Abstract
Cardiac allograft vasculopathy is the major cause of late graft loss in heart transplant recipients. Histological studies of characteristic end stage lesions reveal arterial changes consisting of a diffuse, confluent and concentric intimal expansion containing graft-derived cells expressing smooth muscle markers, extracellular matrix, penetrating microvessels and a host mononuclear cell infiltrate concentrated subjacent to an intact graft-derived luminal endothelial cell lining with little evidence of acute injury. This intimal expansion combined with inadequate compensatory outward remodeling produces severe generalized stenosis extending throughout the epicardial and intramyocardial arterial tree that causes ischemic graft failure. CAV lesions affect at least 50% of transplant recipients and are both progressive and refractory to treatment, resulting in about 5% graft loss per year through the first ten years post-transplant. Lesions typically stop at the suture line, implicating alloimmunity as the primary driver, but pathogenesis may be multifactorial. Here we will discuss six potential contributors to lesion formation: (1) conventional risk factors for atherosclerosis; (2) pre- or peri-transplant injuries; (3) infection; (4) innate immunity; (5) T cell-mediated immunity; and (6) B cell-mediated immunity through production of donor-specific antibody. Finally, we will consider how these various mechanisms may interact with each other.
Keywords: Transplantation, Chronic Rejection, T cells, Donor-specific Antibody
Cardiac allograft vasculopathy (CAV), a pathological process affecting the vasculature of transplanted hearts, is the major cause of late heart graft failure 1, 2. Although changes are found in the blood vascular tree throughout graft, the most clinically relevant feature is the change observed in the arterial circulation and we will focus this review on these arteriosclerotic changes. End stage lesions (analyzed in failing hearts examined at autopsy or in grafts removed during re-transplantion) show extensive, confluent luminal narrowing due to a combination of concentric intimal hyperplasia and inadequate compensation by outward remodeling; the confluent nature of the narrowing has made it difficult to recognize CAV by conventional angiography. Early intimal lesions may be eccentric and focal but these may be adequately compensated by outward remodeling, again making them difficult to recognize by angiography. Diagnosis therefore requires a measure of wall thickness, typically provided by intravascular ultrasound (the current “gold standard”) or possibly coronary computed tomography 3, 4. As the lumen becomes more progressively stenotic, graft failure results from organ hypoperfusion. It is estimated that at least 50% of cardiac allograft recipients develop clinically significant vasculopathy and CAV lesions progress at a rate that causes about 5% of grafts being lost in each of the first ten years post-transplantation 5.
The pathogenesis of CAV inferred from clinical observations has been uncertain6. Therefore, inferences re causality have instead largely been drawn both from analysis of the expanded intima from late stage human lesions and from animal models, typically involving vascular interposition allografts or heterotopic cardiac allografts 7, 8 Because there are profound differences in the manner in which rodents and humans respond to allogeneic blood vessels 9, we will focus our remarks on human tissue analyses and on the human alloresponse to vascular cells studied in vitro or in humanized mouse hosts. The majority of the cells in the expanded intima of human allografts express markers of vascular smooth muscle cells (SMCs) 10. These intimal SMCs may arise from any of five different sources: (a) human coronary arteries contain resident intimal SMCs and these cells may simply expand in number; (b) SMCs from the media may enter into the intima, undergoing cell division in either compartment to expand in number; (c) intimal SMCs may arise from progenitor cells resident at the medial/adventitial border; (d) SMCs may arise from endothelial mesenchymal transition (“endoMT) as occurs in embryonic development of the heart; and (e) circulating host cells may be recruited to the graft vessel wall where they acquire SMC characteristics. It is clear is that the vast majority of intimal SMCs in human allografts are NOT derived from the host, either from adjacent vessels or from the host circulation 11. The relative contributions of the other four sources of SMCs to human CAV lesions is unresolved. The neointima contains considerable extracellular matrix, believed to be produced primarily by the SMCs.
