Abstract
Here we report the discovery of recurrent mutations concentrated at an ultraviolet signature hotspot in KNSTRN, which encodes a kinetochore protein, in 19% of cutaneous squamous cell carcinomas (SCCs). Cancer-associated KNSTRN mutations, most notably those encoding p.Ser24Phe, disrupt chromatid cohesion in normal cells, occur in SCC precursors, correlate with increased aneuploidy in primary tumors and enhance tumorigenesis in vivo. These findings suggest a role for KNSTRN mutagenesis in SCC development.
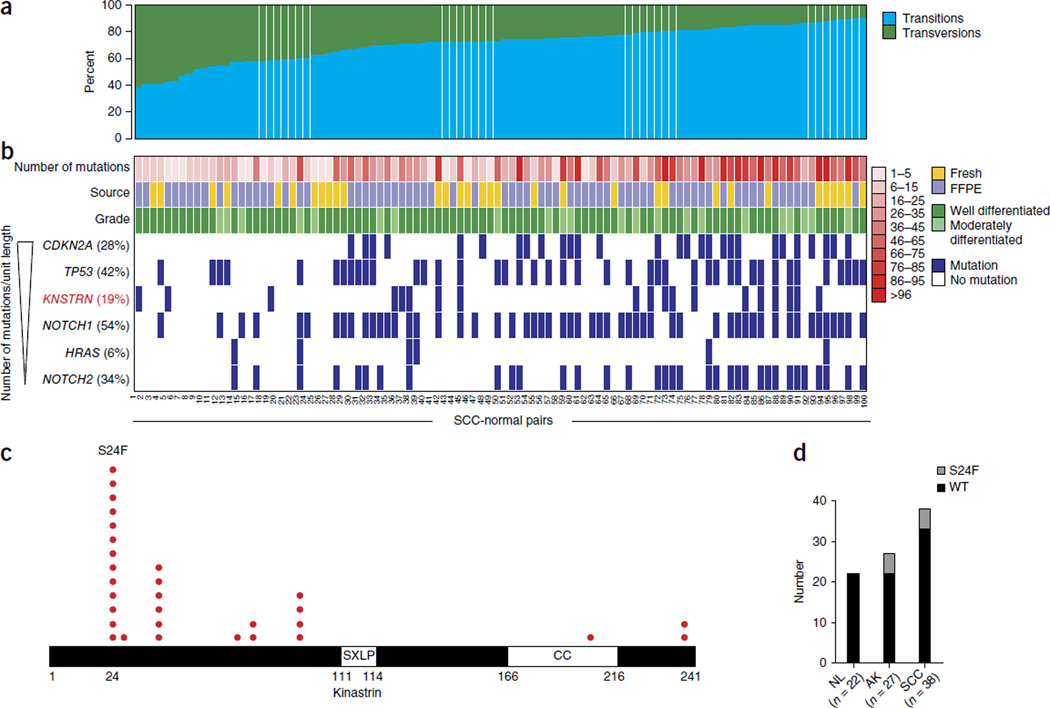
Cutaneous SCC is the second most common cancer, with an annual global incidence exceeding 1 million1. To identify recurrent genomic aberrations that underlie the development of this malignancy, we used single-nucleotide variant (SNV) determinations from the whole-exome sequencing of 12 SCC-normal pairs (Supplementary Tables 1 and 2; ref. 2) to distill a list of 336 candidate genes that were then resequenced in 100 matched SCC-normal pairs as well as in 5 SCC cell lines with an average depth exceeding 1,200× (Supplementary Tables 1–4). Analysis of mutation type showed that the majority of tumors had a mutational profile characteristic of exposure to ultraviolet (UV) light (Fig. 1a), consistent with the known association of this cancer with sunlight. The mutation frequencies in TP53, CDKN2A and HRAS—the three genes most studied in this cancer thus far—were consistent with those previously reported, confirming that the sequenced samples were genetically representative of this malignancy (Fig. 1b and Supplementary Fig. 1). The number of mutations found in archived formalin-fixed, paraffin-embedded samples was not statistically different from that detected in fresh samples (P = 0.55) (Fig. 1b). Previously reported SCC-associated inactivating mutations in TP53 and CDKN2A were identified as well as activating HRAS mutations and frequent disruption of the NOTCH1 and NOTCH2 genes (Fig. 1b and Supplementary Table 2).
