Abstract
Medulloblastoma (MB) is the most common malignant brain tumor in children. Current treatment includes surgery, craniospinal radiation, and high-dose cytotoxic chemotherapy. Despite these aggressive therapies, one-third of patients still succumb to their disease, and survivors suffer devastating side effects, including cognitive deficits, endocrine disorders, and increased incidence of secondary cancers later in life. More effective and less toxic therapies are desperately needed for MB.
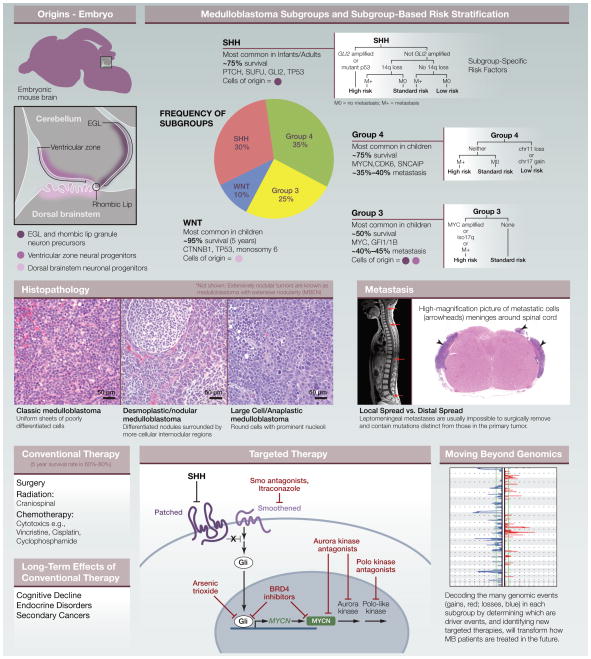
MB Subgroups
Historically, MB was classified based on histopathology, with “classic” tumors having average risk, desmoplastic/nodular tumors having a more favorable prognosis, and large-cell/anaplastic tumors having very poor outcomes. Recent studies have shown that genomics-based classification (i.e., gene expression, CpG methylation, DNA copy number, and mutations) may predict prognosis better and provide valuable information about potential drivers and therapies. Although MB exhibits considerable genomic heterogeneity, the current consensus is that there are four major subgroups of MB: Wingless (WNT), Sonic hedgehog (SHH), group 3, and group 4. Patients with WNT tumors have an extremely favorable prognosis, SHH and group 4 patients have intermediate outcomes, and group 3 patients are most likely to relapse and die of their disease.
WNT tumors, comprising ~10% of MB cases, are characterized by activating mutations in CTNNB1 and can be identified by elevated nuclear β-catenin staining and a WNT-associated gene expression signature. Monosomy 6 is frequent in WNT tumors. Other common genetic alterations include mutations in TP53, RNA helicase DDX3X, and the chromatin modifiers SMARCA4 and MLL2.
SHH tumors represent ~30% of MBs, often show nodular histopathology, and exhibit constitutive SHH pathway activation. Germline mutations in negative regulators PTCH1 (as found in Gorlin syndrome) or Suppressor of Fused (SUFU) predispose to SHH MB. Somatic mutations in PTCH1, SMO, or SUFU and somatic amplifications of transcription factors GLI2 and MYCN are common drivers for this subgroup. Chromosomal aberrations are more common in the SHH subgroup than in WNT tumors, with frequent 9q and 10q deletions. SHH tumors also harbor recurrent mutations in TP53, DDX3X, and MLL2. These tumors are most common in infants and adults and are relatively rare in children.
Group 3 tumors (~25% of MBs) often exhibit large-cell/anaplastic histopathology and have elevated expression of c-MYC (10%–20% of group 3 patients have high-level amplification of the MYC locus on chromosome 8q). The amplified region often encodes a fusion between MYC and PVT1, a noncoding gene that includes a family of microRNAs that may cooperate with MYC to promote transformation. Patients with MYC amplification have a particularly high risk of relapse and the poorest prognosis of all MB patients. TGF-β signaling components and the transcription factor OTX2 (a possible target of the TGF-β pathway) are also frequently amplified in group 3 tumors. In ~30% of group 3 tumors, the transcriptional repressors GFI1 and GFI1B are activated as a result of genomic rearrangements that juxtapose GFI1/GFI1B coding sequences with regions of active chromatin called superenhancers. Group 3 tumors have a peak incidence in childhood and are twice as common in males as in females.
