INTRODUCTION
Introduced a century ago by Bruening as a transoral procedure under general anesthesia, injection laryngoplasty is the oldest surgical management technique for vocal fold insufficiency.1 In 1985, Ward et al. introduced the office-based transcutaneous approach for vocal fold injection augmentation. They described the technique for Teflon injection under local anesthesia using the cricothyroid membrane approach while visualizing the injection using video laryngoscopy. This approach was developed to enable vocal fold augmentation in patients who could not undergo a transoral injection “due to anatomical deformity, trismus, or for other reasons.”2 Although initially described as a transcricothyroid membrane approach, percutaneous injection may also be performed in a transthyroid cartilage route or a transthyrohyoid membrane route.3,4 Office-based approaches are now commonly chosen for injection augmentation laryngoplasty.5,6 The authors’ technique for percutaneous injection laryngoplasty using the transcricothyroid membrane (TCM) and transthyroid cartilage (TTC) approach is described in this article.
MATERIALS
The properties of the material chosen for injection must be considered. The ideal vocal fold implant must be biocompatible, injectable with a small-bore needle, nonvolatile, long-lasting, sized to prevent phagocytosis and migration, and should not adversely affect the viscoelastic properties of the vocal fold.7 Our material of choice in the recent past was bovine collagen (Zyplast; Inamed Aesthetics, Fremont, CA), which was an excellent choice due to its ease of injection, accurate tactile feedback, and consistent results, although the duration of effect was limited to 2 to 4 months. However, as a result of the acquisition of Inamed Aesthetics by Allergan, production of bovine collagen was discontinued. A number of alternate injectable materials are available, including (from least to greatest viscosity): hyaluronic acid-based materials (Restylane; Medicis Aesthetics, Scottsdale, AZ; and Juvederm; Allergan, Irvine, CA); autologous fat, which requires an additional surgical incision and material processing; carboxymethycellulose (Radiesse Voice Gel; Merz Aesthetics, Inc., Franksville, WI); micronized acellular dermal matrix (Cymetra; Life Cell Corporation, Branchburg, New Jersey); and calcium hydroxylapatite (Radiesse Voice, Merz Aesthetics, Inc.).8,9 None of these materials has reached the previous popularity of collagen; significant controversy continues regarding the relative advantages and disadvantages of each. The choice should be based on the patient’s vocal pathology, medical comorbidities, and the clinician’s experience.
Additional materials needed include standard video-laryngoscopy equipment (i.e., video nasolaryngoscope, light source, video processor, monitor), 27-gauge needles, and injectable 1% lidocaine with 1:100,000 epinephrine (optional). Many injectables are packaged with their own needles. We replace these needles with a 1.5-in length, 27-gauge needle, which is critical to the procedure due to its bendability, as discussed below. Although recent literature has documented an increase in heart rate and systolic blood pressure caused by transnasal endoscopy and topical lidocaine use, these changes are not likely clinically significant, and we therefore do not perform physiological monitoring for this office-based procedure.10
METHODS
The first step in percutaneous injection is ascertaining laryngeal landmarks by palpation, which we feel is performed more accurately with a gloveless hand. The most important of these are the inferior border of the thyroid cartilage, the cricoid cartilage, and the cricothyroid membrane. If desired, 0.5 to 1.0 mL of local anesthesia may be diffusely infiltrated using a 1 mL syringe and 30-gauge needle. This is done immediately over the cricothyroid membrane for the TCM approach and over the lower thyroid lamina for the TTC approach to improve patient tolerance during the injection procedure.
