Abstract
Background
Parasites of the genus Trichinella are zoonotic nematodes common in carnivores throughout the world. We determined the prevalence and species of Trichinella infections in Florida panthers (Puma concolor coryi).
Methods
Tongues from Florida panthers were collected at necropsy and examined by pepsin-HCl artificial digestion for infection with Trichinella spp. DNA was extracted from larvae and multiplex PCR using Trichinella species-specific primers was used to genotype the worms.
Results
Trichinella spp. larvae were detected in 24 of 112 (21.4%; 14.6%–30.3%) panthers. Sixteen of the panthers (14.3%) were infected with T. pseudospiralis, 1 (0.9%) was infected with T. spiralis, and 2 (1.8%) had mixed infections of T. pseudospiralis and T. spiralis. Trichinella spp. larvae from 5 panthers were not identified at the species level due to degraded DNA.
Conclusions
This is the highest prevalence of T. pseudospiralis detected in North America up to now and suggests the Florida panther is a key mammalian reservoir of this parasite in southern Florida. Trichinella pseudospiralis can infect both mammals and birds indicating the source of infection for Florida panthers could be broader than believed; however, birds represent a small percentage (0.01%) of the cat’s diet. Since wild pigs (Sus scrofa) can be parasitized by both T. pseudospiralis and T. spiralis and these swine can comprise a large portion (~40%) of a panther’s diet in Florida, we believe that Florida panthers acquired these zoonotic parasites from feeding on wild pigs.
Keywords: Florida panther, Puma concolor coryi, Trichinella spiralis, Trichinella pseudospiralis, Zoonotic
Background
Infection with Trichinella species is common in wild carnivores throughout the world [1]. Transmission of Trichinella spp. in wild animals is largely based on predator–prey relationships and scavenger behavior. Hosts are infected with Trichinella spp. when they ingest muscle tissues containing infective larvae. There are currently 5 species or genotypes of Trichinella known in the United States. Trichinella spiralis, T. murrelli, Trichinella genotype T6, and T. nativa which modify the muscle cell to a nurse cell with a thick collagen capsule, and T. pseudospiralis which modifies the muscle cell to a nurse cell without a collagen capsule [2]. Trichinella spiralis is found primarily in domestic and wild pigs throughout the world and occasionally infects other animals [1,2]. Trichinella murrelli predominately infects wild carnivores in temperate regions of the United States, southern Canada, and possibly Mexico [2]. Trichinella nativa and Trichinella genotype T6 are freeze-resistant species whose natural hosts are carnivores in northern latitudes. Trichinella pseudospiralis has a cosmopolitan distribution, can infect meat-eating mammals and is the only Trichinella sp. that has been found to infect birds. All of these species of Trichinella are zoonotic [2].
The Florida panther (Puma concolor coryi) is an endangered species whose current range is restricted to south Florida, but transient males have been documented as far north as central Georgia [3]. Panther home ranges vary from 435–650 km2 for males and 193–396 km2 for females with both sexes utilizing wetland forests in the daytime and prairie grasslands at night [4,5]. Analysis of prey items from Florida panther kills and scat showed that wild pigs (Sus scrofa), white-tailed deer (Odocoileus virginianus), raccoons (Procyon lotor), 9-banded armadillos (Dasypus novemcinctus), and marsh rabbits (Sylvilagus palustris) comprise the majority of the cat’s diet [6,7]. Because Florida panthers are strict carnivores, their risk for being exposed to Trichinella spp. is high. Previous analysis of 7 Florida panthers demonstrated that 4 (57.1%) were infected with Trichinella sp. [8]. However, in 1985, methodologies necessary to reliably and conveniently distinguish species of Trichinella were not available and the first-stage larvae were not identified further. The objectives of the present paper were to reassess the prevalence of Trichinella spp. infection in Florida panthers and determine which species of Trichinella infect the endangered cats. We report that 24 of 112 (21.4%) Florida panthers were infected with Trichinella spp. Sixteen of the panthers were infected with T. pseudospiralis, 1 had only T. spiralis, and 2 had mixed infections of T. pseudospiralis and T. spiralis.
