Biol. Cell. 2000;11:1959–1972. doi: 10.1091/mbc.11.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 1999;259:3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- Lang J, Fukuda M, Zhang H, Mikoshiba K, Wollheim CB. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic beta-cells: Action of synaptotagmin at low micromolar calcium. EMBO J. 1997;16:5837–5846. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange RH. Crystalline islet B-granules in the grass snake (Natrix natrix (L.)): Tilting experiments in the electron microscope. J. Ultrastruct. Res. 1974;46:301–307. doi: 10.1016/s0022-5320(74)80064-x. [DOI] [PubMed] [Google Scholar]
- Leabu M. Membrane fusion in cells: Molecular machinery and mechanisms. J. Cell Mol. Med. 2006;10:423–427. doi: 10.1111/j.1582-4934.2006.tb00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YM, Sheu L, Kwan E, Wang G, Tsushima R, Gaisano H. Visualization of sequential exocytosis in rat pancreatic islet beta cells. Biochem. Biophys. Res. Commun. 2002;292:980–986. doi: 10.1006/bbrc.2002.6712. [DOI] [PubMed] [Google Scholar]
- Leung YM, Kang Y, Gao X, Xia F, Xie H, Sheu L, Tsuk S, Lotan I, Tsushima RG, Gaisano HY. Syntaxin 1A binds to the cytoplasmic C terminus of Kv2.1 to regulate channel gating and trafficking. J. Biol. Chem. 2003;278:17532–17538. doi: 10.1074/jbc.M213088200. [DOI] [PubMed] [Google Scholar]
- Leung YM, Kang Y, Xia F, Sheu L, Gao X, Xie H, Tsushima RG, Gaisano HY. Open form of syntaxin-1A is a more potent inhibitor than wild-type syntaxin-1A of Kv2.1 channels. Biochem. J. 2005;387:195–202. doi: 10.1042/BJ20041625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luo R, Hooi SC, Ruga P, Zhang J, Meda P, Li G. Ectopic expression of syncollin in INS-1 beta-cells sorts it into granules and impairs regulated secretion. Biochemistry. 2005;44:4365–4374. doi: 10.1021/bi048894d. [DOI] [PubMed] [Google Scholar]
- Ligon B, Boyd AE, Dunlap K. Class A calcium channel variants in pancreatic islets and their role in insulin secretion. J. Biol. Chem. 1998;273:13905–13911. doi: 10.1074/jbc.273.22.13905. [DOI] [PubMed] [Google Scholar]
- Lilja L, Johansson JU, Gromada J, Mandic SA, Fried G, Berggren PO, Bark C. Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J. Biol. Chem. 2004;279:29534–29541. doi: 10.1074/jbc.M312711200. [DOI] [PubMed] [Google Scholar]
- Lopez JA, Kwan EP, Xie L, He Y, James DE, Gaisano HY. The RalA GTPase is a central regulator of insulin exocytosis from pancreatic islet beta-cells. J. Biol. Chem. 2008;283:17939–17945. doi: 10.1074/jbc.M800321200. [DOI] [PubMed] [Google Scholar]
- Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS) Proc. Natl. Acad. Sci. USA. 2002;99:2708–2713. doi: 10.1073/pnas.052005699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Bindokas VP, Kuznetsov A, Rhodes C, Hays L, Edwardson JM, Ueda K, Steiner DF, Philipson LH. Direct imaging shows that insulin granule exocytosis occurs by complete vesicle fusion. Proc. Natl. Acad. Sci. USA. 2004;101:9266–9271. doi: 10.1073/pnas.0403201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MJ, Ammon HP, Patel T, Steinke J. Failure of 6-aminoni-cotinamide to inhibit the potentiating effect of leucine and arginine on glucose-induced insulin release in vitrol. Diabetologia. 1974;10:761–765. doi: 10.1007/BF01219538. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Warner TF, Mertz RJ. High activity of mitochondrial glycerol phosphate dehydrogenase in insulinomas and carcinoid and other tumors of the amine precursor uptake decarboxylation system. Cancer Res. 1990;50:7203–7205. [PubMed] [Google Scholar]
- MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos. Trans. Roy. Soc. Lond. B Biol. Sci. 2005;360:2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse WJ, Sener A, Herchuelz A, Hutton JC. Insulin release: The fuel hypothesis. Metabolism. 1979;28:373–386. doi: 10.1016/0026-0495(79)90111-2. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Gallwitz B, Schmidt WE, Nauck MA. Glucagon-like peptide 1 as a regulator of food intake and body weight: Therapeutic perspectives. Eur. J. Pharmacol. 2002a;440:269–279. doi: 10.1016/s0014-2999(02)01434-6. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Schmidt WE, Gallwitz B. Gastric inhibitory polypeptide: the neglected incretin revisited. Regul. Pept. 2002b;107:1–13. doi: 10.1016/s0167-0115(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Mertz RJ, Worley JF, Spencer B, Johnson JH, Dukes ID. Activation of stimulus-secretion coupling in pancreatic beta-cells by specific products of glucose metabolism. Evidence for privileged signaling by glycolysis. J. Biol. Chem. 1996;271:4838–4845. doi: 10.1074/jbc.271.9.4838. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Proks P, Ashcroft FM, Ashcroft SJ. Expression of functionally active ATP-sensitive K-channels in insect cells using baculovirus. FEBS Lett. 1998;429:390–394. doi: 10.1016/s0014-5793(98)00640-1. [DOI] [PubMed] [Google Scholar]
- Min L, Leung YM, Tomas A, Watson RT, Gaisano HY, Halban PA, Pessin JE, Hou JC. Dynamin is functionally coupled to insulin granule exocytosis. J. Biol. Chem. 2007;282:33530–33536. doi: 10.1074/jbc.M703402200. [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Mojsov S, Weir GC, Habener JF. Insulinotropin: Glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Invest. 1987;79:616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinete M, Irminger JC, Tooze SA, Halban PA. Trafficking/sorting and granule biogenesis in the beta-cell. Semin. Cell Dev. Biol. 2000a;11:243–251. doi: 10.1006/scdb.2000.0173. [DOI] [PubMed] [Google Scholar]
- Molinete M, Lilla V, Jain R, Joyce PB, Gorr SU, Ravazzola M, Halban PA. Trafficking of non-regulated secretory proteins in insulin secreting (INS-1) cells. Diabetologia. 2000b;43:1157–1164. doi: 10.1007/s001250051507. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc. Natl. Acad. Sci. USA. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu. Rev. Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J. Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Katsuta H, Ishida H, Nagamatsu S. Monitoring of exocytosis and endocytosis of insulin secretory granules in the pancreatic beta-cell line MIN6 using pH-sensitive green fluorescent protein (pHluorin) and confocal laser microscopy. Biochem. J. 2002;363:73–80. doi: 10.1042/0264-6021:3630073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Nelson D, Wilson JM, Meglasson MD, Erecinska M. Relationships between energy level and insulin secretion in isolated rat islets of Langerhans. Manipulation of [ATP]/[ADP][Pi] by 2-deoxy-d-glucose. Biochem. Pharmacol. 1992;43:1859–1864. doi: 10.1016/0006-2952(92)90722-u. [DOI] [PubMed] [Google Scholar]
- Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, Rorsman P, Eliasson L. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 2002;444:43–51. doi: 10.1007/s00424-002-0781-5. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L. The insulin factory: a tour of the plant surroundings and a visit to the assembly line. The Minkowski lecture 1973 revisited. Diabetologia. 1985;28:528–546. doi: 10.1007/BF00281987. [DOI] [PubMed] [Google Scholar]
- Orci L, Amherdt M, Malaisse-Lagae F, Rouiller C, Renold AE. Insulin release by emiocytosis: Demonstration with freeze-etching technique. Science. 1973;179:82–84. doi: 10.1126/science.179.4068.82. [DOI] [PubMed] [Google Scholar]
- Orci L, Malaisse W. Hypothesis: Single and chain release of insulin secretory granules is related to anionic transport at exocytotic sites. Diabetes. 1980;29:943–944. doi: 10.2337/diab.29.11.943. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli JD, Anderson RG. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Madsen O, Vassalli JD, Perrelet A. Direct identification of prohormone conversion site in insulin-secreting cells. Cell. 1985;42:671–681. doi: 10.1016/0092-8674(85)90124-2. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch MJ, Anderson RG, Vassalli JD, Perrelet A. Proteolytic maturation of insulin is a post-Golgi event which occurs in acidifying clathrin-coated secretory vesicles. Cell. 1987;49:865–868. doi: 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Perez-Branguli F, Muhaisen, Blasi AJ. Munc 18a binding to syntaxin 1A and 1B isoforms defines its localization at the plasma membrane and blocks SNARE assembly in a three-hybrid system assay. Mol. Cell Neurosci. 2002;20:169–180. doi: 10.1006/mcne.2002.1122. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu S-C, Braun JEA, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994a;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu S-C, Scheller RH. N-Sec1: A neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA. 1994b;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson LH, Steiner DF. Pas de deux or more: The sulfonylurea receptor and K+ channels. Science. 1995;268:372–373. doi: 10.1126/science.7716539. [DOI] [PubMed] [Google Scholar]
- Pobbati AV, Razeto A, Boddener M, Becker S, Fasshauer D. Structural basis for the inhibitory role of tomosyn in exocytosis. J. Biol. Chem. 2004;279:47192–47200. doi: 10.1074/jbc.M408767200. [DOI] [PubMed] [Google Scholar]
- Pouli AE, Emmanouilidou E, Zhao C, Wasmeier C, Hutton JC, Rutter GA. Secretory-granule dynamics visualized in vivo with a phogrin-green fluorescent protein chimaera. Biochem. J. 1998;333(Pt 1):193–199. doi: 10.1042/bj3330193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong WF, Bartley C, Wollheim CB. Single islet beta-cell stimulation by nutrients: Relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990;9:53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Wollheim CB. Cytosolic free Ca2+ in insulin secreting cells and its regulation by isolated organelles. Experientia. 1984;40:1052–1060. doi: 10.1007/BF01971451. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Kahn RA. GTP hydrolysis by ADP-ribosylation factor is dependent on both an ADP-ribosylation factor GTPase-activating protein and acid phospholipids. J. Biol. Chem. 1994;269:10758–10763. [PubMed] [Google Scholar]
- Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995;14:2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J. 1996;15:6951–6959. [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Gribble FM, Ashcroft FM. Differential response of K(ATP) channels containing SUR2A or SUR2B subunits to nucleotides and pinacidil. Mol. Pharmacol. 2000;58:1318–1325. doi: 10.1124/mol.58.6.1318. [DOI] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J. Physiol. 1997;502(Pt 1):105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, Halban PA. Newly synthesized proinsulin/insulin and stored insulin are released from pancreatic B cells predominantly via a regulated, rather than a constitutive, pathway. J. Cell Biol. 1987;105:145–153. doi: 10.1083/jcb.105.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ, Lucas CA, Mutkoski RL, Orci L, Halban PA. Stimulation by ATP of proinsulin to insulin conversion in isolated rat pancreatic islet secretory granules. Association with the ATP-dependent proton pump. J. Biol. Chem. 1987;262:10712–10717. [PubMed] [Google Scholar]
- Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. The cell physiology of biphasic insulin secretion. News Physiol. Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- Rutter GA. Nutrient-secretion coupling in the pancreatic islet beta-cell: Recent advances. Mol. Aspects Med. 2001;22:247–284. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Tsuboi T. Kiss and run exocytosis of dense core secretory vesicles. Neuroreport. 2004;15:79–81. doi: 10.1097/00001756-200401190-00016. [DOI] [PubMed] [Google Scholar]
- Sadoul K, Lang J, Montecucco C, Weller U, Regazzi R, Catsicas S, Wollheim CB, Halban PA. SNAP-25 is expressed in islets of Langerhans and is involved in insulin release. J. Cell Biol. 1995;128:1019–1028. doi: 10.1083/jcb.128.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul K, Berger A, Niemann H, Weller U, Roche PA, Klip A, Trimble WS, Regazzi R, Catsicas S, Halban PA. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J. Biol. Chem. 1997;272:33023–33027. doi: 10.1074/jbc.272.52.33023. [DOI] [PubMed] [Google Scholar]
- Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Schulze KL, Littleton JT, Salzberg A, Halachmi N, Stern M, Lev Z, Bellen HJ. rop, a Drosophila homolog of yeast Sec1 and vertebrate n-Sec1/ Munc-18 proteins, is a negative regulator of neurotransmitter release in vivo . Neuron. 1994;13:1099–1108. doi: 10.1016/0896-6273(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Shin OH, Rizo J, Sudhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat. Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- Sossin WS, Fisher JM, Scheller RH. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J. Cell Biol. 1990;110:1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic beta cells. Mol. Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- Steiner DF, Michael J, Houghten R, Mathieu M, Gardner PR, Ravazzola M, Orci L. Use of a synthetic peptide antigen to generate antisera reactive with a proteolytic processing site in native human proinsulin: Demonstration of cleavage within clathrin-coated (pro)secretory vesicles. Proc. Natl. Acad. Sci. USA. 1987;84:6184–6188. doi: 10.1073/pnas.84.17.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer JA, Horstmann H, Almers W. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature. 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- Stoffers DA. The development of beta-cell mass: Recent progress and potential role of GLP-1. Horm. Metab. Res. 2004;36:811–821. doi: 10.1055/s-2004-826168. [DOI] [PubMed] [Google Scholar]
- Straub SG, Sharp GW. Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 2002;18:451–463. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- Sugita S, Shin OH, Han W, Lao Y, Sudhof TC. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297:1349–1352. doi: 10.1126/science.1073806. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hatakeyama H, Okado H, Miwa A, Kishimoto T, Kojima T, Abe T, Kasai H. Sequential exocytosis of insulin granules is associated with redistribution of SNAP25. J. Cell Biol. 2004;165:255–262. doi: 10.1083/jcb.200312033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Kawanishi M, Niki T, Shinoda H, Araki S, Okazawa H, Kasuga M. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J. Biol. Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Tareste D, Shen J, Melia TJ, Rothman JE. SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc. Natl. Acad. Sci. USA. 2008;105:2380–2385. doi: 10.1073/pnas.0712125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J. Biol. Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- Thiele C, Gerdes HH, Huttner WB. Protein secretion: Puzzling receptors. Curr. Bio. 1997;7:R496–R500. doi: 10.1016/s0960-9822(06)00247-8. [DOI] [PubMed] [Google Scholar]
- Thorens B. Molecular and cellular physiology of GLUT-2, a high-Km facilitated diffusion glucose transporter. Int. Rev. Cytol. 1992;137:209–238. doi: 10.1016/s0074-7696(08)62677-7. [DOI] [PubMed] [Google Scholar]
- Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. Regulation of insulin-stimulated GLUT4 translocation by munc18c in 3T3L1 adipocytes. J. Biol. Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- Thurmond DC, Kanzaki M, Khan AH, Pessin JE. Munc18c function is rquired for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol. Cell. Biol. 2000;20:379–388. doi: 10.1128/mcb.20.1.379-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze SA, Huttner WB. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990;60:837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Kern HF, Fuller SD, Howell KE. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J. Cell Biol. 1989;109:35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- rus M, Wiser O, Goodnough MC, Atlas D. The transmembrane domain of syntaxin 1A negatively regulates voltage-sensitive Ca(2+) channels. Neuroscience. 2001;104:599–607. doi: 10.1016/s0306-4522(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Rutter GA. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr. Biol. 2003;13:563–567. doi: 10.1016/s0960-9822(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Terakawa S, Scalettar BA, Fantus C, Roder J, Jeromin A. Sweeping model of dynamin activity. Visualization of coupling between exocytosis and endocytosis under an evanescent wave microscope with green fluorescent proteins. J. Biol. Chem. 2002;277:15957–15961. doi: 10.1074/jbc.C200051200. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J. Biol. Chem. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Varadi A, Tsuboi T, Johnson-Cadwell LI, Allan VJ, Rutter GA. Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 beta-cells. Biochem. Biophys. Res. Commun. 2003;311:272–282. doi: 10.1016/j.bbrc.2003.09.208. [DOI] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Voets T, Moser T, Lund PE, Chow RH, Geppert M, Sudhof TC, Neher E. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 2001a;98:11680–11685. doi: 10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M. Munc18-1 promotes large dense-core vesicle docking. Neuron. 2001b;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, Muccioli G, Ghigo E, Papotti M. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J. Clin. Endocrinol. Metab. 2002;87:1300–1308. doi: 10.1210/jcem.87.3.8279. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE, Bucher P, Hofmann K. A conserved domain is present in different families of vesicular fusion proteins: A new superfamily. Proc. Natl. Acad. Sci. USA. 1997;94:3046–3051. doi: 10.1073/pnas.94.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Islets of Langerhans: The puzzle of intraislet interactions and their relevance to diabetes. J. Clin. Invest. 1990;85:983–987. doi: 10.1172/JCI114574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MB, Sheu L, Ghai M, Bouquillon A, Grondin G, Weller U, Beaudoin AR, Bennett MK, Trimble WS, Gaisano HY. Characterization of SNARE protein expression in beta cell lines and pancreatic islets. Endocrinology. 1996;137:1340–1348. doi: 10.1210/endo.137.4.8625909. [DOI] [PubMed] [Google Scholar]
- Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: A novel developmentally regulated islet cell in the human pancreas. Regul. Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. USA. 1999a;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. The voltage sensitive Lc-type Ca2+ channel is functionally coupled to the exocytotic machinery. Proc. Natl. Acad. Sci. USA. 1999b;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rammner B, Margittai M, Artalejo AR, Neher E, Jahn R. Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- Yang SN, Larsson O, Branstrom R, Bertorello AM, Leibiger B, Leibiger IB, Moede T, Kohler M, Meister B, Berggren PO. Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca(2+) channels in pancreatic beta cells. Proc. Natl. Acad. Sci. USA. 1999;96:10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC, Jr., Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J. Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC. Influence of pyruvic acid methyl ester on rat pancreatic islets. Effects on insulin secretion, phosphoinositide hydrolysis, and sensitiza-tion of the beta cell. J. Biol. Chem. 1997;272:3527–3531. doi: 10.1074/jbc.272.6.3527. [DOI] [PubMed] [Google Scholar]
- Zawalich WS, Zawalich KC, Rasmussen H. Interactions between cholinergic agonists and enteric factors in the regulation of insulin secretion from isolated perifused rat islets. Acta Endocrinol. (Copenh) 1989;120:702–707. doi: 10.1530/acta.0.1200702. [DOI] [PubMed] [Google Scholar]
- Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta -cell. J. Biol. Chem. 2000;275:41521–41527. doi: 10.1074/jbc.M005479200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Khan A, Ostenson CG, Berggren PO, Efendic S, Meister B. Down-regulated expression of exocytotic proteins in pancreatic islets of diabetic GK rats. Biochem. Biophys. Res. Commun. 2002;291:1038–1044. doi: 10.1006/bbrc.2002.6555. [DOI] [PubMed] [Google Scholar]