While graft-derived SMCs and the matrix they produce form the bulk of the expanded intima, they are not the only elements present. The hyperplastic intima of affected vessels remains covered by an intact luminal endothelial cell (EC) lining, which, in the arterial tree, is also of graft and not host origin. The expanded intima contains microvessels and an infiltrate formed largely of host T cells and macrophages 10, 12 and a majority of the T cells are memory cells that express IFN-γ and TGF-β 13. Nodular aggregrates of host B cells, T cells and myeloid cells are commonly found in the adventitia, possibly as part of rudimentary tertiary lymphoid organs associated with chronic inflammation 14. The media appears generally normal in arteries affected by CAV. Intimal leukocytes tend to be concentrated just subjacent to the EC lining and most of the SMCs are concentrated near the intimal/medial border 10. These varied cell populations show little if any evidence of necrosis 10, but occasional evidence of apoptotic cells has been reported 15. Affected arteries also show evidence of EC dysfunction, e.g. by failing to dilate in response to acetylcholine 16, 17. The barrier created by the expanded intima separating the EC lining from the SMCs of the vessel media as well as potential refractoriness of the medial SMCs to NO may also contribute to this dysfunction 18. The extracellular matrix tends to be collagen-rich and elastin-poor, which also may affect vessel function.
Having described the characteristic features of CAV in the arterial tree, we will focus in the remainder of this Brief Review on potential mechanisms that may contribute to the development of the hyperplastic intima. In a concluding section, we will discuss how these mechanisms may interact.
Conventional risk factors for atherosclerosis
There are points of similarity between arterial changes observed in CAV, sometimes called transplant or graft arteriosclerosis, and the more common metabolic/inflammatory disorder of atherosclerosis 19. For example, early lesions in both cases may involve eccentric formation of a hyperplastic intima that contains SMCs, T cells, macrophages, angiogenic microvessels and extracellular matrix covered by an intact luminal EC lining. Both processes tend to spare the vessel media and are associated with adventitial inflammation that may take the form of lymphoid nodular aggregates. Finally, both processes are associated with endothelial dysfunction in the form of inadequate vasodilation in response to acetylcholine. However, there are important differences, most notably that the intima of atheromas contain lipid-laden foam cells and a lipid rich necrotic core, usually absent in CAV, and when these are seen, they may represent pre-existing atheromas that formed in the donor prior to transplantation. The SMCs in atheromas are concentrated within a fibrous cap subjacent to the EC lining whereas T cells and macrophages are largely localized to shoulder regions of the lesion as opposed to the organization of CAV which localizes SMCs to the deeper regions of the intima adjacent to the media and concentrates the inflammatory infiltrate subjacent to the luminal endothelium. These similarities and differences are readily explained if one posits that both processes are driven by adaptive immune responses to antigen but that the antigens are different (see below for a fuller discussion of adaptive immunity in CAV). Specifically, the principal antigens driving atherosclerosis are likely to be altered (e.g. oxidized) low density lipoproteins that deposit in the extracellular matrix and/or are taken up by macrophages that become foam cells 20 whereas the principal antigen in the case of CAV are non-self MHC molecules, especially HLA-DR, expressed most abundantly on the luminal EC 21. This hypothesis explains both the distinct sites of localization of the inflammatory infiltrates and explains why atherosclerosis is a systemic disease and CAV stops at the suture lines separating donor from host.
While elevated LDL levels are the primary drivers for atherosclerosis, could they contribute to CAV as a secondary driver? Even though the extracellular matrix in CAV is not filled with lipid-rich necrotic cores or foam cells, the lipid content of the intima is often elevated 19. Medical therapy for primary prevention in atherosclerosis involves the use of statins to reduce hepatic synthesis of cholesterol, resulting in lowered LDL levels compared to normal intima, and statins have been shown to reduce the incidence of CAV, although the effect is small 22. Moreover, these benefits may relate more to the inhibitory effects that statins have on T cells than on lipid lowering 23.
Pre- and perioperative injuries to graft vessels
In renal transplantation, cadaver organs do less well long term than do organs from living donors 24, a difference attributed to vascular injury during the period of continued perfusion following brain death. Worse outcome is also associated with renal delayed graft function, a manifestation of pre-transplant injury 25. Post cardiac transplant dysfunction also correlates with poor outcome 26. The link between graft vessel injury and CAV was suggested by the idea that intimal hyperplasia in atherosclerosis may start as a response to injury 27, but as we have noted above, CAV and atherosclerosis are not the same process. An important consideration is that complement, activated either by natural antibodies or the mannose-binding lectin pathways, has been reported to play a role in ischemia reperfusion (I/R) injury of various organs 28 and complement, activated by donor-specific antibody (DSA), has been linked to CAV 29. It is unclear of these other initiators of complement activation may also be linked to CAV. Finally, as we will discuss below, I/R injury of resident vascular cells may release molecules that act as stimulators of innate immunity. While many of these molecules are unknown, injured ECs have been shown to release interleukin (IL)-1α. A potent mediator of inflammation and immunity 30.
