Abstract
The balance of cellular energy levels in response to changes of nutrient availability, stress stimuli or exercise is a critical step in maintaining tissue and whole body homeostasis. Disruption of this balance is associated with various pathologies, including the metabolic syndrome. Recently, accumulating evidence has demonstrated that the AMP-activated protein kinase (AMPK) plays a central role in sensing changes in energy levels. The regulation of AMPK activity is currently the subject of significant investigation since this enzyme is a potential therapeutic target in both metabolic disorders and tumorigenesis. In this review, we present novel evidence of crosstalk between Fyn, one member of the Src kinase family, and AMPK.
Keywords: Fyn kinase, AMPK, LKB1, homeostasis, lipid metabolism
Introduction
Regulation of energy levels is a fundamental process for all living organisms. The ability of cells and tissues to “energy sense” allows for fine control of cellular AMP and ATP ratios, which must be precisely maintained to drive essential metabolic functions. At the whole body level, maintaining an appropriate energy balance depends on the ability of molecular and cellular mechanisms to efficiently couple the energy intake with that of energy expenditure. Thus, obesity and other disorders known collectively as the metabolic syndrome (insulin resistance, Type II diabetes mellitus, steatosis, atherosclerosis) are often a result of disordered balance between these two components.
For these reasons, interest has recently focused upon the identification of basic cellular metabolic pathways involved in energy sensing at the cellular, tissue and organism levels. The molecules controlling this energy sensing mechanism have become the focus of significant research given their inherently attractive roles as potential therapeutic targets. At present, a variety of molecules have been identified in the energy sensing pathways including hypoxia-inducible factor 1 (Ebert et al., 1995), peroxisome proliferator-activated receptors (Tanaka et al., 2003), Sirt1 (Chung et al., 1992) and mTORC complex (Wullschleger et al., 2006). However, much interest has been given to the AMP-activated protein kinase (AMPK) and its downstream signalling pathway (Sim and Hardie, 1988). Many studies have described AMPK as one of the main energy sensors and a key regulator of the energy homeostasis at cellular level. More recently, evidence also points to AMPK’s role in whole body energy balance by its response to nutrient and hormonal signals that modulate feeding behaviour thereby regulating energy expenditure (Lage et al., 2008).
Clearly, the identification of upstream factors regulating AMPK is likely to have a significant impact on future therapeutic and medical interventions for insulin resistance, obesity and other related disorders.
AMPK structure
AMPK is a heterotrimeric complex composed of one catalytic α-subunit and two regulatory subunits, β and γ (Davies et al., 1994). This structure is highly conserved and orthologues are found in all eukaryotic species (Stapleton et al., 1994). Each functional AMPK complex is composed of multiple isoforms (α1, α2, β1, β2, γ1, γ2, γ3) that are encoded by different genes and have overlapping tissue distribution (Kajita et al., 2008). Muscle primarily expresses the α2 subunit as well as both β and all three γ isoforms whereas adipose tissue primarily expresses the α1 subunit with both β and the γ1 and 2 isoforms (Woods et al., 1996a, 1996b, 2000). Although the precise role of the different subunits of the AMPK is not known, hetero-trimeric complexes containing the catalytic α1 subunit appear to be less AMP sensitive (Salt et al., 1998). The β-subunit appears to function as part of the heterotrimeric assembly mechanism though its C-terminus. In addition, the β-subunit contains a glycogen-binding domain (GBD/KIS) closely related to that of enzymes which metabolize glycans such as starch and glycogen. It has been suggested that the GBD domain of the β subunit localizes AMPK, at least in part, to glycogen particles (Polekhina et al., 2003). The γ-subunit has a variable N-terminus and contains two pairs of a structural module called cystathionine-β-synthase (CBS) but the function of these modules is still not clearly defined (Hardie and Hawley, 2001).
AMPK activity regulation
The primary mechanism for AMPK activation occurs from an increase in the AMP/ATP ratio. AMP first binds to the γ subunit inducing a conformational change in AMPK (Hawley et al., 1995; Hardie et al., 1999) structure and triggers an upstream kinase to phosphorylate T172 on the α subunit. AMP also appears to prevent α subunit dephosphorylation (Davies et al., 1995), thereby maintaining AMPK in an activated state.
While AMP was originally thought to be solely responsible for this activation, it has become increasingly clear that AMPK is regulated to a far more complex level. For example, changes in the NAD/NADH ratio have been shown to activate AMPK independently of the adenine nucleotide levels (Hawley et al., 2002; Rafaeloff-Phail et al., 2004). In addition, AMPK activity is negatively regulated by Akt. Akt-dependent phosphorylation of the S485 and S491 residues inhibits AMPK T172 phosphorylation (Kovacic et al., 2003).