Figure 1.
Recurrent mutations in KNSTRN encoding p.Ser24Phe in cutaneous SCC. (a) The percentages of somatic point mutations in 100 primary cutaneous SCCs that are transitions compared to transversions. (b) Characterization of SCC–matched normal pairs. Mutation frequency is shown in parentheses next to each gene name. FFPE, formalin fixed and paraffin embedded. (c) Distribution of the SCC-associated alterations identified in this study across the kinastrin coding sequence. SXLP, SXLP motif; CC, coiled-coil region. (d) Detection of KNSTRN mutation encoding p.Ser24Phe in SCC precursor actinic keratoses (AKs) as well as primary SCCs by allelic discrimination quantitative PCR. NL, freshly excised normal skin. Data shown represent technical triplicates. WT, wild type.
Among the recurrently mutated genes in SCC, KNSTRN ranked third behind CDKN2A and TP53 after normalizing for ORF length (Fig. 1b). KNSTRN encodes a kinetochore-associated protein that modulates anaphase onset and chromosome segregation during mitosis3. It is expressed in a broad range of human tissues, including in skin (Supplementary Fig. 2). Somatic mutations in KNSTRN were present in 2 of 12 (17%) and 19 of 100 (19%) SCCs analyzed by whole-exome and targeted sequencing, respectively. Over half of these mutations mapped to a 17-amino-acid N-terminal region, with a ‘hotspot’ serine-to-phenylalanine substitution present at codon 24 (p.Ser24Phe) (Fig. 1c and Supplementary Fig. 3) that was also detected in the cutaneous SCC cell line SCC-12B.2 (Supplementary Table 4). This pattern of clustered somatic missense mutations is characteristic of dominant mutations in oncogenes4, although KNSTRN has thus far not been implicated in any published study of human cancer.
Notably, the KNSTRN mutation encoding p.Ser24Phe involves a C>T transition that is characteristic of UV-induced mutagenesis. To determine whether KNSTRN mutagenesis might be an early event in SCC development, we screened 38 additional primary SCCs as well as 27 actinic keratoses, representing the earliest SCC precursor, for the presence of KNSTRN mutation encoding p.Ser24Phe. The mutation was detected in 5 of 27 (19%) and 5 of 38 (13%) actinic keratoses and SCCs, respectively, but was never identified in normal skin (0 of 122), indicating that it arises early in tumorigenesis (Fig. 1d). We next parsed all publicly available data sets from The Cancer Genome Atlas (TCGA). We identified KNSTRN mutations in 23 of 490 (4.7%) melanomas, another major sunlight-associated cancer, with 15 (65%) mapping to the 17-amino-acid N-terminal region and 10 (44%) specifically inducing the p.Ser24Phe substitution (Supplementary Fig. 4 and Supplementary Table 5). KNSTRN mutations were rare events in the other surveyed cancers, with none displaying mutations resulting in the p.Ser24Phe substitution (Supplementary Fig. 4a). Thus, recurrent mutation of KNSTRN and, in particular, mutation encoding p.Ser24Phe appear selective for UV-associated malignancies.
