Abstract
Reelin protein (RELN) level is reduced in the cerebral cortex and cerebellum of subjects with autism. RELN is synthesized and secreted by a subpopulation of neurons in the developing cerebral cortex termed Cajal-Retzius (CR) cells. These cells are abundant in the marginal zone during cortical development, many die after development is complete, but a small population persists into adulthood. In adult brains, RELN is secreted by the surviving CR cells, by a subset of GABAergic interneurons in layer I, and by pyramidal cells and GABAergic interneurons in deeper cortical layers. It is widely believed that decreased RELN in layer I of the cerebral cortex of subjects with autism may result from a decrease in the density of RELN expressing neurons in layer I; however, this hypothesis has not been tested. We examined RELN expression in layer I of the adult human cortex and found that 70% of cells express RELN in both control and autistic subjects. We quantified the density of neurons in layer I of the superior temporal cortex of subjects with autism and age-matched control subjects. Our data show that there is no change in the density of neurons in layer I of the cortex of subjects with autism, and therefore suggest that reduced RELN expression in the cerebral cortex of subjects with autism is not a consequence of decreased numbers of RELN-expressing neurons in layer I. Instead reduced RELN may result from abnormal RELN processing, or a decrease in the number of other RELN-expressing neuronal cell types.
Keywords: Autism, Reelin, Superior temporal cortex, layer I, Cajal-Retzius cells, Postmortem
Introduction
Autism is a disorder characterized by abnormalities in social interaction, communication, and repetitive interest and behavior. Autism is more common in males than females (four to one) and the prevalence of reported cases of autism is increasing dramatically. While the underlying etiology remains poorly understood, evidence suggests that neuropathological changes contribute to ASD. Cajal-Retzius (CR) cells are located in the marginal zone of the developing cerebral cortex and secrete the protein Reelin (RELN) to the extracellular matrix [1]. The definition of these cells has remained somewhat confusing, in part because Cajal and Retzius studied different species and different stages of brain development [2,3]. The absence of RELN impairs cellular migration and results in an altered lamination of the developing cerebral cortex [4] large population of CR cells die after the completion of cortical development [5], but some persist into adulthood [6–12]. In the adult cerebral cortex, CR cells continue to express RELN, but RELN is also expressed by GABAergic interneurons in layer I, pyramidal neurons, and interneurons in deeper cortical layers [6–9,13–16]. We previously determined that virtually all the pyramidal neurons in the adult monkey isocortex, mesocortex and archicortex contain significant amounts of intracellular Reelin in their soma [8]. In contrast, Reelin-containing pyramidal neurons are less numerous in ferret and in rodent, where only some layer V pyramidal cells and the layer II pyramidal cells of the entorhinal cortex, are immunoreactive for Reelin [7,9,17,18]. Reelin has recently been shown to be a marker of GABAergic neurons; it is expressed in subpopulations of both MGE- and CGE-derived interneurons [17,19].
Altered expression of the RELN gene and protein has been implicated in neurological conditions including lissencephaly, temporal lobe epilepsy, schizophrenia Alzheimer’ disease, and mood disorders [20]. RELN protein and mRNA deficiencies have also been reported in the cortex and cerebellum of subjects with autism [21,22]. Genome scans indicate a linkage of autism to the chromosome 7q21–q36 region, and the RELN gene may be one of the loci contributing to this positive linkage in autism [23]. It is widely believed that a decrease in the density of RELN+ cells in layer I of the cerebral cortex may underlie the decreased level of RELN in subjects with autism, but this hypothesis has not yet been thoroughly tested. To address this gap in our knowledge we quantified the density of neurons in layer I, in which we determined that 70% of neurons express RELN in control and autistic brain, in layer I of the superior temporal lobe of six subjects with autism and six age-matched controls using stereological methods. Our evidence indicates that an alteration in layer I neuronal density in the superior temporal cortex may not associated with autism.
Material and Methods
Brain specimens
Blocks of the human temporal lobe were collected from 6 autism and 6 control subjects (Autism Tissue Program and Department of Pathology, UC Davis). The cases with autism were all diagnosed as typical autism, as confirmed by ADI-R. The control cases were free of neurological disorders. Cause of death for control cases included accidents, cardiac and respiratory arrest. All samples were male and were age-matched. Cases with autism, on average were younger (average 28.5yr, range 13–56) than controls (average 31.1, range 14–57) and had higher brain weight (1,616g, range 1470–1990g.) than controls (1,293g, range 1130–1420g), although these differences were not statistically significant. Hemisphere, brain weight, severity of symptoms, and fixation time varied for each case (Supplementary tables 1 and 2). All sections were examined by a board certified neuropathologist and findings compared with the clinical history. Macro- and microscopically, tissue did not show any abnormalities.