AMPK activity is also regulated by other serine/threonine protein kinases. Among them is TAK1 (transforming factor beta activated kinase 1) which was identified as an AMPK kinase in yeast (Momcilovic et al., 2006) and subsequently in mice (Xie et al., 2006). It has been shown to associate with TAK-1 binding proteins (TAB1, 2 or 3) to form heterotrimeric complexes that involve TAK1-TAB-1 and either TAB 2 or TAB3.
However, there are two other AMPK kinases that have also been shown to directly phosphorylate the AMPKα subunit resulting in its activation. CAMKKs appear to function as upstream AMPK kinases in neuronal cells (Hawley et al., 2005). The activity of CAMKKα requires an increase in intracellular calcium levels suggesting that AMPK may have a role in calcium mediated signal transduction pathways (Woods et al., 2005).
Given that the CAMKK family is poorly expressed in peripheral tissues (liver, skeletal muscle and adipose) the identity of the primary kinase that phosphorylates AMPK at T172 in these tissues has been ascribed to LKB1. LKB1 is a serine/threonine kinase multi-tasking enzyme, which is involved in cell polarity, tumour growth, and energy metabolism (Alessi et al., 2006). In this role, LKB1 has been shown to regulate glucose uptake and fatty acid oxidation as well as multiple metabolic actions via AMPK. LKB1 itself is regulated by phosphorylation on diverse serine and threonine residues by protein kinase A (PKA) and p90 S6 kinase (Collins et al., 2000; Sapkota et al., 2001). LKB1 is assembled into a functional hetero-trimeric complex composed of Ste20 related adaptor protein (STRAD) and mouse protein 25 (MO25) (Baas et al., 2003; Boudeau et al., 2003; Alessi et al., 2006). STRAD, although able to bind ATP molecules, appears to function as a pseudo-kinase since it lacks intrinsic catalytic activity. Nevertheless, STRAD is essential for LKB1 to phosphorylate AMPK and appears to re-localize LKB1 from the nucleus into the cytoplasm. MO25 stabilizes the LKB1/STRAD complex into a heterotrimeric structure that has approximately 100-fold more kinase activity towards substrates than LKB1 alone (Hawley et al., 2003).
AMPK phosphorylation is also regulated by protein phosphatases 2A and 2C (PP2A, PP2C) (Davies et al., 1995; Marley et al., 1996). PPC2A and PP2C dephosphorylate AMPK at T172 in a process regulated by AMP and palmitate (Wu et al., 2007). AMP regulates PP2A and PP2C dephosphorylation by blocking the action of these phosphatases at T172.
More recently, we have uncovered a novel signalling pathway regulating AMPK activity (Bastie et al., 2007). The remainder of this review will focus on the role of Fyn kinase, a member of the Src family kinase, in AMPK activity and regulation.
Fyn kinase structure
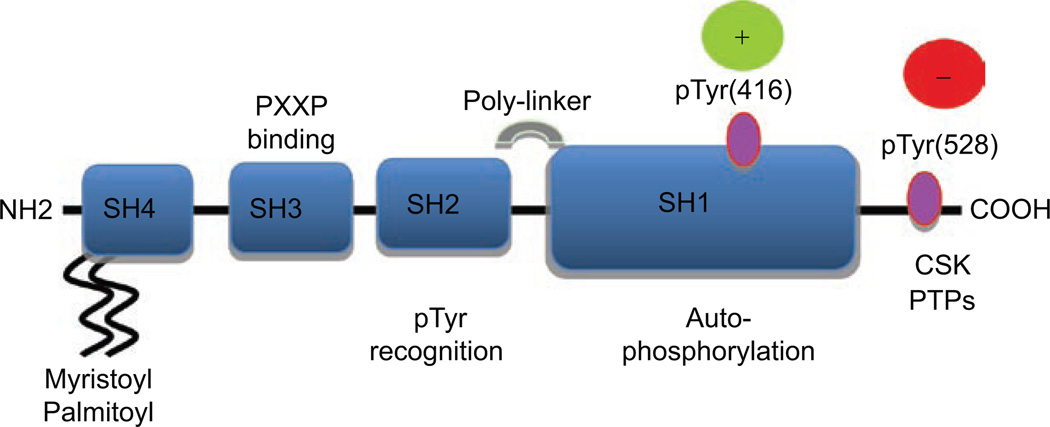
Fyn is a tyrosine specific phospho-transferase that is a member of the large Src family of non-receptor tyrosine kinases. Although no formal crystal structure exists for the full length Fyn protein, the mode of regulation of Fyn tyrosine kinase activity is likely to be similar to that of other Src family kinases. The members of this family share a conserved structure consisting of consecutive SH1, SH2 and SH3 domains (Figure 1). The SH1 domain is the catalytic tyrosine kinase and the SH2 domain binds to tyrosine-phosphorylated substrates. In particular, the SH2 domain of Fyn binds the phosphorylated tyrosine Y528 residue in its carboxyl terminal tail under basal conditions in vivo (Zheng et al., 2000), thereby stabilizing the structure into an inactive conformation and inhibiting the tyrosine kinase SH1 domain (Sicheri and Kuriyan, 1997; Boggon and Eck, 2004). Additionally, repression of Fyn kinase activity is achieved by intra-molecular interactions between the SH3 domain and a polyproline type II linker helix that connects the SH2 and the SH1 domains.