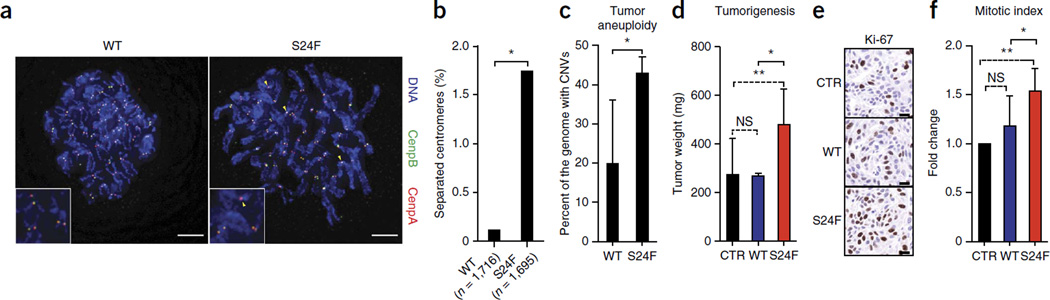
Aberrant KNSTRN expression has previously been shown to result in loss of chromatid cohesion in HeLa cells3,5; however, the effects of mutant kinastrin protein in normal primary cells have not been described. To evaluate whether Ser24Phe kinastrin is functionally relevant in this context, we expressed wild-type or Ser24Phe kinastrin in primary human keratinocytes (Fig. 2a and Supplementary Fig. 5) and assessed chromosome segregation during mitosis. Expression of mutant kinastrin disrupted sister chromatid cohesion, as demonstrated by a subset of cells containing unpaired chromatids in normal cells as well as SCC-13 cells (P = 0.0002) (Fig. 2b and Supplementary Fig. 6). Kinastrin proteins corresponding to four additional cancer-associated KNSTRN mutations (encoding p.Arg11Lys, p.Pro26Ser, p.Pro28Ser and p.Ala40Glu substitutions), including those present in melanoma, similarly disrupted chromosome segregation (Supplementary Figs. 4b and 7). These functional data support a role for cancer-associated KNSTRN mutations in controlling chromosomal stability in normal as well as cancer cells.
Figure 2.
Ser24Phe kinastrin promotes aneuploidy and enhances tumorigenesis in vivo. (a) Disrupted sister chromatid cohesion in early-passage, primary human keratinocytes pooled from multiple donors and transduced to express wild-type or Ser24Phe kinastrin. Arrowheads mark unpaired chromatids. Scale bars, 5 µm. (b) Quantification of unpaired chromatids (n = 2 biological replicates). *P = 0.0002. (c) Percentage of the genome affected by chromosomal gains and losses in SCC tissues with either wild-type or Ser24Phe mutant kinastrin. Data shown represent means ± s.d.; n = 5 independent primary tumors per group. *P = 0.007. CNVs, copy number variants. (d) Weight of mouse xenograft tumors 23 d after injection. Primary human keratinocytes were transduced to express human Cdk4 and oncogenic Ras as well as LacZ (CTR), wild-type kinastrin or Ser24Phe kinastrin and were injected subcutaneously into NOD SCID mice. Data shown represent means ± s.d.; n = 4 tumors per group. *P = 0.03, **P = 0.04; NS, not significant. (e) Ki-67 immunohistochemistry of the tumors from d. Representative fields are shown. Scale bars, 30 µm. (f) Quantification of the mitotic index for the tumors from d. Two high-power fields each containing an average of 408 cells were quantified per tumor (n = 4 tumors per group). Error bars, s.d. *P = 0.019, **P = 0.002; NS, not significant.
We next performed whole-genome copy number analysis on five primary SCCs with the KNSTRN mutation encoding p.Ser24Phe and on five histologically matched SCCs with wild-type KNSTRN to determine whether the observed perturbations in sister chromatid cohesion correlated with tumor aneuploidy. Affymetrix OncoScan arrays were used to interrogate genomic DNA and identify chromosomal gains and losses in each sample (Supplementary Table 6). Whereas both groups of tumors were aneuploid, SCCs with the KNSTRN mutation encoding p.Ser24Phe were significantly more so (P = 0.007), with a greater percentage of their genomes affected by copy number aberrations (Fig. 2c). We further observed that Ser24Phe kinastrin was capable of enhancing aneuploidy in TP53-depleted primary human keratinocytes in the presence of the aneugen paclitaxel (Supplementary Fig. 8). Neither wild-type nor mutant kinastrin significantly altered cell growth or cell cycle kinetics in two-dimensional culture (Supplementary Fig. 9). However, mutant kinastrin selectively enhanced tumorigenesis in a mouse model of human Ras-driven SCC, resulting in a threefold increase in tumor weight in comparison to control tumors driven by Ras and Cdk4, along with an associated 53.7% increase in the mitotic index (Fig. 2d–f). Ser24Phe kinastrin thus disrupts sister chromatid cohesion, is associated with increased tumor aneuploidy and augments oncogene-driven tumor growth in vivo.