Tissue processing
Brain tissue was immersed in 10% formalin for at least 8 weeks. A 4 cm block of temporal cortex containing the entire rostro-caudal extent of the amygdala was cut, placed into a cryoprotectant, freeze, and serially sectioned into 100 µm thick sections for Nissl, and 14 µm thick sections for immunostaining. Nissl staining was performed following a standard protocol.
Sampling region
We established rostral, caudal, dorsal and ventral boundaries for cortical areas. We established the dorsal boundary of temporal cortex at the Sylvian fissure and the ventral boundary at the depth of the superior temporal sulcus. The rostral boundary was the section in which the lateral or basal nuclei of the amygdala first appeared, and the caudal boundary was the first section in which the basal and accessory basal nuclei of the amygdala could no longer be identified. The cortical areas included for analysis were the superior temporal gyrus (Brodmann’s Area 22), the planum temporale, the planum operculare, and the anterior transverse temporal gyrus (Brodmann’s Area 41), within the established rostro-caudal boundaries [24]. Layer I and supragranular layers in the superior and medial temporal gyri have a similar structure. However, the infragranular layers of the medial temporal gyrus are thinner and the layer VI is denser than in the superior temporal gyrus. The insular area has a denser and more robust layer V than the temporal regions [25].
Immunostaining
Sections were block with 1% H2O2, citrate buffer (microwaved at 700 W, 2min), and 10% horse serum, 3% bovine serum, and 1% Triton in 0.1 M PBS. Sections were incubated (24h, RT) in anti-RELN mouse monoclonal IgG G142 (1:400, Abcam), followed by biotinylated horse anti-mouse IgG (1:200, Jackson, 90min). Sections were subsequently incubated in ABC (Vector, 2h, RT) and developed with 0.001% H2O2 and 0.04% DAB in acetate buffer. The specificity of the monoclonal antibodies used is well characterized. We included primary antibody-free controls in each experiment. Sections were mounted, air-dried, dehydrated, cleared, and cover-slipped. Imaging was performed on an Olympus BX61 microscope.
Stereology
The disector method was used on an Olympus microscope with StereoInvestigator Optical Fractionator Workflow (MicroBrightField). A section sampling interval of ten sections was employed. The neuroanatomical region was defined at low magnification (2×). The optical fractionator probe was run within the selected neuroanatomical region with a 100× oil objective. To prevent sampling bias, postprocessing tissue thickness was measured at each sampling. Cells were counted if the nucleolus came into focus within the disector counting frame. The optimal sampling scheme was carried out using the population estimator followed by a sampling of each case. The population estimate was used to determine the section sampling fractionx (0.09), area sampling fraction (0.0031), and height sampling fraction (0.66) to reach a coefficient of error of less than 10% [26,27].
Statistics
The density of neurons was compared between brains from control and autistic subjects and between hemispheres using two-sample t-tests using GraphPad software.
Results
To quantify the proportion of layer I neurons that expressed RELN, we first performed immunochemistry for RELN, co-stained the tissue with Nissl, and analyzed cells in layer I from a subset of three control and three autistic subjects (Fig. 1). We identified neurons in Nissl stained tissue by the presence of cresyl violet-positive cytoplasm, and a nucleolus and euchromatin material within the nucleus [8]. Glial cells were distinguished from neurons by the lack of cytoplasmic staining, lack of a nucleolus, and presence of heterochromatin clumps within the nucleus (Fig. 2). We determined that in control tissue 71.43± 0.35% of neurons (70.5%, 72.6% y 73.0%; n = 1,379 neurons) in layer I expressed RELN, and in autistic tissue 70.16 ± 0.51% of neurons in layer I expressed RELN (70.9%, 68.4% y 71.2%; n = 1,541 neurons). Therefore, we did not find a significant difference between the density of cells that expressed RELN in control versus tissue with autism (p = 0.18).
Figure 1.
RELN+ neurons in layer I of human cortex had a variety of morphologies, orientations and staining intensity. Some neurons had a pear-shaped body and a single, thick, descending dendrite and bulged on the cortical surface (C). Other neurons displayed one tangentially oriented dendrite (B). Horizontal line represent the pia matter. Scale bar: 10 µm.
Figure 2.