Methods
As part of ongoing studies to assess mortality of Florida panthers, tongues from dead panthers were collected at necropsy from July 1999–January 2011. Samples originated from an area ranging from 25°08′–30°20′ North and 80°03′–82°12′ West (Figure 1). Sex, age class (kitten [≤2 months, still in the den]; dependent juveniles [≤1.1 years, out of the den but still with dam]; adult [≥1.2 years]), and collection locations were recorded in Universal Transverse Mercator (UTM) for each panther. Tongues in varying degrees of decomposition (panther dead a few hours to a day or more) were frozen (−20°C) until they were shipped on ice packs to the Center for Veterinary Health Sciences at Oklahoma State University (OSU) in January 2011. Once the tongues arrived at OSU, they were still frozen and, subsequently, were placed at −20°C until they were processed to determine infection with Trichinella.
Figure 1.
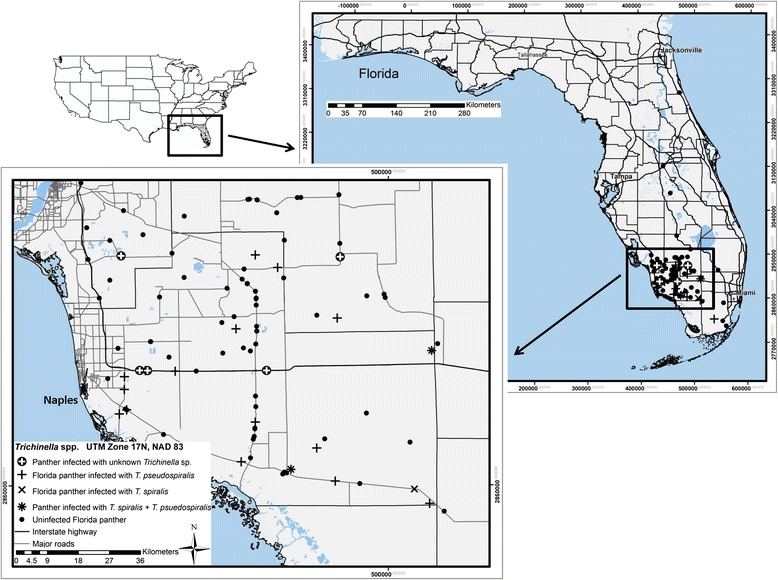
Locations where Florida panthers ( Puma concolor coryi ) were collected and where Trichinella pseudospiralis and Trichinella spiralis were found from July 1999 until January 2011.
Trichinella spp. detection
Panther tongues were tested for infection with Trichinella spp. by artificial digestion [9]. Approximately 5.0 g of tissue were weighed (to the nearest 0.1 gram) and homogenized with a Polytron (Kinematica GmbH, Kriens-Luzern, Switzerland). Homogenized samples were mixed with 10 mL of artificial digestive fluid (1% pepsin 1:10,000 IU and 1% hydrochloric acid) per 1.0 g of tissue. Digests were mixed vigorously on magnetic stir plates at 37°C for 30 minutes. After 30 minutes, digests were immediately cooled on ice and allowed to settle for 20 minutes [10]. Sediment was washed with tap water 3 to 5 times, depending on the amount of cellular debris, and samples were scanned for larvae using a stereomicroscope at 40× magnification. Results were recorded as the number of Trichinella sp. larvae per g (LPG) of tissue digested.
Molecular characterization of Trichinella spp. Larvae
Trichinella spp. larvae recovered by artificial tissue digestion from panthers were washed in saline, preserved in absolute ethyl alcohol, and submitted to the International Trichinella Reference Center (ITRC, www.iss.it/site/Trichinella/) in Rome, Italy for genotyping. Individual Trichinella spp. larvae were identified by multiplex PCR analysis following the protocol described by Zarlenga et al. [11] and modified by Pozio and La Rosa [10]. Briefly, DNA was extracted from 10 individual worms of each isolate. PCR was performed using ExTaq DNA polymerase (Takara) in 50 ml containing 1.5 mM MgCl2, 200 mM dNTPs, 50 pmol of each primer and 0.5 unit of ExTaq DNA polymerase. The PCR-amplified fragments from purified DNA were visualised by agarose gel electrophoresis (2.0% standard agarose). Single Trichinella sp. larvae from one reference strain (ITRC code) for each taxa circulating in North America, were used for comparison: T. spiralis (ISS003), T. nativa (ISS010), T. pseudospiralis (ISS470), T. murrelli (ISS035), and Trichinella genotype T6 (ISS040).