Here we identify what are, to our knowledge, the first cancer-associated missense mutations in KNSTRN with dominant, protumorigenic consequences. The clustered distribution of KNSTRN mutations in skin cancer, their annotation as heterozygous events by allele frequency and the ability of mutant KNSTRN to enhance tumorigenesis all suggest that KNSTRN might be a previously unrecognized oncogene in human cancer. This possibility is consistent with results from a recently developed modeling algorithm predicting that KNSTRN is 14.3 times more likely to be an oncogene than a tumor suppressor6. Although our whole-genome copy number analysis did not detect deletions in KNSTRN, we do note that the gene appears to be lost in a subset of human cancers. Interrogation of GISTIC analyses performed on the TCGA Pan-Cancer data set7 showed that KNSTRN was not located within a focal peak region of deletion in the aggregated collection of 4,934 primary tumors representing 11 cancer types. Moreover, closer examination of regions annotated as having KNSTRN loss in the Catalogue of Somatic Mutations in Cancer (COSMIC) and TCGA databases showed that nearly all (>99%) were part of a larger locus that included genes with established tumor-suppressive roles, making it difficult to specifically attribute tumorigenic activity to KNSTRN loss in these contexts. We further note that, although several SCCs were found to contain more than one KNSTRN mutation, this observation might relate to intratumoral heterogeneity. Consistent with this idea, we also observed multiple independent PIK3CA and BRAF mutations within the same tumor in a subset of the SCCs we analyzed.
Our data support a model wherein mutant KNSTRN disrupts the chromatid cohesion required for faithful cellular replication, driving cells toward aneuploidy and culminating in tumor development. A systematic search of all sequenced genes for mutations that might co-occur or be mutually exclusive with KNSTRN mutations did not identify any statistically significant pairings to provide initial mechanistic insight, perhaps owing to the limited scope of this gene set (Supplementary Table 7). Detection of KNSTRN mutation encoding p.Ser24Phe in actinic keratoses, which are known to be aneuploid8 and frequently exhibit TP53 mutations9 that may permit escape from cell cycle arrest and/or apoptosis, is consistent with this model of tumorigenesis and demonstrates that the UV signature–associated hotspot mutation encoding p.Ser24Phe occurs early during the progression of skin cancer precursors to frank carcinoma. The presence of KNSTRN mutation encoding p.Ser24Phe at comparable frequencies in actinic keratoses and SCCs is notably reminiscent of other early events in SCC tumorigenesis, such as mutational inactivation of TP53 (ref. 10). Clinically, our findings imply that tumors with KNSTRN mutation encoding p.Ser24Phe or similar dominant mutations might be more prone to aneuploidy, and the presence of this mutation might therefore predict aggressive tumor behavior with potential implications for disease-specific survival. Further exploration of how cancer-specific mutations in KNSTRN contribute to tumor development seems to be warranted.
Online methods
Tumor tissues
Cutaneous SCCs and case-matched normal adjacent skin samples as well as actinic keratoses were collected under a protocol approved by the Institutional Review Board at Stanford University Medical Center. Individuals donating fresh surgical tissue provided informed consent. The archived specimens used fall under exemption 4. All diagnoses were verified by histological review. Samples with heavy neutrophilic infiltrate or widespread necrosis were excluded. Each SCC used for allelic discrimination contained ≥80% tumor tissue (US Biomax). Genomic DNA was isolated from all specimens using the DNeasy Blood and Tissue kit (Qiagen). A sample size of 100 was selected to approximate the number of cases needed to adequately represent this malignancy in the absence of prespecified mutation frequencies.