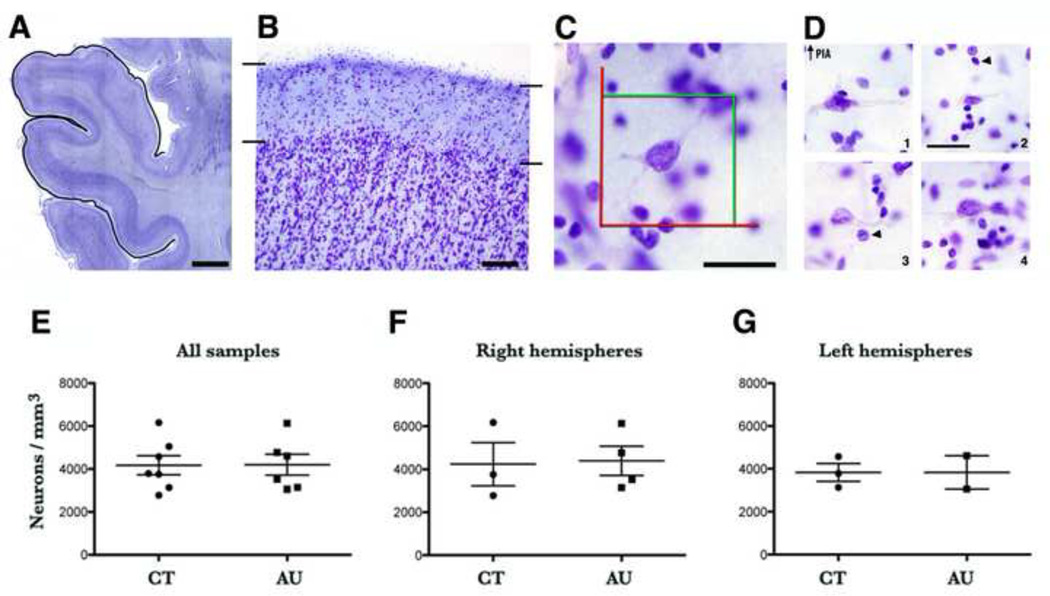
A. Coronal, Nissl-stained 100 µm thick section through the superior temporal lobe. The black line defines the region of interest. B. Layer I of the superior temporal cortex delineated by black lines (10× magnification). C. Example of a neuron in layer I of the superior temporal lobe shown at the magnification at which stereological estimates were performed (60×). This neuron is present inside the counting frame (red and green lines). D. Nissl-staining of neurons from layer I of the superior temporal cortex (60×). 1. Neurons with distinct features that are outlined with the dashed line: radially ascending process extending toward the pia and a horizontal axon plexus. 2. Most adult neurons in layer I have a small or bursiform body. 3, 4. Some neurons have a large soma with one or two thick, long and poorly branched dendrites that expand parallel to the cortical surface just beneath the pia. Arrowheads point to glial nuclei. E-G: There was no difference in the density of neurons between groups (control versus autism) when compared all samples (p = 0.27), right hemispheres (p = 0.90), or left hemispheres (p = 23). Scale: A. 5 mm, B. 200 µm, C, D. 30 µm.
Since the majority of cells in layer I expressed RELN and since the density of RELN-expressing cells in layer I was similar in control and autistic tissue, we reasoned that quantifying the total density of neurons in layer I would estimate the total density of RELN-expressing neurons in layer. Therefore, we quantified the total density of neurons in layer I of the superior temporal lobe in Nissl-stained sections of 6 subjects with autism and six control subjects through stereology. The density of neurons was quantified in 189.7 ± 29 neurons/mm3 of layer I in control cases, and 162.83 ± 24 neurons/mm3 of layer I in AU cases. In control subjects, the density of layer I cells ranged from 2,779.4 to 6173.6 neurons/mm3 with a mean density of 4,181.3 ± 479.2 cells/mm3. The corresponding density of layer I cells from subjects with autism was 3,054.3 to 6,129.6 neurons/mm3 with a mean density of 4,205.7 ± 486.9 neurons/mm3. Statistical analysis on the subjects and brain regions we quantified using the two-sample t-test did not detect a significant difference in the density of neurons in layer I of the superior temporal cortex between subjects with autism compared to the control brains (p = 0.97, Supplementary table 3).