In the TCM approach, the cricoid and cricothyroid membrane are palpated, then the index finger of the noninjecting hand is placed over the cricothyroid membrane at the level of the inferior thyroid ala. This is done to maintain a visual position of the laryngeal landmarks (Fig. 1). The 27-gauge needle is inserted at the inferior border of the thyroid cartilage, just 5 mm lateral to the midline, in a nearly perpendicular fashion to the thyroid ala. It is then advanced until the needle tip makes contact with the cartilage (Fig. 2). Using the index finger, the needle tip is pushed inferiorly to guide the tip under the inferior border of the thyroid cartilage (Fig. 3). The tactile feedback of the needle going under the thyroid border is important, as it essentially confirms that the needle will then be guided into the paraglottic space. With the needle tip in this position, and taking care not to retract the needle, the injecting hand can then be used to create a bend in the needle to an angle of approximately 30° at the hub. This is done by pushing the syringe against the neck skin while the needle tip is pointed superiorly and laterally (Fig. 4). The needle should then be slowly advanced 5 to 10 mm in this position, aiming superiorly and laterally while imagining the posterolateral paraglottic space, and simultaneously watching on the video monitor (Fig. 5). At this time, gentle manipulation of the needle, or injecting a small amount of the augmenting material, should help identify the intralaryngeal position of the tip. The augmentation material is then injected, while watching for adequate medialization of the vocal fold on the video monitor. Holding the needle hub and distal end of the syringe with the noninjecting hand braced against the skin is helpful in keeping the injection position steady.
Fig. 1.
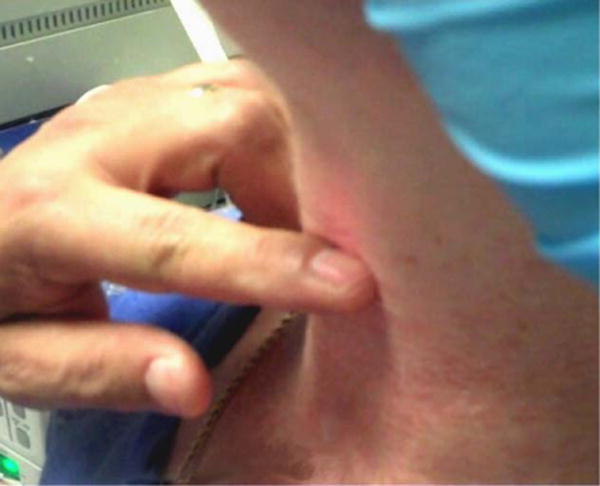
In both the transcricothyroid membrane and transthyroid cartilage approaches, the index finger of the noninjecting hand is placed at the cricothyroid membrane to mark the inferior border of the thyroid lamina. The needle is not yet inserted. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig. 2.
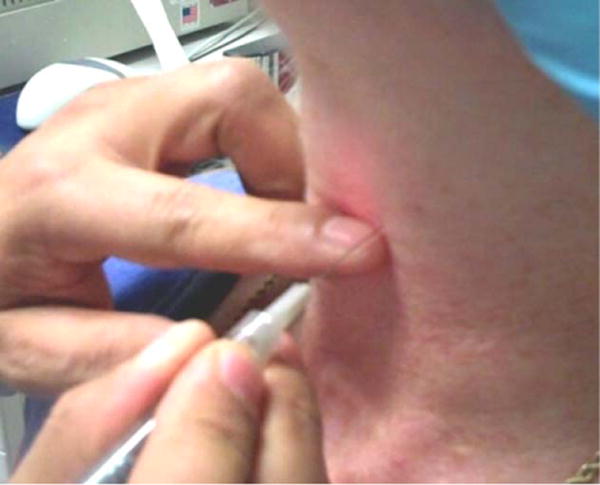
Transmembrane and transcartilage approach. The needle is inserted at the inferior border of the thyroid cartilage (2–3 mm above the inferior border for the transthyroid cartilage approach) until needle tip-to-cartilage contact is made. [Color figure can be viewed in the online issue, which is available at wileyonline library.com.]
Fig. 3.