Infection
There is considerable evidence that concurrent infection, including infections that do not directly involve the vasculature such as chronic gingivitis, respiratory infections or gastritis, is a risk factor for atherosclerosis 31, perhaps by increasing circulating factors that act on the endothelium to promote inflammation. In the case of CAV, only infection by cytomegalovirus (CMV) is well established as a factor for increased risk 32. Risk of CAV increases with viral load but even subclinical infection is sufficient to contribute. Furthermore, pre-emptive treatment of subclinical infection with gancyclovir can reduce (but not eliminate) the risk of developing CAV. Efforts to demonstrate infection of the coronary graft vasculature have generally been negative and the association of CMV with CAV has led to two alternative hypotheses. First, some CMV component or a host factor generated in response to CMV may activate graft endothelium to increase inflammation, similar to the hypothesis linking bacterial infections to atherosclerosis. Second, the presence of CMV may alter the host immune system in some unspecified manner that increases the contributions of adaptive immunity (see below) to CAV development. Neither of these ideas has been proven and it remains unknown how CMV infection contributes to the risk of developing CAV.
Innate immunity
Innate immunity refers to elements of the host defense to microbes that can act independently of lymphocyte populations that require gene rearrangements to generate antigen receptors, i.e. T cells, B cells and NK/T cells. Among the cell types that contribute to innate immunity are both leukocytes and tissue cells. The principal innate leukocytes that have been implicated in the pathogenesis of CAV, based on mouse models of transplant vasculopathy, are NK cells and macrophages 33. NK cells can recognize allogeneic cells because these cells express germ-line encoded killer inhibitory receptors that are selected to recognize self-allelic forms of class I HLA antigens, particularly (but not exclusively) HLA-C alleles. This phenomenon, known as activation to “absent self,” has been shown to play a major role in the rejection of non-self hematopoietic stem cells and their bone marrow-derived progeny 34 but has been considered less important for rejection of solid organ allografts. Upon activation, NK cells can be a source of cytokines, such as IFN-γ, that can serve as a mitogen for SMCs. While several recent studies have shown a role for NK cells in murine models of CAV, NK cells appear require the presence of T cells 35 and/or DSA 36. In other words, this may not be so much an example of innate immunity but rather an example of how an innate leukocytic effector cells can be co-opted and targeted by the adaptive immune system. Unlike NK cells, macrophages lack receptors that can distinguish self from non-self human cells, but like NK cells, macrophages express Fc receptors capable of binding antibodies and may be targeted to respond to non-self by DSA. Macrophages also express germ-line encoded pattern recognition receptors (PRRs) that can recognize microbe-derived molecules, known as pathogen-associated molecular patterns (PAMPs) not expressed by eukaryotic cells. These same PRRs may also enable macrophages to respond to sequestered eukaryotic molecules that have been released and/or structurally altered as a result of tissue injury (damage-associated molecular patterns or DAMPs) 37. Although allograft vessels are likely to be sterile and devoid of PAMPs (unless infected by CMV, see above), they may be injured and release PAMPs that can activate macrophages to produce mitogens for SMCs. Depletion of macrophages can reduce CAV in mouse models, but the adaptive immune system is intact in these models and activated T cells can serve to direct the macrophage response 38. It seems likely that NK cells and macrophages also play a role in human CAV as such cells are present in the lesions in patterns similar to what has been seen in mouse models 39, but there are no data in humans to support a role for these cells independent of adaptive immunity. In addition to their role as a source of mitogens for SMCs, NK cells and macrophages also release cytokines that cause and direct the activation of T cells. In other words, even if they do not play an independent role in CAV, innate leukocytes may contribute both as modulators and effectors of T cell responses. Like macrophages, resident vascular cells express PRRs 40. Consequently, PAMPs and DAMPs may also induce these cells to produce cytokines that modulate T cell responses. ECs, activated through PRRs, may also be a source of growth factors for SMCs, contributing to CAV. However, there is no evidence to suggest that CAV can develop in settings in which the adaptive immune system is absent.