Cells and cell lines
Primary human keratinocytes were isolated from fresh surgical specimens and grown in a 1:1 mixture of KSF-M (Gibco) and Medium 154 for keratinocytes (Gibco), supplemented with epidermal growth factor (EGF) and bovine pituitary extract (BPE). Keratinocyte differentiation was induced in vitro by introducing 1.2 mM calcium to the medium and then growing the cells at full confluence for up to 5 d. The human SCC cell lines SCC-12B.2 and SCC-13 (a generous gift from J.G. Rheinwald, Dana-Farber/Harvard Cancer Center) were cultured in DMEM (Gibco) supplemented with 20% bovine calf serum and 0.4 µg/ml hydrocortisone and KSF-M supplemented with 25 µg/ml BPE, 0.2 ng/ml EGF and 0.3 mM CaCl2, respectively. The human SCC cell lines A431, CAL-27 and SCC-25 were obtained from the American Type Culture Collection and grown in DMEM supplemented with 10% FBS. All cells were grown at 37 °C in a humidified chamber with 5% CO2. All cell lines were negative for mycoplasma with MycoAlert (Lonza) immediately before use.
Library preparation and sequencing
Sequencing libraries were prepared with the Ovation Ultralow kit (NuGEN) using 50–100 ng of genomic DNA as input. Libraries were barcoded and pooled, and exon enrichment was then performed using a custom-designed capture measuring 1.4 Mb (SeqCap EZ Choice, NimbleGen). Enriched libraries were sequenced with the Illumina HiSeq platform with 101-bp paired-end reads.
Selection of targeted sequencing genes
To prioritize candidates for targeted exome sequencing, genes containing somatic mutations by whole-exome sequencing were first filtered for expression in primary human keratinocytes11. Genes with somatic mutations distinct from established SNP positions (dbSNP Build 137), occurring in COSMIC cancer census genes and for which variants were predicted to be damaging12 were assigned a higher priority, as were genes observed to be mutated recurrently. Genes not known to be causally implicated in cancer that were mutated in ≥2 of the 12 non-SCC exomes sequenced in parallel were removed from further analysis to minimize the likelihood of studying a non-pathogenic SNP or a sequencing artifact.
SNV analysis
Paired-end alignment was performed with the Burrows-Wheeler Aligner (BWA)13 to the hg19 reference using default parameters. SNV calling was performed with the Genome Analysis Toolkit (GATK)14, VarScan15 and SeqGene16. GATK was run following Best Practices v3 for exomes, using Indel Realignment, the Unified Genotyper, the Variant Quality Score Recalibrator and Variant Filtration as recommended17. Quality scores of 50 were required for a call, whereas a quality of 10 was accepted for emitting. Recalibration was performed to the 1000 Genomes Project and HapMap 3.3 SNPs provided in the resource bundle. Resequencing analysis was recalibrated to the Mills and 1000 Genomes Project Gold Standard package with a maximum Gaussians parameter of 4. Variants were further filtered for clusters of greater than three SNVs in a 10-bp window. VarScan was run with default parameters. SeqGene was run with a threshold of 0.1 for SNV calling, and all other parameters were default. No minimum threshold was set for the resequencing analysis.
Exome sequencing downstream analysis of GATK-called SNVs was performed on calls in a tranche of 99.0 or better. VarScan and SeqGene results were further filtered for false positives by removing any calls not supported by at least one read in each direction in the exome sequencing analysis. Unequal forward and reverse read distributions (with more than an 80%/20% split) were also removed from analysis. Low-coverage calls (<6 reads) were not held to this standard, but variant calls instead had to comprise at least 20% of the reads at that position.