Statistics
The prevalence of Trichinella spp. infection in Florida panthers was calculated according to Bush et al. [12]. 95% confidence intervals were calculated according to Sterne’s exact method [13] using Quantitative Parasitology 3.0 [14]. Comparisons of the prevalence of Trichinella spp. between sex and age class of panthers and year collected were done using Chi-square or Fisher’s Exact tests [15]. Mann–Whitney Rank Sum tests [15] were used to compare Trichinella sp. LPG between sex and age of infected panthers and year collected. Chi-square, Fisher’s Exact and Mann–Whitney Rank Sum tests were performed using SigmaPlot 12.5 statistical software (Systat Software Inc, San Jose, California, United States).
Ethics
Tongues from Florida panthers were collected opportunistically at necropsy. The panthers were being necropsied in part to determine the cause of death following a natural mortality event. As the panthers, while alive, were not manipulated for the purposes of the current research, ethical approval was not necessary.
Results
Trichinella Larvae detection
Tongues from 112 Florida panthers collected across southern Florida (Figure 1) were tested for infection with Trichinella spp. (Table 1). The prevalence of Trichinella spp. infection (95% confidence interval) was 21.4% (14.6%–30.3%). Significantly more (X2 = 3.977, df = 1, P = 0.046) male panthers were infected with Trichinella spp. 28.1% (18.2%–40.6%) than females 12.5% (5.6%–24.8%). Infection of Trichinella spp. was not detected in 4 kittens sampled. Of 18 dependent juvenile Florida panthers tested for infection with Trichinella spp., 4 (all males) were infected (22.2%; 8.0%–47.1%). The prevalence of Trichinella spp. in adult panthers was 22.2% (14.5%–32.1%) and although greater, was not statistically discernible (X2 = 1.131, df = 2, P = 0.568) from other age classes. Prevalence of Trichinella spp. infection was not statistically impacted by the year samples were collected (Table 2).
Table 1.
Number infected and median Trichinella spp. larvae per g (LPG) in tongues of Florida panthers determined by tissue digestion according to sex and age
| Sex | Age | Number of samples | Number infected (%; 95% CI) | Median LPG (SE; min–max) |
|---|---|---|---|---|
| Male | Kitten | 0 | 0 (0.0%; 0.0–0.0) | 0.0 (0.0; 0.0–0.0) |
| Dependent | 10 | 4 (40.0%; 15.0%–70.9%) | 13.4 (80.1; 0.4–329.0) | |
| Adult | 54 | 14 (25.9%; 15.5%–39.7%) | 0.9 (0.5; 0.2– 7.2) | |
| Female | Kitten | 4 | 0 (0.0%; 0.0%–0.0%) | 0.0 (0.0; 0.0–0.0) |
| Dependent | 8 | 0 (0.0%; 0.0%–0.0%) | 0.0 (0.0; 0.0–0.0) | |
| Adult | 36 | 6 (16.7%; 7.5%–32.0%) | 0.9 (2.1; 0.2–11.6) | |
| Total | 112 | 24 (21.4%; 14.6%–30.3%) | 1.0 (13.6; 0.2–329.0) |
Table 2.