T cell-mediated immunity
Effector T cells mediate host defense via direct cytolysis of infected cells or by elaboration of cytokines that recruit and activate effector cells of innate immunity (such as neutrophils, eosinophils, macrophages or NK cells). Both functions are triggered by recognition of non-self antigens, generally in the form of non-self peptides, such as those derived from the proteins of a microbe, bound to a self-allelic form of an HLA molecule that is displayed on the surface of another cell 41. HLA molecules are extremely polymorphic, a feature which allows different alleles to bind different peptides but also ensures that every graft, unless from an identical twin, will express HLA molecules not expressed by the host. The actual epitope recognized by the T cell receptor for antigen is comprised of amino acid residues in the peptide and in the polymorphic peptide binding region of the HLA molecule. Cytolysis is typically mediated by T cells expressing CD8 and recognition involves peptide bound to a class I HLA molecule (HLA-A, B or C) whereas cytokine-producing effector cells more typically express CD4 and respond to peptides bound to class II HLA molecules (HLA-DR. DP or DQ). Allorecognition can involve recognition of peptides derived from polymorphic proteins bound to self-allelic MHC molecules displayed on the surface of a host cell (referred to as “indirect recognition”) or can be a cross reaction in which T cells that normally recognize non-self protein-derived peptides bound to self HLA molecules instead respond to peptides (which may be self- or non-self-derived) bound to non-self HLA molecules (“direct recognition”) expressed on the surface of a graft cell. The principal cellular target of direct recognition in coronary arteries are the graft EC lining the lumen as these cells express high levels of both class I and class II HLA molecules whereas graft SMC express only minimal levels of HLA 10, 42. As there is little evidence for cytolysis in CAV lesions, the host T cells that are present subjacent to the EC likely function through release of cytokines and an analysis of T cells recovered from lesions reveal that the principal cytokines made by these cells are IFN-γ and TGF-β 13. We have modeled this process by implanting human coronary artery segments into immunodeficient mice along with adoptively transferred T cells from a donor allogeneic to the artery donor 18, 43–45. Neutralizing IFN-γ prevents the development of intimal expansion 44, 46 and addition of human IFN-γ in the absence of T cells induces intimal expansion 42, 46. In contrast, neutralizing TGF-β increases cytolysis, especially of medial SMC, with little effect on intimal size 47. Cultured human EC, but not SMC, can directly present non-self HLA molecules to human peripheral blood T cells and induce the production of IFN-γ 10, 48. Based on these observations, we have proposed that IFN-γ, produced by host T cells in the intima that are responding to the HLA antigens expressed on the graft EC, is the driver of CAV 49. This conclusion is derived from a reductionist model in which human T cells alone can produce CAV-like lesions in vivo, but our data do not exclude the possibility that in patients, NK cells and other innate lymphocytes or myeloid cells, including macrophages, may also contribute to CAV by secreting IFN-γ or other growth factors. We also cannot exclude the possibility that myeloid cells, such as host macrophages or dendritic cells, may present peptides derived from graft-derived SMC or other cell types, leading to indirect allorecognition.
The failure of conventional immunosuppressive drugs, such as calcineurin inhibitors, mTOR inhibitors, anti-proliferative agents and corticosteroids, which primarily target T cells, to prevent CAV is discouraging and suggests that T cells and IFN-γ may not be central to CAV pathogenesis. However, the use of these drugs is limited by their toxicity as well as by the danger to patients of infection and cancer from overly suppressing the immune system. In other words, the therapeutic window that effectively controls acute rejection without undue risk of infection may not be adequate to suppress T cell responses that drive CAV. It is also possible that these drugs may themselves contribute to injury of vessel wall cells, leading to release of DAMPs and increased local T cell responses.
Donor-specific antibody
Observational studies have established that patients who develop a DSA, typically reactive with a non-self HLA-DQ molecule expressed by graft EC, are at greater risk for developing CAV 50. Such antibodies typically activate complement via the classical pathway and lead to deposits of C4d on the EC lining graft microvessels observed on endomyocardial biopsy 51. It seems probable that the same antibodies are binding to and then activating complement is on the EC lining graft arteries, but it is not been possible to examine these in living patients. The binding of mouse anti-human monoclonal antibody to class I HLA molecules can activate certain effector pathways in EC that can promote inflammation independent of complement activation 52. Repeated injection of one such mouse-anti-human antibody (W6/32) into immunodeficient mice bearing human artery interposition grafts can produce CAV-like lesions independent of T cells 53, 54. It is not clear, however, how this antibody, which is much more strongly reactive with EC than SMC, causes SMC proliferation and accumulation in an expanded intima. Our own experience, using a single injection of high titer human anti-HLA antibodies present in presensitized patients on the transplant waiting list, does not produce CAV-like changes in this model 55. Based on mouse models that lack T cells, we suspect that W6/32 antibody functions by recruiting and activating NK cells 36, which, as we have noted above, are a non-T cell source source of IFN-γ. Antibodies directed against molecules other than HLA may also contribute to CAV. Some of these may detect alloantigens but others (autoantibodies) may react with molecules shared by the graft and host 56. In particular, there has been a reported correlation with the development of autoantibodies to cardiac myosin 57. However, it is difficult to understand how this works as the relevant antigen is not expressed in cells of the vessel wall. It has also been reported that “natural” IgM antibodies that react with leukocytes in graft recipients may reduce acute rejection 58, but the effect of such antibodies on CAV is unknown.