We also analyzed the data taking into account cell density in layer I of the right versus left hemispheres. In the right hemisphere of control subjects layer I cell density ranged from 2,779.4 to 6,173.6 neurons/mm3, with a mean density of 4,236.8 ± 1,008.6 neurons/mm3. In the right hemisphere of subjects with autism the layer I cell density ranged from 3,134.6 to 6,129.6 neurons/mm3, with a mean density of 4,391.8 ± 671.1 neurons/mm3. In the left hemisphere of control subjects, the layer I cell density ranged from 3054.2 to 4,612.5 neurons/mm3, with a mean density of 3,833.42 ± 779.1 neurons/mm3. In the left hemisphere of subjects with autism the layer I cell density ranged from 3,139.3 to 4,578.6 neurons/mm3 with a mean density of 3,835.5 ± 416.1 neurons/mm3. We performed statistical analysis on the cases used in our study to determine if the groups differed. The two-sample t-test did not detect significant differences in the density of neurons in layer I of the superior temporal cortex in the right hemisphere of subjects with autism compared to the right hemisphere of control subjects (p = 0.89), or in the left hemisphere of subjects with autism compared to the left hemisphere of control subjects (p = 0.99, Supplementary table 3).
We also performed multivariance ANOVA, using condition (autism versus control) as a fixed factor, and age and hemisphere as covariances. Using Pillai's trace we obtained a p-value of 0.967 indicating there is no relation between the fixed factor and the covariances. Therefore, neither age nor hemisphere affected the results we obtained.
Overall, we found that neither the percentage of cells in layer I that express RELN, nor the density of neurons in layer I was altered in the superior temporal cortex of subjects with autism.
Discussion
Fatemi et al. first reported that dysregulation of RELN may be responsible for structural and behavioral abnormalities observed in autism. They compared the level of RELN expression in the cerebellar cortex of brains from control and autistic subjects through SDS-gel electrophoresis and Western blotting using specific anti-Reelin antibodies and found a 44% reduction of RELN in the cerebellum of five subjects with autism compared to eight control subjects [21]. They also found that reelin protein and mRNA were significantly reduced in the frontal cortex, that the mRNA for the adaptor Dab-1 was significantly reduced, and that the mRNA for the reelin receptor VLDLR was significantly elevated in the superior frontal lobe and cerebellar areas of brains from subjects with autism versus controls [22]. Fatemi and colleagues also measured the blood level of unprocessed RELN in 28 individuals with autism and 8 controls through SDS-PAGE and western blotting. Results indicated a significant reduction in a 410 kDa Reelin variant in autistic twins (−70%, p < 0.01) versus controls [28]. We investigated if this proven decrease in Reln protein is due to a decrease in the number of RELN+ neurons in layer I.
Due to the current lack of sufficient human tissue for quantitative anatomical study, our study did not sample the full rostro-caudal extent of the superior temporal lobe, but sampled the region of the superior temporal lobe defined by the rostro-caudal extent of the amygdalar formation. This anatomical boundary could be influenced by factors that affect development of the amygdala or adult size of the amygdala. With this caveat in mind, we therefore quantified neuronal density in layer I of the superior temporal lobe rather than the total number of neurons (Supplementary table 3). Cases included in this study were age and gender-matched and included both adult and adolescents, all male. The lack of perinatal cases, when the brain development in autism may diverge from that of controls, is a limitation confronted by current studies. Despite these limitations, our findings provide important information concerning specific RELN+ neurons that have been hypothesized to contribute to the cellular basis of autism.
Since we found that 70% of neurons in layer I expressed RELN both in control and tissue with autism, we reasoned that quantifying the total density of neurons in layer I would allow us to estimate the total density of RELN-expressing neurons in layer of control and autistic subjects. We tested the hypothesis that the number of neurons is decreased in autism by quantifying the density of neurons in the layer I of the superior temporal lobe of six subject with autism and six age-matched control subjects using stereological methods. We did not find a statistically significant difference in the density of neurons in the superior temporal lobe between autistic and control cases. Nevertheless, additional factors must be taken into account. We cannot rule out the possibility that even though the density of neurons is similar in the autistic and control cerebral cortex, individual neurons may produce or secrete lower amounts of RELN protein in the cerebral cortex of autistic subjects. The decrease of the amount of Reelin reported in autism could also result from abnormal RELN processing in RELN+ neurons leading to a lack or decreased synthesis and/or secretion of RELN, as has been shown for other conditions [29,30]. Nevertheless, Fatemi et al. showed that not only the 410 kDa RELN isoform is decreased in human cortical areas associated to autism, but also the 330 kDa and 180 kDa isoforms, as well as RELN mRNA levels. These data indicate that if there is an alteration in the process or synthesis of RELN, it may occur at the level of DNA transduction. It is also possible that decreased levels of RELN could result from decreased densities of other cortical neuronal cell types that have been shown to express Reelin, such as pyramidal neurons or deep layer interneurons [6–9,13–15]. Consistent with this idea, a decrease in the density or density of pyramidal cells in specific areas of the autistic cerebral cortex has been reported [31].