Figure 1.
Fyn kinase structure and regulation. Fyn kinase consists of SH1-4 domains. The SH2 domain binds the phosphorylated Y528 in the C-terminus, locking Fyn in an inactive conformation. Y528 is dephosphorylated by phosphatases (PTPs), opening the structure and allowing Y416 in the catalytic SH1 domain to be phosphorylated.
In Fyn kinase, the tyrosine Y528 negative regulatory site is phosphorylated by C-terminal src Kinase (Csk), a cytoplasmic protein-tyrosine kinase, first isolated from neonatal rat brain (Nada et al., 1991). Csk homology kinase (CHK) is a second enzyme that catalyses the phosphorylation of this inhibitory tyrosine Y528 (Chong et al., 2005a). While Csk is expressed in all mammalian cells, CHK expression is limited to breast, haematopoietic cells, neurons and testes (Brown and Cooper, 1996). CHK binds to Src family members with a high affinity, independent of CHK catalytic activity. This binding may be sufficient to inhibit Src family kinase activity (Chong et al., 2004). The dephosphorylation of the Y528 residue by protein tyrosine phosphatases rPTPα, SHP1/2, PTP1B, PTPε and CD45 (Chan et al., 1994; Sefton and Taddie, 1994; Asante-Appiah and Kennedy, 2003; Chong et al., 2005b; Poole and Jones, 2005; Roskoski, 2005) can release the SH2 domain and activate the enzyme.
In addition, the subfamily composed of Fyn, Src and Lyn kinases contains dual acylation sites in the amino-terminal SH4 domain, which is thought to be partially responsible for lipid raft micro-domain association (Boggon and Eck, 2004). Studies have observed that disruption of adipocyte lipid raft organization can have large effects on adipocyte function and differentiation (Saltiel and Pessin, 2002). In the case of Fyn, the amino terminal glycine residue, in the SH4 domain, is modified by myristoylation and cysteine 3 is S-acylated with palmitate (as well as palmitoleate, stearate or oleate) (van’t Hof and Resh, 1997; Liang et al., 2001, 2004). Alterations in the sub-cellular localization of Fyn can also modulate its activity by regulating its accessibility to substrates localized in membranes or membrane microdomains sometimes referred to as lipid rafts (Sicheri and Kuriyan, 1997). Depalmitoylation or substitution of the palmitate by un-saturated fatty acids, results in the release of Fyn from the membrane and the subsequent inhibition of Fyn-mediated phosphorylation of membrane bound substrates (Liang et al., 2001, 2004). It has also been recently demonstrated that nonpalmitoylated Src-family tyrosine kinase is rapidly exchanged between the plasma membrane and late endosomes, suggesting that Fyn (and other src kinases) trafficking is specified by the palmitoylation state of the SH4 domain (Sato et al., 2009).
Role of Fyn protein kinase in insulin signalling
Studies have suggested that the Src kinase family plays a significant regulatory role in propagating a subset of insulin signalling events. For example, IGF-1 stimulated adipocyte differentiation was reported to activate Csk and inhibit Src kinase activity (Sekimoto and Boney, 2003). The Src kinase family has also been implicated in the activation of the MAPkinase cascade and as transducers of signals via G protein coupled receptors (Della Rocca et al., 1997; Luttrell et al., 1999). The Src kinase family has been reported to cross talk with insulin and IGF1 receptors as well as IRS1 by inducing tyrosine phosphorylation, mimicking their downstream biological action (Muller et al., 2000). In addition, the Src kinase family activates the PI3-kinase signalling pathway, this being a well established link to the stimulation of glucose uptake in skeletal muscle and adipocytes (Choudhury et al., 2006). More recently, it has been shown that a constitutively active form of Src inhibits pyruvate kinase (Christofk et al., 2008a, 2008b). Additionally, the role of Fyn was further strengthened by a study using a pharmacological approach which showed that Src family kinase inhibitors prevented 3T3L1 adipocyte differentiation (Sun et al., 2005).