An integrated model of CAV pathogenesis
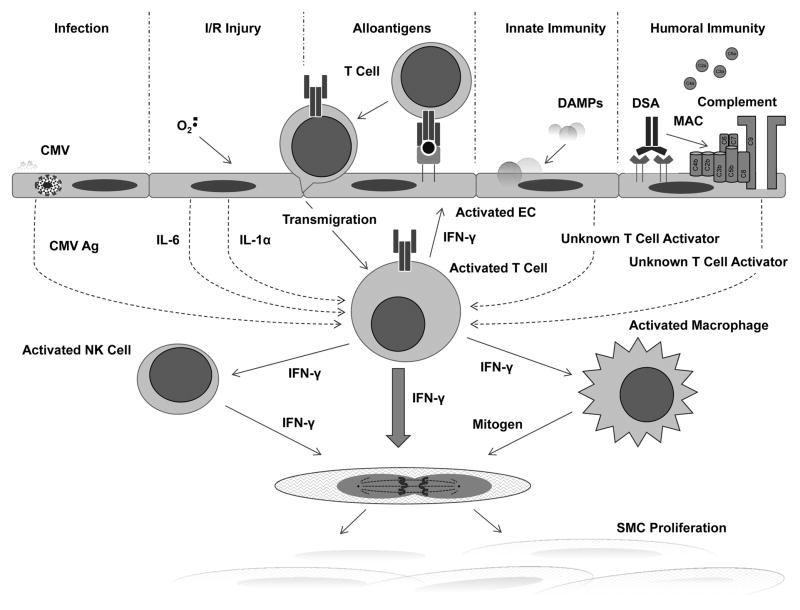
In our view, the primary mediator of CAV is IFN-γ that is produced by infiltrating host T cells. These cells are positioned subjacent to graft EC and are activated by direct recognition of non-self class I (for CD8+ T cells) and class II (for CD4+ T cells) HLA molecules expressed by graft EC. Host myeloid cells may permit cells T cells involved in indirect recognition to participate as well. Graft injury leads to the production of cytokines by injured graft cells that enhance T cell activation, proliferation and IFN-γ secretion 59. In our humanized mouse model, the principal cytokine that exerts this effect is IL-6 60, although IL-1α, released by injured graft cells as a DAMP, may contribute as well 61. The presence of CMV in the recipient will increase the number of T cells that can be activated by EC by responding to CMV antigens acquired and then presented by EC, even without infection 62. NK cells and macrophages may contribute to the response by acting as a source of IFN-γ or by producing other growth factors that positively interact with IFN-γ. Innate leukocytic effector cells may also produce cytokines, such as IL-1β or IL-6, that boost T cell responses. DSA can further contribute by activating NK cells or macrophages via Fc receptors and, as we have recently shown, by enhancing the immunogenic properties of EC in a response mediated by complement membrane attack complex and new EC gene expression downstream of activation of non-canonical NF-κB signaling 55. These ideas are summarized in Figure 1. Finally, we acknowledge that such a unifying hypothesis is likely to be an overly simplistic explanation for what is probably a complicated and multifactorial process. Nevertheless, this idea presents a way forward to develop new therapeutic approaches to what is currently an intractable disease. For example, if effector T cells responding to graft EC are the major drivers of CAV, then neutralizing IFN-γ 44, 45 or reducing effector effector T cell activation by induction of inhibitory signals on the EC, such as PD-1 ligands 62, or depriving them of activating signals by preventing complement activation 55, 63 or by adoptively transferring regulatory T cells 64 could all be developed as new therapeutic approaches.
Figure 1.
Acknowledgments
Sources of funding: Supported by NIH grant R01-HL109455 to JSP. DJ-w was supported by NIH training grant T32-AI089704 and by an ACC-Merck fellowship.
Footnotes
Disclosures: The authors have no conflicts to declare.