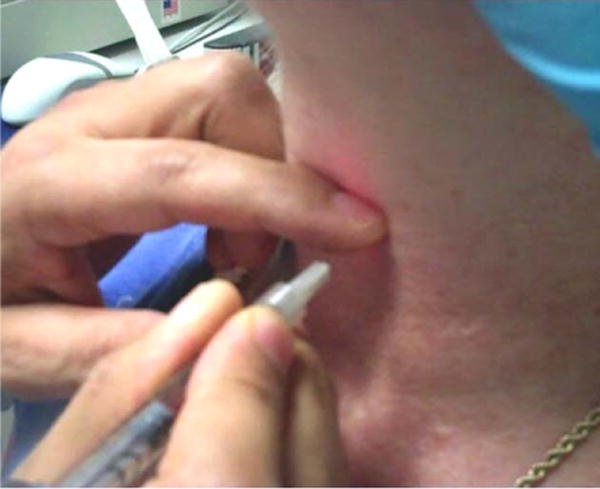
Transmembrane approach. The needle tip is pressed down and under the inferior thyroid cartilage into the paraglottic space with the index finger of the noninjecting hand. [Color figure can be viewed in the online issue, which is available at wileyonline library.com.]
Fig. 4.
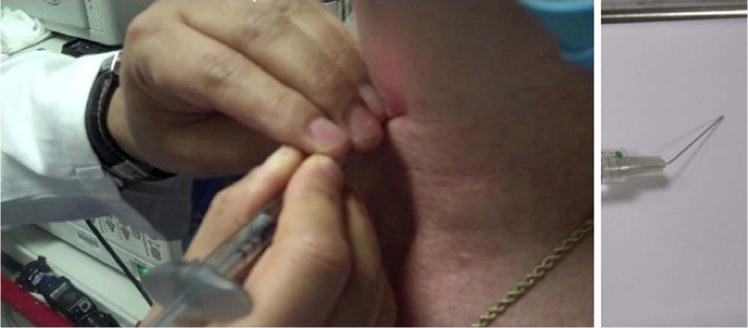
Transmembrane and transcartilage approach. After the injection needle has been inserted, it is bent laterally toward the ipsilateral paraglottic space. In the transmembrane approach, the needle tip is bent slightly superiorly as well to aim toward the paraglottic space. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig. 5.
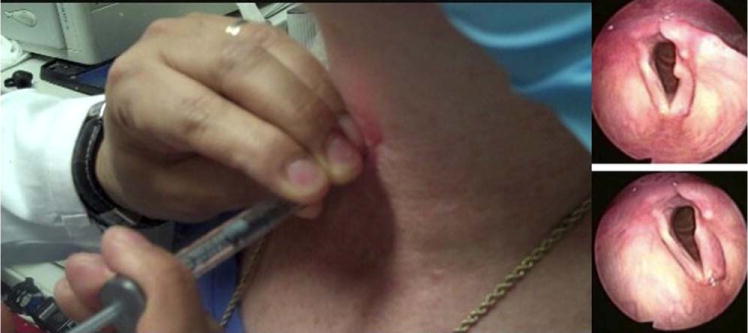
Transmembrane and transcartilage approach. Injection is performed while watching the vocal fold augmentation on the video monitor. Stabilizing the syringe and needle with the noninjecting hand will facilitate a controlled injection. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the TTC approach, the laryngeal landmarks are palpated as above, and the index finger is again placed at the level of the cricothyroid membrane. The needle is then inserted perpendicular to the thyroid cartilage, 5 mm lateral to the midline and 2 to 3 mm above the inferior border. If landmarks are easily palpable, one may also palpate the levels of the thyroid notch and inferior thyroid cartilage border to generate a mental picture of the superior vocal fold level, which generally lies halfway between the thyroid notch and the inferior border of the thyroid ala. The needle can then be inserted at a vertical level halfway between the superior vocal fold level and the inferior thyroid cartilage border. Gentle pressure is applied until the needle is felt to “pop” through the cartilage. It is then bent slightly at the hub to point laterally, toward the paraglottic space, while maintaining its position without slipping out of the thyroid cartilage. Injection is then performed as above, while watching for medialization on the video monitor as the injection is layered in the paraglottic space.