Interestingly, several studies have implicated Fyn in the regulation of insulin signalling through lipid raft dependent signalling (Mastick and Saltiel, 1997; Newcomb and Mastick, 2002). The integrity of the lipid raft micro-domain organization plays an important role in insulin signalling, independently of the classical IRS/PI3K/Akt pathway (Saltiel and Pessin, 2002, 2003). Disruption of the lipid raft microdomains result in a marked reduction of adipogenesis associated with a drastic impairment of insulin signalling and subsequent marked insulin resistance in mice (Cohen et al., 2003; Oshikawa et al., 2004; Capozza et al., 2005). Fyn localizes in the lipid raft micro-domains of the plasma membrane where it is associated to lipid raft proteins such as flotilin and CD36 (Huang et al., 1991; Bull et al., 1994). CD36, also known as FAT (fatty acid translocase), facilitates long-chain fatty acid uptake in skeletal muscle and adipose tissue and is linked to phenotypic features of the metabolic syndrome including insulin resistance and dyslipidemia (Pravenec et al., 2003; Drover and Abumrad, 2005; Meex et al., 2005). Loss of CD36 expression was also observed to impair adipogenesis in cultured pre-adipocytes (Sfeir et al., 1999). The physical association of Fyn with CD36 suggests a functional coupling between lipid raft organization and the regulation of fatty acid translocation.
Role of Fyn protein kinase in peripheral tissue fatty acid oxidation
Despite the apparent relationship between insulin signalling, lipid raft organization and Fyn function in cultured adipocytes, only a limited analysis of Src kinase family function in insulin sensitivity and action in vivo has been performed. To date, studies of Src family kinase knockout mice have only been investigated in terms of immunological function, neuronal development or tumorigenesis. In particular, Fyn null mice display various defects in immune signalling such as reduced capacity of natural killer T cells to proliferate and reduced mast cell degranulation (Parravicini et al., 2002; Gadue et al., 2004). In addition, Fyn null mice display aberrant oligodendrocyte morphogenesis and hypomyelination (Colognato et al., 2004; Perez et al., 2008). However, these are generally mild phenotypes since these mice have a normal lifespan and are fertile.
Our initial studies demonstrated that the Fyn null mice have reduced body weight and decreased total fat volume quantified by microcomputed tomography (microCT). This decrease was due to a 60% reduction in adipocyte size. Interestingly, the Fyn null mice showed markedly improved (reduced) triglyceride and non-esterified-fatty-acid content in both plasma and tissue. In addition, Fyn null mice displayed improved glucose tolerance and increased insulin sensitivity as assessed by conscious non-stressed euglycemic-hyperglycemic clamps. Tissue lipid accumulation is often coupled to states of insulin resistance (Shulman, 2000). Thus, it is likely that the decrease of tissue lipid accumulation accounts, at least in part, for the increased insulin sensitivity observed in the Fyn null mice.
In addition, Fyn null mice demonstrated increased whole body fatty acid utilization, with specific increases in both skeletal muscle and adipose tissue, resulting in a greater state of catabolism in the fasted state. Taking the above data, and coupling it to the observation that these animals have reduced adiposity, suggests that the likely cause for the decreased tissue lipids is increased fatty acid oxidation.