References
- 1.Lee MS, Finch W, Weisz G, Kirtane AJ. Cardiac allograft vasculopathy. Rev Cardiovasc Med. 2011;12:143–152. [PubMed] [Google Scholar]
- 2.Colvin-Adams M, Agnihotri A. Cardiac allograft vasculopathy: current knowledge and future direction. Clin Transplant. 2011;25:175–184. doi: 10.1111/j.1399-0012.2010.01307.x. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A, Nazif T, Mancini D, Weisz G. Detection and imaging of cardiac allograft vasculopathy. JACC Cardiovasc Imaging. 2013;6:613–623. doi: 10.1016/j.jcmg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Wever-Pinzon O, Romero J, Kelesidis I, Wever-Pinzon J, Manrique C, Budge D, Drakos SG, Pina I, Kfoury AG, Garcia MJ, Stehlik J. Coronary Computed Tomography Angiography for the Detection of Cardiac Allograft Vasculopathy: A Meta-Analysis of Prospective Trials. J Am Coll Cardiol. 2014;63:1992–2004. doi: 10.1016/j.jacc.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 5.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report--2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Braga JR, Santos IS, McDonald M, Shah PS, Ross HJ. Factors associated with the development of cardiac allograft vasculopathy--a systematic review of observational studies. Clin Transplant. 2012;26:E111–E124. doi: 10.1111/j.1399-0012.2011.01565.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss MJ, Madsen JC, Rosengard BR, Allan JS. Mechanisms of chronic rejection in cardiothoracic transplantation. Front Biosci. 2008;13:2980–2988. doi: 10.2741/2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- 9.Yacoub-Youssef H, Marcheix B, Calise D, Thiers JC, Benoist H, Blaes N, Segui B, Dambrin C, Thomsen M. Chronic vascular rejection: histologic comparison between two murine experimental models. Transplant Proc. 2005;37:2886–2887. doi: 10.1016/j.transproceed.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Salomon RN, Hughes CC, Schoen FJ, Payne DD, Pober JS, Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- 11.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 12.Seipelt IM, Pahl E, Seipelt RG, Mavroudis C, Backer CL, Stellmach V, Cornwell M, Crawford SE. Neointimal inflammation and adventitial angiogenesis correlate with severity of cardiac allograft vasculopathy in pediatric recipients. J Heart Lung Transplant. 2005;24:1039–1045. doi: 10.1016/j.healun.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Huibers M, De Jonge N, Van Kuik J, Koning ES, Van Wichen D, Dullens H, Schipper M, De Weger R. Intimal fibrosis in human cardiac allograft vasculopathy. Transpl Immunol. 2011;25:124–132. doi: 10.1016/j.trim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Wehner JR, Fox-Talbot K, Halushka MK, Ellis C, Zachary AA, Baldwin WM., 3rd B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89:1141–1148. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong C, Wilson JE, Winters GL, McManus BM. Human transplant coronary artery disease: pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft arteriopathy. Lab Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- 16.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 17.Colvin-Adams M, Harcourt N, Duprez D. Endothelial dysfunction and cardiac allograft vasculopathy. J Cardiovasc Transl Res. 2013;6:263–277. doi: 10.1007/s12265-012-9414-3. [DOI] [PubMed] [Google Scholar]
- 18.Koh KP, Wang Y, Yi T, Shiao SL, Lorber MI, Sessa WC, Tellides G, Pober JS. T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysregulation of NO synthase. J Clin Invest. 2004;114:846–856. doi: 10.1172/JCI21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 20.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaczmarek I, Deutsch MA, Rohrer ME, Beiras-Fernandez A, Groetzner J, Daebritz S, Schmoeckel M, Spannagl M, Meiser B, Reichart B. HLA-DR matching improves survival after heart transplantation: is it time to change allocation policies? J Heart Lung Transplant. 2006;25:1057–1062. doi: 10.1016/j.healun.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6:1248–1256. doi: 10.1111/j.1600-6143.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 23.Yi T, Rao DA, Tang PC, Wang Y, Cuchara LA, Bothwell AL, Colangelo CM, Tellides G, Pober JS, Lorber MI. Amelioration of human allograft arterial injury by atorvastatin or simvastatin correlates with reduction of interferon-gamma production by infiltrating T cells. Transplantation. 2008;86:719–727. doi: 10.1097/TP.0b013e318183eefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecka JM. Kidney transplantation in the United States. Clin Transpl. 2008:1–18. [PubMed] [Google Scholar]