Group 4 is the most common form of MB (~35% of patients), but it remains the least understood. A subset of these tumors (termed group 4α) exhibits tandem duplication and elevated expression of synuclein alpha interacting protein (SNCAIP). Group 4β tumors have lower SNCAIP expression but may exhibit amplification or overexpression of MYCN or CDK6. Other common genetic changes in group 4 tumors include isochromosome 17q (also found in some group 3 tumors), loss of one copy of chromosome X in female patients, and mutations in histone demethylase KDM6A. Group 4 tumors occur at all ages and are three times more common in males than in females.
Epigenetic Regulators
Among the most commonly altered genes across all subtypes are chromatin regulators, including the histone methyltransferases MLL2, MLL3, and EHMT1; the histone demethylases KDM6A, KDM6B, JMJD2C, and JMJD2B; and the chromatin remodeling genes SMARCA4, CHD7, and ARID1B. Analysis of DNA methylation reveals that many of the genes expressed specifically within each MB subgroup exhibit unique methylation patterns at and downstream of their promoters. For example, the mRNA processing gene LIN28B is hypomethylated and overexpressed in most group 3 and group 4 MBs. Epigenome dysregulation is a critical element of tumorigenesis, suggesting that drugs that target chromatin modifying enzymes might be effective for therapy.
Animal Models
MB animal models have been invaluable for studying tumor biology and identifying new therapies. The first and most widely used genetically engineered mouse model of MB was the Ptch-LacZ mouse, which carries a mutant allele of Ptch1; 15%–20% of these mice develop MB resembling the SHH subtype. Since then, strains lacking Sufu or expressing activated alleles of Smo or Gli2 have been developed as models of SHH-driven MB. Tumor incidence in these strains increases dramatically when crossed to mice lacking Trp53. Conditional deletion of Ptch1 or activation of Smo in granule neuron precursors also leads to highly penetrant MB, suggesting that these cells may represent cells of origin for this subtype of MB. The availability of multiple models of SHH-associated MB has facilitated preclinical testing of therapeutic agents, including small-molecule Smo antagonists, which are now in clinical trials for MB.
Recently, models have been created for other MB subgroups. Animals expressing activated β-catenin and lacking p53 develop tumors resembling WNT-associated MB. These tumors appear to arise outside the cerebellum, from progenitors in the dorsal brainstem, suggesting that different MB subtypes may have distinct origins. Group 3 MB models have been made by overexpressing Myc and inactivating p53 (or overexpressing Myc and Gfi1/Gfi1b) in cerebellar neural stem/progenitor cells. Mice overexpressing MYCN in neural progenitors also develop tumors that resemble Group 3 or 4 MB. These models have recently been used to identify chemotherapeutic agents and small molecules that are effective at inhibiting tumor growth.
Figure 1.
References
- Adamski J, Ramaswamy V, Huang A, Bouffet E. F1000Prime Rep. 2014;6:56. doi: 10.12703/P6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson RJ, Ellison DW. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- Hovestadt V, Jones DT, Picelli S, Wang W, Kool M, Northcott PA, Sultan M, Stachurski K, Ryzhova M, Warnatz HJ, et al. Nature. 2014;510:537–541. doi: 10.1038/nature13268. [DOI] [PubMed] [Google Scholar]
- Kool M, Jones DT, Jäger N, Northcott PA, Pugh TJ, Hovestadt V, Piro RM, Esparza LA, Markant SL, Remke M, et al. ICGC PedBrain Tumor Project . Cancer Cell. 2014;25:393–405. doi: 10.1016/j.ccr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant SL, Wechsler-Reya RJ. Neuropathol Appl Neurobiol. 2012;38:228–240. doi: 10.1111/j.1365-2990.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM. Nat Rev Cancer. 2012;12:818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Lee C, Zichner T, Stütz AM, Erkek S, Kawauchi D, Shih DJ, Hovestadt V, Zapatka M, Sturm D, et al. Nature. 2014;511:428–434. doi: 10.1038/nature13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, Witt H, Korshunov A, Read TA, Sun JL, et al. Cancer Cell. 2012;21:155–167. doi: 10.1016/j.ccr.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DJ, Northcott PA, Remke M, Korshunov A, Ramaswamy V, Kool M, Luu B, Yao Y, Wang X, Dubuc AM, et al. J Clin Oncol. 2014;32:886–896. doi: 10.1200/JCO.2013.50.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]