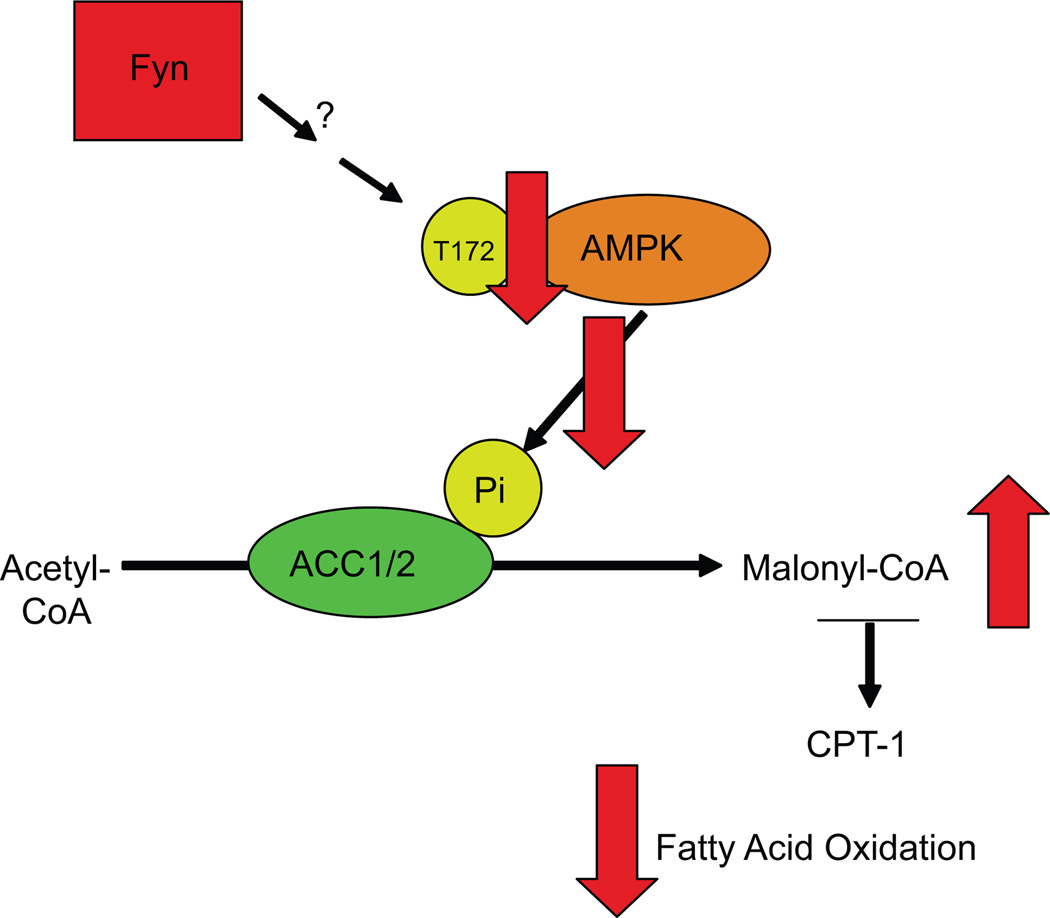
It is well established that fatty acid oxidation is co-ordinately regulated by the allosteric regulation of CPT-1 (carnitine palmitoyl-transferase) activity, the rate-limiting step in the transport of acyl-CoA across the outer mitochondrial membrane. CPT-1 activity is inhibited when malonyl-CoA levels are high and activated when malonyl-CoA levels are low. The two isoforms of acetyl-CoA carboxylase (ACC1 and ACC2) catalyze the production of malonyl-CoA from acetyl-CoA. The ACCs are regulated by an inhibitory phosphorylation on the serine 221 in ACC1 and serine 79 in ACC2 by AMPK (Figure 2). Consistent with the increased fatty acid oxidation, the phosphorylation of ACC on these inhibitory residues was increased in skeletal muscle and adipose tissue of Fyn null animals. This was directly correlated with increased AMPK T172 α subunit phosphorylation and increased AMPK activity in these tissues. In addition, acute inhibition of Fyn by different Src-family inhibitors resulted in the phosphorylation of the activating site of AMPK in 3T3L-1 adipocytes, strongly suggesting that Fyn activity, and not its expression, is coupled to a signalling pathway leading to the repression of AMPK activity.
Figure 2.
Fyn kinase regulates fatty acid oxidation. Fatty acid oxidation is co-ordinately regulated by the allosteric regulation of carnitine palmitoyl-transferase (CPT-1) activity. CPT-1 activity is inhibited when malonyl-CoA levels are high and activated when malonyl-CoA levels are low. The acetyl-CoA carboxylase (ACC) is the enzyme catalysing the production of malonyl-CoA from acetyl-CoA. ACC is inhibited by AMPK that phosphorylates ACC. AMPK itself is activated when phosphorylated on T172.
Fyn kinase decreases T172 phosphorylation on AMPK, activating ACC. This results in increased malonylCoA levels that inhibit CPT1 activity and decrease fatty acid oxidation.