- 25.Sharif A, Borrows R. Delayed graft function after kidney transplantation: the clinical perspective. Am J Kidney Dis. 2013;62:150–158. doi: 10.1053/j.ajkd.2012.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Kwon MH, Wong SY, Ardehali A, Laks H, Zhang ZK, Deng MC, Shemin RJ. Primary graft dysfunction does not lead to increased cardiac allograft vasculopathy in surviving patients. J Thorac Cardiovasc Surg. 2013;145:869–873. doi: 10.1016/j.jtcvs.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Mechanisms of atherosclerosis--a review. Adv Nephrol Necker Hosp. 1990;19:79–86. [PubMed] [Google Scholar]
- 28.Chan RK, Ibrahim SI, Takahashi K, Kwon E, McCormack M, Ezekowitz A, Carroll MC, Moore FD, Jr, Austen WG., Jr The differing roles of the classical and mannose-binding lectin complement pathways in the events following skeletal muscle ischemia-reperfusion. J Immunol. 2006;177:8080–8085. doi: 10.4049/jimmunol.177.11.8080. [DOI] [PubMed] [Google Scholar]
- 29.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., 3rd Antibody and complement in transplant vasculopathy. Circ Res. 2007;100:191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 30.Rao DA, Tracey KJ, Pober JS. IL-1alpha and IL–1beta are endogenous mediators linking cell injury to the adaptive alloimmune response. J Immunol. 2007;179:6536–6546. doi: 10.4049/jimmunol.179.10.6536. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld ME. Inflammation and atherosclerosis: direct versus indirect mechanisms. Curr Opin Pharmacol. 2013;13:154–160. doi: 10.1016/j.coph.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potena L, Valantine HA. Cytomegalovirus-associated allograft rejection in heart transplant patients. Curr Opin Infect Dis. 2007;20:425–431. doi: 10.1097/QCO.0b013e328259c33b. [DOI] [PubMed] [Google Scholar]
- 33.Millington TM, Madsen JC. Innate immunity and cardiac allograft rejection. Kidney Int Suppl. 2010:S18–S21. doi: 10.1038/ki.2010.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamin JE, Gill S, Negrin RS. Biology and clinical effects of natural killer cells in allogeneic transplantation. Curr Opin Oncol. 2010;22:130–137. doi: 10.1097/CCO.0b013e328335a559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehara S, Chase CM, Kitchens WH, Rose HS, Colvin RB, Russell PS, Madsen JC. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 36.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvin RB, Russell PS, Madsen JC. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7:2675–2682. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 39.Akiyoshi T, Hirohashi T, Alessandrini A, Chase CM, Farkash EA, Neal Smith R, Madsen JC, Russell PS, Colvin RB. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73:1226–1232. doi: 10.1016/j.humimm.2012.07.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell JA, Ryffel B, Quesniaux VF, Cartwright N, Paul-Clark M. Role of pattern-recognition receptors in cardiovascular health and disease. Biochem Soc Trans. 2007;35:1449–1452. doi: 10.1042/BST0351449. [DOI] [PubMed] [Google Scholar]
- 41.Lakkis FG, Lechler RI. Origin and biology of the allogeneic response. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a014993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tellides G, Tereb DA, Kirkiles-Smith NC, Kim RW, Wilson JH, Schechner JS, Lorber MI, Pober JS. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 43.Lorber MI, Wilson JH, Robert ME, Schechner JS, Kirkiles N, Qian HY, Askenase PW, Tellides G, Pober JS. Human allogeneic vascular rejection after arterial transplantation and peripheral lymphoid reconstitution in severe combined immunodeficient mice. Transplantation. 1999;67:897–903. doi: 10.1097/00007890-199903270-00018. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Burns WR, Tang PC, Yi T, Schechner JS, Zerwes HG, Sessa WC, Lorber MI, Pober JS, Tellides G. Interferon-gamma plays a nonredundant role in mediating T cell-dependent outward vascular remodeling of allogeneic human coronary arteries. Faseb J. 2004;18:606–608. doi: 10.1096/fj.03-0840fje. [DOI] [PubMed] [Google Scholar]