Number infected and median Trichinella spp. larvae per g (LPG) in tongues of Florida panthers determined by tissue digestion according to year collected
| Year sampled | Number of samples | Number infected (%; 95% CI) | Median LPG (SE; min–max) |
|---|---|---|---|
| 2000 | 1 | 0 (0.0%; 0.0%–0.0%) | 0.0 (0.0; 0.0–0.0) |
| 2001 | 8 | 1 (12.5%; 0.6%–50.0%) | 1.2 (NA; 1.2–1.2) |
| 2002 | 7 | 0 (0.0%; 0.0%–0.0%) | 0.0 (0.0; 0.0–0.0) |
| 2004 | 14 | 4 (28.6%; 10.4%–57.4%) | 1.2 (0.6; 0.2–2.4) |
| 2005 | 10 | 4 (40.0%; 15.0%–70.9%) | 5.1 (81.3; 0.8–329.0) |
| 2006 | 12 | 4 (33.3%; 12.3%–63.0%) | 1.8 (5.5; 0.2–23.0) |
| 2007 | 16 | 3 (18.8%; 5.3%–43.6%) | 7.2 (3.3; 0.2–11.6) |
| 2008 | 9 | 2 (22.2%; 4.1%–55.8%) | 0.8 (0.0; 0.8–0.8) |
| 2009 | 14 | 0 (0.0%; 0.0%–0.0%) | 0.0 (0.0; 0.0–0.0) |
| 2010 | 19 | 5 (26.3%; 11.0%–50.0%) | 0.4 (0.7; 0.2–3.8) |
| 2011 | 2 | 1 (50.0%; 2.5%–97.5%) | 0.5 (NA; 0.5–0.5) |
| Total | 112 | 24 | 1.0 (13.6; 0.2–329.0) |
The median LPG (SE; range) of Trichinella spp. observed in tongues according to sex and age classes of Florida panthers are presented in Table 1. Median Trichinella spp. LPG was 1.0 (13.6; 0.2–329.0). Statistically distinguishable differences in Trichinella spp. LPG were not detected among sex (U = 52.5, P = 0.947) nor age class (U = 17.0, P = 0.081) of panthers. Median Trichinella spp. LPG was not statistically noticeable among years samples were collected.
Molecular identification
Amplifiable DNA from Trichinella spp. first-stage larvae was obtained from 19 of the 24 infected Florida panthers. No PCR amplification was obtained from larvae of 5 isolates; probably due to DNA degradation from multiple freeze-thaw events. Banding patterns from multiplex PCR amplifications showed 16 Florida panthers infected with T. pseudospiralis (ITRC codes: ISS5109, ISS5111, ISS5112, ISS5113, ISS5114, ISS5115, ISS5116, ISS5117, ISS5118, ISS5119, ISS5120, ISS5196, ISS5197, ISS5201, ISS5202, and ISS5203), 2 co-infected with T. pseudospiralis and T. spiralis (ISS5198 and ISS5199), and 1 infected with T. spiralis (ISS5200). The expansion segment five of the large subunit ribosomal DNA of T. pseudospiralis larvae displayed a band pattern of 340 bp.
Discussion
Previous examination of tongues and/or diaphragms collected from 7 Florida panthers between March 1978 to December 1983 using tissue squash or pepsin-HCl digestion showed 4 (57.1%) were infected with Trichinella spp. [8].Odds ratio analysis showed that Florida panthers sampled by Forrester et al. were 4.9 times more likely to be infected with Trichinella spp. than those in the current study. Differences in the prevalence of Trichinella spp. infection detected between the two studies are likely explained by several factors. We examined 16 times more Florida panthers from a larger geographical area over a longer period of time. Interestingly, this difference may also be attributed to genetic introgression of Florida panthers in 1995 when 8 female Texas pumas (P. c. cougar) were released into the population to increase depleted genetic diversity [3].
Infection of Trichinella spp. has been documented in other wildlife from Florida. In 1962, Scholtens and Norman [16] sampled diaphragms from 224 fur-bearing animals collected in Marion County (north-central), Florida. Trichinella spp. larvae were detected by artificial digestion in 1 of 17 (5.9%) foxes (Urocyon cinereoargenteus and Vulpes [fulva] vulpes), 3 of 65 (4.6%) opossums (Didelphis [marsupialis] virginiana), 4 of 109 (3.7%) raccoons, and 1 of 22 (4.5%) skunks (Mephitis mephitis and Spilogale putorius) [16]. Eleven bobcats (Lynx rufus) were sampled; however, Trichinella sp. was not detected in any of the bobcats. Because the Scholtens and Norman survey was conducted before the advent of current taxonomy which identifies 12 taxa in this genus [2], it is uncertain whether these wild animals were actually infected with T. spiralis or a different species. Nonetheless, the routine occurrence of Trichinella spp. in Florida panthers and some of their prey animals suggests it is common for this endangered wild felid to be infected.