Conclusion
While these studies demonstrate a connection between Fyn kinase and AMPK, the precise molecular mechanisms underlying their coupling requires further characterization. Whether Fyn kinase directly interacts with AMPK or represses the upstream cascade of events activating AMPK remains to be determined. In addition, although there is only one fyn gene, three splice variants generate mRNAs presumably encoded for three distinct Fyn proteins. Non-quantitative PCR has indicated that FynB (exon 7A) is expressed in the brain with much reduced expression in lymphoid tissue (Picard et al., 2002). In contrast, FynT (exon 7B) is primarily expressed in cells of the hematopoietic lineage with reduced levels in the brain (Sudol et al., 1993; Takeuchi et al., 1993). The mRNA for a third splice variant that is devoid of exon 7 (FynΔ7) has been detected in cultured cells (Goldsmith et al., 2002). FynT isoform appears to have increased catalytic activity compared to the FynB isoform (Goldsmith et al., 2002). Experimental evidence has demonstrated that different Fyn isoforms couple to distinct signalling pathways leading to tissue-specific biological responses (Cooke and Perlmutter, 1989). Therefore, the regulation of AMPK may be differently modulated, accordingly to the Fyn isoform present in a particular tissue. Thus, additional work is required to understand the mechanism linking Fyn activity to AMPK regulation and to determine the role of the different Fyn isoforms in this process. Nevertheless, these findings support a model in which Fyn signalling limits fatty acid oxidation in the fasted state due to decreased activation of AMPK. Consequently, the loss of Fyn function allows increased energy production from lipid stores. In turn, the integrated physiological response to these changes in fatty acid oxidation improves metabolic lipid profiles and insulin sensitivity. Importantly, this study implicates Fyn kinase as a novel nutrient-sensor system coupled to AMPK.
Acknowledgements
This work was supported by The Ellison Medical Foundation (New Scholars Award in Aging) (CB), the National Institutes of Health (DK78886, DK20541, DK020541) and the American Diabetes Association (1-07-RA-142) (JP). MV is grateful to the Fulbright Commission for support.
Footnotes
Declaration of interest: The authors report no conflict of interest.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signalling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Asante-Appiah E, Kennedy BP. Protein tyrosine phosphatases: the quest for negative regulators of insulin action. Am J Physiol Endocrinol Metab. 2003;284:E663–E670. doi: 10.1152/ajpendo.00462.2002. [DOI] [PubMed] [Google Scholar]
- Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastie CC, Zong H, Xu J, Busa B, Judex S, Kurland IJ, Pessin JE. Integrative metabolic regulation of peripheral tissue fatty acid oxidation by the SRC kinase family member Fyn. Cell Metab. 2007;5:371–381. doi: 10.1016/j.cmet.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- Bull HA, Brickell PM, Dowd PM. Src-related protein tyrosine kinases are physically associated with the surface antigen CD36 in human dermal microvascular endothelial cells. FEBS Lett. 1994;351:41–44. doi: 10.1016/0014-5793(94)00814-0. [DOI] [PubMed] [Google Scholar]
- Capozza F, Combs TP, Cohen AW, Cho YR, Park SY, Schubert W, Williams TM, Brasaemle DL, Jelicks LA, Scherer PE, Kim JK, Lisanti MP. Caveolin-3 knockout mice show increased adiposity and whole body insulin resistance, with ligand-induced insulin receptor instability in skeletal muscle. Am J Physiol Cell Physiol. 2005;288:C1317–C1331. doi: 10.1152/ajpcell.00489.2004. [DOI] [PubMed] [Google Scholar]
- Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- Chong YP, Ia KK, Mulhern TD, Cheng HC. Endogenous and synthetic inhibitors of the Src-family protein tyrosine kinases. Biochim Biophys Acta. 2005a;1754:210–220. doi: 10.1016/j.bbapap.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Chong YP, Mulhern TD, Cheng HC. C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)-endogenous negative regulators of Src-family protein kinases. Growth Factors. 2005b;23:233–244. doi: 10.1080/08977190500178877. [DOI] [PubMed] [Google Scholar]
- Chong YP, Mulhern TD, Zhu HJ, Fujita DJ, Bjorge JD, Tantiongco JP, Sotirellis N, Lio DS, Scholz G, Cheng HC. A novel non-catalytic mechanism employed by the C-terminal Src-homologous kinase to inhibit Src-family kinase activity. J Biol Chem. 2004;279:20752–20766. doi: 10.1074/jbc.M309865200. [DOI] [PubMed] [Google Scholar]