- 45.Yi T, Cuchara LA, Wang Y, Koh KP, Ranjbaran H, Tellides G, Pober JS, Lorber MI. Human allograft arterial injury is ameliorated by sirolimus and cyclosporine and correlates with suppression of interferon-gamma. Transplantation. 2006;81:559–566. doi: 10.1097/01.tp.0000198737.12507.19. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 47.Lebastchi AH, Khan SF, Qin L, Li W, Zhou J, Hibino N, Yi T, Rao DA, Pober JS, Tellides G. Transforming growth factor beta expression by human vascular cells inhibits interferon gamma production and arterial media injury by alloreactive memory T cells. Am J Transplant. 2011;11:2332–2341. doi: 10.1111/j.1600-6143.2011.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray AG, Libby P, Pober JS. Human vascular smooth muscle cells poorly co-stimulate and actively inhibit allogeneic CD4+ T cell proliferation in vitro. J Immunol. 1995;154:151–161. [PubMed] [Google Scholar]
- 49.Tellides G, Pober JS. Interferon-gamma axis in graft arteriosclerosis. Circ Res. 2007;100:622–632. doi: 10.1161/01.RES.0000258861.72279.29. [DOI] [PubMed] [Google Scholar]
- 50.Ticehurst EH, Molina MR, Frank R, Kearns J, Lal P, Goldberg LR, Tsai D, Wald J, Kamoun M. Antibody-mediated rejection in heart transplant patients: long-term follow up of patients with high levels of donor-directed anti-DQ antibodies. Clin Transpl. 2011:409–414. [PubMed] [Google Scholar]
- 51.Frank R, Molina MR, Wald JW, Goldberg LR, Kamoun M, Lal P. Correlation of circulating donor-specific anti-HLA antibodies and presence of C4d in endomyocardial biopsy with heart allograft outcomes: a single-center, retrospective study. J Heart Lung Transplant. 2013;32:410–417. doi: 10.1016/j.healun.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela NM, McNamara JT, Reed EF. Antibody-mediated graft injury: complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant. 2014;19:33–40. doi: 10.1097/MOT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galvani S, Trayssac M, Auge N, Thiers JC, Calise D, Krell HW, Sallusto F, Kamar N, Rostaing L, Thomsen M, Negre-Salvayre A, Salvayre R. A key role for matrix metalloproteinases and neutral sphingomyelinase-2 in transplant vasculopathy triggered by anti-HLA antibody. Circulation. 2011;124:2725–2734. doi: 10.1161/CIRCULATIONAHA.111.021790. [DOI] [PubMed] [Google Scholar]
- 54.Galvani S, Auge N, Calise D, Thiers JC, Canivet C, Kamar N, Rostaing L, Abbal M, Sallusto F, Salvayre R, Bohler T, Zou Y, Stastny P, Negre-Salvayre A, Thomsen M. HLA class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/beige mice. Am J Transplant. 2009;9:2607–2614. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 55.Jane-Wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS. Alloantibody and complement promote T cell-mediated cardiac allograft vasculopathy through noncanonical nuclear factor-kappaB signaling in endothelial cells. Circulation. 2013;128:2504–2516. doi: 10.1161/CIRCULATIONAHA.113.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nath DS, Tiriveedhi V, Basha HI, Phelan D, Moazami N, Ewald GA, Mohanakumar T. A role for antibodies to human leukocyte antigens, collagen-V, and K-alpha1-Tubulin in antibody-mediated rejection and cardiac allograft vasculopathy. Transplantation. 2011;91:1036–1043. doi: 10.1097/TP.0b013e318211d2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187:1023–1030. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lobo PI, Brayman KL, Okusa MD. Natural IgM Anti-leucocyte Autoantibodies (IgM-ALA) Regulate Inflammation Induced by Innate and Adaptive Immune Mechanisms. J Clin Immunol. 2014 doi: 10.1007/s10875-014-0027-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi T, Fogal B, Hao Z, Tobiasova Z, Wang C, Rao DA, Al-Lamki RS, Kirkiles-Smith NC, Kulkarni S, Bradley JR, Bothwell AL, Sessa WC, Tellides G, Pober JS. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human-mouse chimeric artery model. Arterioscler Thromb Vasc Biol. 2012;32:353–360. doi: 10.1161/ATVBAHA.111.239285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fogal B, Yi T, Wang C, Rao DA, Lebastchi A, Kulkarni S, Tellides G, Pober JS. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. J Immunol. 2011;187:6268–6280. doi: 10.4049/jimmunol.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C, Yi T, Qin L, Maldonado RA, von Andrian UH, Kulkarni S, Tellides G, Pober JS. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123:1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, Schiopu A, Taggart DP, Wood KJ. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]