The presence of T. pseudospiralis and T. spiralis in Florida panthers and the absence T. murrelli was surprising. Trichinella murrelli is the predominant species that infects wild carnivores, but not swine, in temperate regions of the US [17] and suggests that the main source of Trichinella spp. infections for Florida panthers were wild pigs. This is in agreement with the diet of Florida panthers in which wild pigs can represent ~42% [6] of their prey and with the detection of anti-Trichinella IgG in 5.6% of wild pigs from Florida [18]. In Europe, T. pseudospiralis is common in wild pigs and carnivores even if its prevalence is much lower than that of T. spiralis and T. britovi (www.iss.it/Trichinella/).
Infection of T. pseudospiralis in North American animals had been documented only three times previously: in a black vulture (Coragyps atratus) from Alabama in 1985 [19], in a wild pig from Texas in 2005, and in a mountain lion (P. c. cougar) from British Columbia in 2010 [20]. Prior to these confirmed cases, T. pseudospiralis was suspected in a Cooper’s hawk (Accipiter cooperi) from California in 1982 [21,22], a great horned owl (Bubo virginianus) from Iowa in 1959 [23], and a pomarine jaeger (Stercorarius pomarinus) from Alaska in 1956 [24]. The band pattern of 340 bp for T. pseudospiralis is a hallmark identifying isolates which belong to the North American population of this parasite [25]. The common occurrence of T. pseudospiralis in Florida panthers reported in the current study suggests it is likely these wild felids are a key mammalian species in the transmission and ecology of this parasite in Florida. The current study comprises the first report of T. pseudospiralis in Florida.
Trichinella spiralis is adapted to and most commonly occurs in domestic and wild pigs [26]. Today the occurrence of T. spiralis in the US is rare [2]. It is sporadically reported in free-ranging pigs and poorly managed domestic swine in the US [18,26]. Trichinella spiralis spills over into wildlife when there is a current or historic occurrence of the parasite in pigs [18,27,28]. For example, on a poorly managed pig farm in Maryland, the overall prevalence of T. spiralis in adjacent raccoon and opossum populations was 41% (7 of 17) 6 months after pigs had been depopulated from the farm [27].The prevalence of T. spiralis dropped to 10% (1 of 10) one year after pigs were depopulated and was undetectable (0 of 15) in wild scavengers 18 months after pigs were removed from the farm [27]. A study conducted in 1993 demonstrated that 0.3% (4 of 1294) of domestic and 2.8% (5 of 179) of wild pigs in Florida had anti-Trichinella IgG in their sera [29]. However, the specificity of the serological test used at that time can be questioned [30].
Mixed infections of T. pseudospiralis and T. spiralis had been documented in a wild pig from Germany [31], a raccoon dog (Nyctereutes procyonoides) and a red fox from Germany, a red fox from Bulgaria, and a domestic pig from Bosnia-Herzegovina (www.iss.it/Trichinella/). In the current study, mixed infections of T. pseudospiralis and T. spiralis were documented in two Florida panthers. It is likely these two panthers became infected with T. pseudospiralis and T. spiralis on separate occasions but exactly how they became infected is unknown. Laboratory studies demonstrated that immunity to re-infection is influenced by the host immune response, the Trichinella species of first exposure, and the worm burden [32].
The prevalence (21.4%) of T. pseudospiralis in Florida panthers is, to our knowledge, one of the highest of this species detected in a single host species in the world reported to date. A higher prevalence of T. pseudospiralis (30%; 46 of 153) was detected in Tasmanian devils (Sarcophilus harrisii) [33] from Tasmania. However, the epidemiology of T. pseudospiralis in Florida and Tasmania should be dissimilar due to different environmental and ecological patterns including the presence of wild pigs in Florida but not Tasmania. The different transmission patterns suggest a high plasticity of T. pseudospiralis, which is one of the most widespread parasitic nematodes circulating in wild animals.