Resequencing downstream analysis was performed on all acquired mutation calls on the basis of the genotype designated by the SNV caller. It was further required that the SCC samples contain ≥2-fold enrichment of reads supporting the mutation in comparison to the control samples. For cell lines, a minimum variant allele frequency of 0.1 was required. Annotations to all mutation calls were performed with SeattleSeq18.
Allelic discrimination
Probe sets and primers for wild-type KNSTRN and KNSTRN encoding p.Ser24Phe were custom designed (Custom TaqMan SNP Genotyping Assay Mix). Reactions were performed in triplicate with TaqMan Universal PCR Master Mix (Applied Biosystems).
Immunofluorescence and immunohistochemistry
Site-directed mutagenesis was performed on isoform 3 (NM_001142762) of KNSTRN, which was then cloned into the LZRS retroviral backbone for transduction into primary keratinocytes. For transduction into SCC-13 cells, KNSTRN was cloned into the pLEX lentiviral backbone with a sequence encoding a Flag-HA-poly(His) tag at the N terminus. Protein blotting was performed to confirm overexpression of kinastrin (Abcam, ab122769; 1:1,000 dilution), Cdk4 (clone h-303, Santa Cruz Biotechnology, sc-749; 1:1,000 dilution) and Ras (clone c-20, Santa Cruz Biotechnology, sc-520; 1:1,000 dilution), and equivalent loading was verified with antibody to β-actin (Sigma). Kinastrin staining (1:50 dilution; Abcam, ab122769) was performed on a skin cancer and normal tissue microarray (Biomax). Ki-67 staining (1:200 dilution; Dako, M7240) was performed on mouse xenograft tumors.
Chromosome spreads
Primary human keratinocytes transduced to express LacZ, wild-type kinastrin or Ser24Phe kinastrin were grown in medium containing 100 ng/ml nocodazole (Sigma) for 12 h. Mitotic cells were selected by brief trypsinization followed by shaking off. Cells were resuspended in 75 mM KCl and cytospun onto glass coverslips with a cytocentrifuge (Shandon Cytospin 4). The resulting spreads were washed with KCM buffer (120 mM KCl, 20 mM NaCl, 10 mM Tris-HCl (pH 8.0), 0.5 mM EDTA and 0.1% Triton X-100), permeabilized in KCM buffer with 0.5% Triton X-100, stained with antibodies to CenpA and CenpB (a gift from the Straight laboratory at Stanford University; 1:1,000 and 1:500 dilution, respectively), fixed in 3.7% formaldehyde in KCM and stained with Hoechst (10 µg/ml). The investigator was blinded to the identities of the experimental groups during centromere quantification.
Imaging
Images were collected in a z stack using an Olympus IX70 microscope and Softworx software (Applied Precision). The final images shown are maximum-intensity projections of deconvolved z stacks.
Copy number analysis
Genomic DNA purified from ten fresh primary SCCs was interrogated using Affymetrix OncoScan arrays (v3) according to the manufacturer’s instructions. Chromosomal gains and losses were identified using Nexus Express software.
Flow cytometry
Primary human keratinocytes transduced to express dominant-negative p53 (Arg248Trp) as well as LacZ, wild-type kinastrin or Ser24Phe kinastrin were grown in medium containing 5 nM Taxol (Sigma) for 68 h, fixed in ethanol for at least 2 h at room temperature and stained for 30 min at room temperature (1% Triton X-100, 20 µg/ml propidium iodide, 0.1% BSA and 10 mg/ml RNase A). At least 100,000 cells were collected per event using a Scanford or BD FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Tree Star). Doublets and debris were removed by forward scatter (FSC) versus side scatter (SSC) gating followed by propidium iodide width gating. FlowJo software was used to generate cell cycle kinetics using the Watson Pragmatic algorithm and to place G0/G1, S and G2 gates. Polyploid events were gated on mean fluorescence intensities greater than those for the G2 gate.
Cell growth assays
We plated 2 × 103 cells per well in 24-well plates and analyzed them at various time points after seeding using the CellTiter-Blue Cell Viability Assay (Promega). Reactions were performed in triplicate.