- Choudhury GG, Mahimainathan L, Das F, Venkatesan B, Ghosh-Choudhury N. c-Src couples PI 3 kinase/Akt and MAPK signalling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal. 2006;18:1854–1864. doi: 10.1016/j.cellsig.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008a;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008b;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Chung AB, Stepien G, Haraguchi Y, Li K, Wallace DC. Transcriptional control of nuclear genes for the mitochondrial muscle ADP/ATP translocator and the ATP synthase beta subunit. Multiple factors interact with the OXBOX/REBOX promoter sequences. J Biol Chem. 1992;267:21154–21161. [PubMed] [Google Scholar]
- Cohen AW, Razani B, Wang XB, Combs TP, Williams TM, Scherer PE, Lisanti MP. Caveolin-1-deficient mice show insulin resistance and defective insulin receptor protein expression in adipose tissue. Am J Physiol Cell Physiol. 2003;285:C222–C235. doi: 10.1152/ajpcell.00006.2003. [DOI] [PubMed] [Google Scholar]
- Collins SP, Reoma JL, Gamm DM, Uhler MD. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem J. 2000;345(Pt 3):673–680. [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MP, Perlmutter RM. Expression of a novel form of the fyn proto-oncogene in hematopoietic cells. New Biol. 1989;1:66–74. [PubMed] [Google Scholar]
- Davies SP, Hawley SA, Woods A, Carling D, Haystead TA, Hardie DG. Purification of the AMP-activated protein kinase on ATP-gamma-sepharose and analysis of its subunit structure. Eur J Biochem. 1994;223:351–357. doi: 10.1111/j.1432-1033.1994.tb19001.x. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, van Biesen T, Daaka Y, Luttrell DK, Luttrell LM, Lefkowitz RJ. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk2, and Src kinase. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- Drover VA, Abumrad NA. CD36-dependent fatty acid uptake regulates expression of peroxisome proliferator activated receptors. Biochem Soc Trans. 2005;33:311–315. doi: 10.1042/BST0330311. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- Gadue P, Yin L, Jain S, Stein PL. Restoration of NK T cell development in fyn-mutant mice by a TCR reveals a requirement for Fyn during early NK T cell ontogeny. J Immunol. 2004;172:6093–6100. doi: 10.4049/jimmunol.172.10.6093. [DOI] [PubMed] [Google Scholar]
- Goldsmith JF, Hall CG, Atkinson TP. Identification of an alternatively spliced isoform of the fyn tyrosine kinase. Biochem Biophys Res Commun. 2002;298:501–504. doi: 10.1016/s0006-291x(02)02510-x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338(Pt 3):717–722. [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The anti-diabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2???? activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita K, Mune T, Ikeda T, Matsumoto M, Uno Y, Sugiyama C, Matsubara K, Morita H, Takemura M, Seishima M, Takeda J, Ishizuka T. Effect of fasting on PPARgamma and AMPK activity in adipocytes. Diabetes Res Clin Pract. 2008;81:144–149. doi: 10.1016/j.diabres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Liang X, Lu Y, Wilkes M, Neubert TA, Resh MD. The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J Biol Chem. 2004;279:8133–8139. doi: 10.1074/jbc.M311180200. [DOI] [PubMed] [Google Scholar]
- Liang X, Nazarian A, Erdjument-Bromage H, Bornmann W, Tempst P, Resh MD. Heterogeneous fatty acylation of Src family kinases with polyunsaturated fatty acids regulates raft localization and signal transduction. J Biol Chem. 2001;276:30987–30994. doi: 10.1074/jbc.M104018200. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Marley AE, Sullivan JE, Carling D, Abbott WM, Smith GJ, Taylor IW, Carey F, Beri RK. Biochemical characterization and deletion analysis of recombinant human protein phosphatase 2C alpha. Biochem J. 1996;320(Pt 3):801–806. doi: 10.1042/bj3200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick CC, Saltiel AR. Insulin-stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3-L1 cells. J Biol Chem. 1997;272:20706–20714. doi: 10.1074/jbc.272.33.20706. [DOI] [PubMed] [Google Scholar]
- Meex SJ, van der Kallen CJ, van Greevenbroek MM, Eurlings PM, El Hasnaoui M, Evelo CT, Lindsey PJ, Luiken JJ, Glatz JF, de Bruin TW. Up-regulation of CD36/FAT in preadipocytes in familial combined hyperlipidemia. FASEB J. 2005;19:2063–2065. doi: 10.1096/fj.04-2403fje. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Muller G, Wied S, Frick W. Cross talk of pp125(FAK) and pp59(Lyn) non-receptor tyrosine kinases to insulin-mimetic signalling in adipocytes. Mol Cell Biol. 2000;20:4708–4723. doi: 10.1128/mcb.20.13.4708-4723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Newcomb LF, Mastick CC. Src family kinase-dependent phosphorylation of a 29-kDa caveolin-associated protein. Biochem Biophys Res Commun. 2002;290:1447–1453. doi: 10.1006/bbrc.2002.6371. [DOI] [PubMed] [Google Scholar]