Judging the quantity that should be injected is quite subjective. Ideally, the vocal fold should be slightly overinjected, such that its position is at least at midline, with a mild convexity of the hemiglottic contour. The overinjection is important to obtain satisfactory longer-lasting results, as some of the injectate fluid will be resorbed in the days following the procedure. In fact, the immediate postinjection voice will ideally sound a bit strained from overinjection. The patient should be reassured that his or her voice will improve over the course of 2 to 3 days. If any of the injection material is seen in the submucosal plane, the procedure must be stopped immediately. This indicates that the superficial lamina propria compartment is being injected, which will result in significant dysphonia. Following injection, the patient should cough and clear his or her throat in order to “massage” the injection bolus and help contour a smooth vocal fold edge. He or she should then phonate /eee/ in order to assess for improvement in vocal quality.
The TTC technique is our approach of choice in nearly all women, and in men under the age of 40. In older men (as well as in a minority of women), the thyroid cartilage is too calcified to allow this approach. Rather than employing a larger-gauge needle, we prefer to switch to the TCM approach. It should be noted that less injectate material is generally needed for TTC injection than the TCM approach.
Cartilage plugging of the needle is an occasional and inconsistent complication with the TTC technique. When the plunger of the syringe does not advance with the expected degree of pressure (as judged by tactile feedback), we simply change the needle and try again. We do not advocate proceeding with forcible injection without needle replacement once a cartilage plug is suspected, as injectate may extrude uncontrollably; we therefore do not use luer lock syringes in this technique for the same reason. We suspect several reasons why we encounter cartilage plugging so infrequently: 1) we use a 27-gauge needle; 2) we routinely prime the needle with injectate prior to needle insertion, which may help prevent a cartilage plug from entering the hollow needle bore; and 3) we regularly switch from a TTC to a TCM approach when the thyroid cartilage feels too calcified to allow for easy needle entry.
DISCUSSION
Percutaneous laryngeal injection through the transmembrane or transcartilaginous approach critically depends on appreciation of external and internal anatomy of the laryngeal framework. Palpation of the thyroid cartilage, cricoid cartilage, and cricothyroid membrane allows for accurate needle insertion at the skin. The next critical step is to bend the needle to point the tip toward the paraglottic space. Visualization on the video monitor helps prevent inaccurate needle placement, in addition to helping judge the quantity of injectate needed to sufficiently medialize the vocal fold and improve glottic closure. Care should be taken not to inject superficially in the submucosal plane or to overinject an already compromised airway. Mastery of this technique improves patient care by limiting surgical wait time, avoiding general anesthesia and the risks of direct laryngoscopy, allowing direct visualization and titration of medialization material based on the patient’s voice result without obstruction by an endotracheal tube, and affording the patient rapid procedure and recovery times. We have found that this approach is thus the safest in patients with a narrow airway due to bilateral glottic paralysis or paresis who still desire improvement in their voice.
There are a number of potential disadvantages of injection laryngoplasty, particularly with a viscous and long-lasting substance such as calcium hydroxylapatite.11 Although a number of studies have demonstrated biocompatibility and a lack of major complications, this experience has not been uniform.7, 12–14 Others have noted development of marked inflammatory reactions, migration of injectate, and compromise of vocal fold function, with a particular effect on vibration.15 This highlights the importance of appropriate injectate choice in addition to accurate needle placement.
There is certainly a learning curve for those with limited experience in the percutaneous technique. For those practitioners who are accustomed to performing injection laryngoplasty under general anesthesia, one option to facilitate comfort with this technique is to initially perform percutaneous injections in the operating room, using microdirect laryngoscopy as a visual guide. This will still require an assistant to hold a 0° telescope in place following laryngeal suspension; however, it allows the surgeon time to become comfortable with this new approach while minimizing patient discomfort. Thus, with practice and familiarity, mastery of office-based percutaneous injection laryngoplasty is achievable for the general otolaryngologist.