Statistical analysis
Two-tailed, unpaired t tests with Welch’s correction were performed to compare mean values between experimental groups using GraphPad Prism software. Fisher’s exact test was used to evaluate the significance of mutational co-occurrence. The resulting P values were corrected for multiple-hypothesis testing using the Benjamini-Hochberg method, and a threshold for calling significantly co-occurring events was determined on the basis of an empirical q value (0.0142) derived from the false discovery rate inherent in multiple-testing correction.
Mouse xenografts
All mouse husbandry and experimental procedures were performed in compliance with policies approved by the Stanford University Administrative Panel of Laboratory Animal Care. Transduced primary human keratinocytes (1 × 106) were resuspended in 100 µl of PBS and 100 µl of Matrigel (BD Biosciences) and injected with a 27-gauge needle into the subcutaneous space of 6-week-old female NOD SCID mice (Charles River). Mice were randomly assigned to experimental groups. NCBI guidelines were consulted when calculating sample size for continuous variables to establish significance for α = 0.05. As tumor weight is an objective measurement, the investigator was not blinded to experimental group during this assessment.
Supplementary Material
Acknowledgments
We thank J.G. Rheinwald (Dana-Farber/Harvard Cancer Center) for his generous gift of SCC cell lines. We thank A.E. Oro, H.Y. Chang, M. Diehn, M.P. Scott, S.E. Artandi, G.R. Crabtree and T. Waldman as well as A. Zehnder, X. Bao, B.K. Sun, R.J. Flockhart and V. Lopez-Pajares for presubmission review and helpful comments. This work was supported by the US Veterans Affairs Office of Research and Development and by US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) grant AR43799 to P.A.K. C.S.L. is the recipient of career award K08 AR064732 from the NIH/NIAMS. A.S. is supported by NIH/National Institute of General Medical Science (NIGMS) grant GM074728 and American Cancer Society grant 120161-RSG. W.L.J. is the recipient of National Science Foundation (NSF) graduate research fellowship grant DGE-114747.
Footnotes
Accession codes. Sequence and array data have been deposited in dbGaP under accession phs000785.v1.p1.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
C.S.L. designed and executed experiments, analyzed data and wrote the manuscript. A.M., A.B., C.J.A., C.B.N., W.L.J., E.J.R., A.U. and Z.S. helped execute experiments, analyzed data and contributed to the design of experimentation. A.S. helped design experiments and analyzed data. J.K. and S.Z.A. performed tumor tissue acquisition and analysis. P.A.K. designed experiments, analyzed data and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. Br. J. Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Durinck S, et al. Cancer Discov. 2011;1:137–143. doi: 10.1158/2159-8290.CD-11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang L, Seki A, Fang G. Cell Cycle. 2009;8:2819–2827. doi: 10.4161/cc.8.17.9514. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, et al. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunsch AK, Linnane E, Barr FA, Gruneberg U. J. Cell Biol. 2011;192:959–968. doi: 10.1083/jcb.201008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davoli T, et al. Cell. 2013;155:948–962. doi: 10.1016/j.cell.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zack TI, et al. Nat. Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biesterfeld S, Pennings K, Grussendorf-Conen EI, Böcking A. Br. J. Dermatol. 1995;133:557–560. doi: 10.1111/j.1365-2133.1995.tb02704.x. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler A, et al. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler A, et al. Proc. Natl. Acad. Sci. USA. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretz M, et al. Genes Dev. 2012;26:338–343. doi: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Jian X, Boerwinkle E. Hum. Mutat. 2013;34:E2393–E2402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Durbin R. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna A, et al. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koboldt DC, et al. Bioinformatics. 2009;25:2283–2285. doi: 10.1093/bioinformatics/btp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng X. BMC Bioinformatics. 2011;12:267. doi: 10.1186/1471-2105-12-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePristo MA, et al. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng SB, et al. Nature. 2009;461:272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.