Conclusions
In conclusion, our data indicate that an alteration in the density of layer I RELN+ neurons in the superior temporal lobe may not be associated with autism. Similar results have been obtained in other diseases where RELN levels have been shown decreased. For example, the overall density of CR cells in layer I of the prefrontal cortex did not differ significantly between controls and schizophrenic subject [32], and there were no major changes in the density of CR cells in the Alzheimer disease entorhinal cortex when compared with those from normal elderly individuals [33].
Future studies will be necessary to investigate if there is an alteration in the number of neurons in layer I, or in the number of other RELN-producing cell types in the superior temporal lobe and other cortical regions of the cerebral cortex from individuals with autism [34].
Supplementary Material
All control cases were free of neurological disorders, seizures and mental retardation. The diagnosis of autism was confirmed by ADI-R (social score, non-verbal score, and repetition score). PMI= postmortem interval.
The autism cases, on average, were younger and had higher brain weight than the control cases. Samples from subjects with autism were taken primarily from the right hemisphere.
Data show the range and mean of neuron density. Statistically significant differences in neuron density between control and autism cases were not found. Analysis also failed to find statistically significant differences in the density of neurons in the right hemisphere and left hemisphere of autistic and control subjects.
Highlights.
In cortical layer I, 70% of neurons express RELN in both control and subjects with autism.
The density of layer I neurons in the superior temporal lobe is similar in autism and control tissue.
An alteration in the density of layer I RELN+ neurons in the superior temporal lobe may not be associated with autism.
Acknowledgements
This project was funded by NIMH R01-MH094681 and Shriners Hospitals
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, et al. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer G, Goffinet AM, Fairen A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- 3.Martinez Cerdeno V, Noctor C. Cjal, Retzius, and Cajal-Retzius cells. Frontiers in Neuroscience. 2014 doi: 10.3389/fnana.2014.00048. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 5.Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain visualized with horseradish peroxidase and electron microscopy. Neuroscience. 1990;36:839–856. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Cerdeno V, Clasca F. Reelin immunoreactivity in the adult neocortex: a comparative study in rodents, carnivores, and non-human primates. Brain Res Bull. 2002;57:485–488. doi: 10.1016/s0361-9230(01)00718-3. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Cerdeno V, Galazo MJ, Cavada C, Clasca F. Reelin immunoreactivity in the adult primate brain: intracellular localization in projecting and local circuit neurons of the cerebral cortex, hippocampus and subcortical regions. Cereb Cortex. 2002;12:1298–1311. doi: 10.1093/cercor/12.12.1298. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Cerdeno V, Galazo MJ, Clasca F. Reelin-immunoreactive neurons, axons, and neuropil in the adult ferret brain: evidence for axonal secretion of reelin in long axonal pathways. J Comp Neurol. 2003;463:92–116. doi: 10.1002/cne.10748. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Moreno T, Galazo MJ, Porrero C, Martinez-Cerdeno V, Clasca F. Extracellular matrix molecules and synaptic plasticity: immunomapping of intracellular and secreted Reelin in the adult rat brain. Eur J Neurosci. 2006;23:401–422. doi: 10.1111/j.1460-9568.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- 10.Abraham H, Toth Z, Bari F, Domoki F, Seress L. Novel calretinin and reelin expressing neuronal population includes Cajal-Retzius-type cells in the neocortex of adult pigs. Neuroscience. 2005;136:217–230. doi: 10.1016/j.neuroscience.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Belichenko PV, Vogt Weisenhorn DM, Myklossy J, Celio MR. Calretinin-positive Cajal-Retzius cells persist in the adult human neocortex. Neuroreport. 1995;6:1869–1874. doi: 10.1097/00001756-199510020-00012. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury TG, Jimenez JC, Bomar JM, Cruz-Martin A, Cantle JP, et al. Fate of Cajal-Retzius neurons in the postnatal mouse neocortex. Front Neuroanat. 2010;4:10. doi: 10.3389/neuro.05.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deguchi K, Inoue K, Avila WE, Lopez-Terrada D, Antalffy BA, et al. Reelin and disabled-1 expression in developing and mature human cortical neurons. J Neuropathol Exp Neurol. 2003;62:676–684. doi: 10.1093/jnen/62.6.676. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RC, Xu L, Roche JK, Kirkpatrick B. Ultrastructural localization of reelin in the cortex in post-mortem human brain. J Comp Neurol. 2005;482:294–308. doi: 10.1002/cne.20408. [DOI] [PubMed] [Google Scholar]