CONCLUSION
Office-based injection laryngoplasty offers a number of unique advantages compared with performing the procedure on the intubated patient under general anesthesia. Choosing the appropriate injection material and mastery of this unique skill set will facilitate vocal rehabilitation in the dysphonic patient population.
Footnotes
Level of Evidence: NA
Editor–s Note: This Manuscript was accepted for publication August 28, 2013.
Presented at the Triological Society Combined Sections Meeting, Scottsdale, Arizona, U.S.A., January 27, 2011.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Bruening W. Uber eine neue behandlungsmethode der rekurrensslahmung. Ver Deutsch Laryng. 1911;18:93–151. [Google Scholar]
- 2.Ward PH, Hanson DG, Abemayor E. Transcutaneous Teflon injection of the paralyzed vocal fold: a new technique. Laryngoscope. 1985;95:644–649. doi: 10.1288/00005537-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Amin MR. Thyrohyoid approach for vocal fold augmentation. Ann Otol Rhinol Laryngol. 2006;115:699–702. doi: 10.1177/000348940611500909. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Kim JW, Koh YW, Shim SS, Son YI. Comparative analysis of efficiency of injection laryngoplasty technique for with or without neck treatment patients: a transcartilaginous approach versus the cricothyroid approach. Clin Exp Otorhinolaryngol. 2010;3:37–41. doi: 10.3342/ceo.2010.3.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luu Q, Tsai V, Mangunta V, Berke GS, Chhetri DK. Safety of percutaneous injection of bovine dermal crosslinked collagen for glottic insufficiency. Otolaryngol Head Neck Surg. 2007;136:445–449. doi: 10.1016/j.otohns.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Mallur PS, Rosen CA. Office-based laryngeal injections. Otolaryngol Clin North Am. 2013;46:85–100. doi: 10.1016/j.otc.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Chhetri DK, Jahan-Parwar B, Hart SD, Bhuta SM, Berke GS. Injection laryngoplasty with calcium hydroxylapatite gel implant in an in vivo canine model. Ann Otol Rhinol Laryngol. 2004;113:259–264. doi: 10.1177/000348940411300402. [DOI] [PubMed] [Google Scholar]
- 8.Havas TE, Priestley KJ. Autologous fat injection laryngoplasty for unilateral vocal fold paralysis. ANZ J Surg. 2003;73:938–943. doi: 10.1046/j.1445-2197.2003.02824.x. [DOI] [PubMed] [Google Scholar]
- 9.Lisi C, Hawkshaw MJ, Sataloff RT. Viscosity of materials for laryngeal injection: a review of current knowledge and clinical implications. J Voice. 2013;27:119–123. doi: 10.1016/j.jvoice.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Ongkasuwan J, Yung KC, Courey MS. The physiologic impact of transansal fexible endoscopy. Laryngoscope. 2012;122:1331–1334. doi: 10.1002/lary.23358. [DOI] [PubMed] [Google Scholar]
- 11.Dursun G, Boynukalin S, Ozgursoy OB, Coruh I. Long-term results of different treatment modalities for glottic insufficiency. Am J Otolaryngol. 2008;29:7–12. doi: 10.1016/j.amjoto.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CA, Thekdi AA. Vocal fold augmentation with injectable calcium hydroxylapatite: short-term results. J Voice. 2004;18:387–391. doi: 10.1016/j.jvoice.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Belafsky PC, Postma GN. Vocal fold augmentation with calcium hydroxylapatite. Otolaryngol Head Neck Surg. 2004;131:351–354. doi: 10.1016/j.otohns.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Rosen CA, Gartner-Schmidt J, Casiano R, et al. Vocal fold augmentation with calcium hydroxylapatite (CaHA) Otolaryngol Head Neck Surg. 2007;136:198–204. doi: 10.1016/j.otohns.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 15.DeFatta RA, Chowdhury FR, Sataloff RT. Complications of injection laryngoplasty using calcium hydroxylapatite. J Voice. 2012;26:614–618. doi: 10.1016/j.jvoice.2011.08.005. [DOI] [PubMed] [Google Scholar]