- Oshikawa J, Otsu K, Toya Y, Tsunematsu T, Hankins R, Kawabe J, Minamisawa S, Umemura S, Hagiwara Y, Ishikawa Y. Insulin resistance in skeletal muscles of caveolin-3-null mice. Proc Natl Acad Sci USA. 2004;101:12670–12675. doi: 10.1073/pnas.0402053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Perez MJ, Ortiz EH, Roffe M, Soto EF, Pasquini JM. Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin. J Neurosci Res. doi: 10.1002/jnr.21962. (Forthcoming). [DOI] [PubMed] [Google Scholar]
- Picard C, Gilles A, Pontarotti P, Olive D, Collette Y. Cutting edge: recruitment of the ancestral fyn gene during emergence of the adaptive immune system. J Immunol. 2002;168:2595–2598. doi: 10.4049/jimmunol.168.6.2595. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Pravenec M, Landa V, Zidek V, Musilova A, Kazdova L, Qi N, Wang J, St Lezin E, Kurtz TW. Transgenic expression of CD36 in the spontaneously hypertensive rat is associated with amelioration of metabolic disturbances but has no effect on hypertension. Physiol Res. 2003;52:681–688. [PubMed] [Google Scholar]
- Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, McClure D, Guo H, Emerson K, Brooks H. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescot A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334(Pt 1):177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signalling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signalling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JS, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90(RSK) and cAMP-dependent protein kinase, but not its farnesylation at Cys(433), is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci. 2009;122:965–975. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- Sefton BM, Taddie JA. Role of tyrosine kinases in lymphocyte activation. Curr Opin Immunol. 1994;6:372–379. doi: 10.1016/0952-7915(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Sekimoto H, Boney CM. C-terminal Src kinase (CSK) modulates insulin-like growth factor-I signalling through Src in 3T3-L1 differentiation. Endocrinology. 2003;144:2546–2552. doi: 10.1210/en.2003-0187. [DOI] [PubMed] [Google Scholar]
- Sfeir Z, Ibrahimi A, Amri E, Grimaldi P, Abumrad N. CD36 antisense expression in 3T3-F442A preadipocytes. Mol Cell Biochem. 1999;192:3–8. [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–785. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- Sim AT, Hardie DG. The low activity of acetyl-CoA carboxylase in basal and glucagon-stimulated hepatocytes is due to phosphorylation by the AMP-activated protein kinase and not cyclic AMP-dependent protein kinase. FEBS Lett. 1988;233:294–298. doi: 10.1016/0014-5793(88)80445-9. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Gao G, Michell BJ, Widmer J, Mitchelhill K, Teh T, House CM, Witters LA, Kemp BE. Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994;269:29343–29346. [PubMed] [Google Scholar]
- Sudol M, Greulich H, Newman L, Sarkar A, Sukegawa J, Yamamoto T. A novel Yes-related kinase, Yrk, is expressed at elevated levels in neural and hematopoietic tissues. Oncogene. 1993;8:823–831. [PubMed] [Google Scholar]
- Sun Y, Ma YC, Huang J, Chen KY, McGarrigle DK, Huang XY. Requirement of SRC-family tyrosine kinases in fat accumulation. Biochemistry. 2005;44:14455–14462. doi: 10.1021/bi0509090. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Kuramochi S, Fusaki N, Nada S, Kawamura-Tsuzuku J, Matsuda S, Semba K, Toyoshima K, Okada M, Yamamoto T. Functional and physical interaction of protein-tyrosine kinases Fyn and Csk in the T-cell signalling system. J Biol Chem. 1993;268:27413–27419. [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, Watanabe M, Magoori K, Ioka RX, Tachibana K, Watanabe Y, Uchiyama Y, Sumi K, Iguchi H, Ito S, Doi T, Hamakubo T, Naito M, Auwerx J, Yanagisawa M, Kodama T, Sakai J. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Cheung PC, Smith FC, Davison MD, Scott J, Beri RK, Carling D. Characterization of AMP-activated protein kinase beta and gamma subunits. Assembly of the heterotrimeric complex in vitro. J Biol Chem. 1996a;271:10282–10290. doi: 10.1074/jbc.271.17.10282. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2????dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996b;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signalling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]