- 15.Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci U S A. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, et al. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Garcia CG, Gonzalez-Delgado FJ, Suarez-Sola ML, Castro-Fuentes R, Martin-Trujillo JM, et al. Reelin-immunoreactive neurons in the adult vertebrate pallium. J Chem Neuroanat. 2001;21:41–51. doi: 10.1016/s0891-0618(00)00104-6. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatemi SH, Kroll JL, Stary JM. Altered levels of Reelin and its isoforms in schizophrenia and mood disorders. Neuroreport. 2001;12:3209–3215. doi: 10.1097/00001756-200110290-00014. [DOI] [PubMed] [Google Scholar]
- 21.Fatemi SH, Stary JM, Halt AR, Realmuto GR. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord. 2001;31:529–535. doi: 10.1023/a:1013234708757. [DOI] [PubMed] [Google Scholar]
- 22.Fatemi SH. Reelin glycoprotein in autism and schizophrenia. Int Rev Neurobiol. 2005;71:179–187. doi: 10.1016/s0074-7742(05)71008-4. [DOI] [PubMed] [Google Scholar]
- 23.Persico AM, D'Agruma L, Maiorano N, Totaro A, Militerni R, et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol Psychiatry. 2001;6:150–159. doi: 10.1038/sj.mp.4000850. [DOI] [PubMed] [Google Scholar]
- 24.Broadmann K. Verleichende localisationslehre der gorbhirnrinde. Verlag von Johann Ambrosius Brath; 1909. [Google Scholar]
- 25.Von Economo C, Koskinas G. Cellular Structure of teh Humman Cerrebral Cortex. New English edition. 1928 [Google Scholar]
- 26.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 28.Fatemi SH, Stary JM, Egan EA. Reduced blood levels of reelin as a vulnerability factor in pathophysiology of autistic disorder. Cell Mol Neurobiol. 2002;22:139–152. doi: 10.1023/A:1019857620251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duveau V, Madhusudan A, Caleo M, Knuesel I, Fritschy JM. Impaired reelin processing and secretion by Cajal-Retzius cells contributes to granule cell dispersion in a mouse model of temporal lobe epilepsy. Hippocampus. 2011;21:935–944. doi: 10.1002/hipo.20793. [DOI] [PubMed] [Google Scholar]
- 30.Tinnes S, Schafer MK, Flubacher A, Munzner G, Frotscher M, et al. Epileptiform activity interferes with proteolytic processing of Reelin required for dentate granule cell positioning. FASEB J. 2011;25:1002–1013. doi: 10.1096/fj.10-168294. [DOI] [PubMed] [Google Scholar]
- 31.van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, et al. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- 32.Kalus P, Senitz D, Beckmann H. Cortical layer I changes in schizophrenia: a marker for impaired brain development? J Neural Transm. 1997;104:549–559. doi: 10.1007/BF01277671. [DOI] [PubMed] [Google Scholar]
- 33.Riedel A, Miettinen R, Stieler J, Mikkonen M, Alafuzoff I, et al. Reelin-immunoreactive Cajal-Retzius cells: the entorhinal cortex in normal aging and Alzheimer's disease. Acta Neuropathol. 2003;106:291–302. doi: 10.1007/s00401-003-0729-7. [DOI] [PubMed] [Google Scholar]
- 34.Zubenko GS, Moossy J, Martinez AJ, Rao GR, Kopp U, Hanin I. A brain regional analysis of morphologic and cholinergic abnormalities in Alzheimer's disease. Arch Neurol. 1989;46(6):634–638. doi: 10.1001/archneur.1989.00520420054022. 1989 Jun. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All control cases were free of neurological disorders, seizures and mental retardation. The diagnosis of autism was confirmed by ADI-R (social score, non-verbal score, and repetition score). PMI= postmortem interval.
The autism cases, on average, were younger and had higher brain weight than the control cases. Samples from subjects with autism were taken primarily from the right hemisphere.
Data show the range and mean of neuron density. Statistically significant differences in neuron density between control and autism cases were not found. Analysis also failed to find statistically significant differences in the density of neurons in the right hemisphere and left hemisphere of autistic and control subjects.