Conclusions
Trichinella pseudospiralis seems to be a common zoonotic parasite of the entozoic habitat of the Florida panthers, whereas T. spiralis seems to be less prevalent. Wild pigs are likely the main source of T. pseudospiralis and T. spiralis to Florida panthers as these swine can be host to both parasites and are a main food source for the cats. The prevalence (21.4%) of T. pseudospiralis in Florida panthers is one of the highest of this parasite detected in the world. The high prevalence of T. pseudospiralis in Florida panthers, in combination with reports of this parasite in a variety of other mammals and birds from distinct geographic locations, suggests T. pseudospiralis is one of the most widespread nematodes in wild animals.
Acknowledgements
The study was funded in part by Oklahoma State University, the Florida Fish and Wildlife Conservation Commission with funds collected through “Protect the Panther” vehicle license plate sales, and the World Organization for Animal Health Reference Laboratory for Trichinellosis (Rome, Italy).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MR, MCr, JT, MCu, KL, and EP conceived and designed the study. MCf, MCu and DO organized sample collection and transport. MR, JT, and JP performed digests and performed statistical analyses. MI, GM, and EP performed the genotyping. MR, MCr, JT, and EP drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Mason V Reichard, Email: mason.reichard@okstate.edu.
Marc Criffield, Email: marc.criffield@myfwc.com.
Jennifer E Thomas, Email: jennifer.e.thomas@okstate.edu.
Jacqueline M Paritte, Email: paritte@okstate.edu.
Mark Cunningham, Email: mark.cunningham@myfwc.com.
Dave Onorato, Email: dave.onorato@myfwc.com.
Kenneth Logan, Email: ken.logan@state.co.us.
Maria Interisano, Email: maria.interisano@iss.it.
Gianluca Marucci, Email: gianluca.marucci@iss.it.
Edoardo Pozio, Email: edoardo.pozio@iss.it.
References
- 1.Pozio E, Murrell KD. Systematics and epidemiology of Trichinella. Adv Parasitol. 2006;63:367–439. doi: 10.1016/S0065-308X(06)63005-4. [DOI] [PubMed] [Google Scholar]
- 2.Pozio E, Zarlenga DS. New pieces of the Trichinella puzzle. Int J Parasitol. 2013;43(12–13):983–97. doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Johnson WE, Onorato DP, Roelke ME, Land ED, Cunningham M, Belden RC, et al. Genetic restoration of the Florida panther. Science. 2010;329(5999):1641–5. doi: 10.1126/science.1192891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onorato DP, Criffield M, Lotz M, Cunningham M, McBride R, Leone EH, et al. Habitat selection by critically endangered Florida panthers across the diel period: implications for land management and conservation. Anim Conserv. 2011;14(2):196–205. doi: 10.1111/j.1469-1795.2010.00415.x. [DOI] [Google Scholar]
- 5.Onorato D, Belden C, Cunningham M, Land D, McBride R, Roelke M. Long-term research on the Florida panther (Puma concolor coryi): historical findings and future obstacles to population persistence. In: Macdonald D, Loveridge A, editors. Biology and conservation of wild felids. Oxford: Oxford University Press; 2010. pp. 453–69. [Google Scholar]
- 6.Maehr DS, Belden RC, Land ED, Wilkins L. Food habits of panthers in southwest Florida. J Wildl Manage. 1990;54(3):420–3. doi: 10.2307/3809651. [DOI] [Google Scholar]
- 7.Dalrymple GH, Bass OL. The diet of the Florida panther in Everglades National Park, Florida. Bull Fla Mus Nat Hist. 1996;39(5):173–93. [Google Scholar]
- 8.Forrester DJ, Conti JA, Belden RC. Parasites of the Florida panther (Felis concolor coryi) Proc Helminthol Soc Wash. 1985;52(1):95–7. [Google Scholar]
- 9.Webster P, Maddox-Hyttel C, Nockler K, Malakauskas A, van der Giessen J, Pozio E, et al. Meat inspection for Trichinella in pork, horsemeat and game within the EU: available technology and its present implementation. Euro Surveill. 2006;11(1):50–5. [PubMed] [Google Scholar]
- 10.Pozio E, La Rosa G. PCR-derived methods for the identification of Trichinella parasites from animal and human samples. Methods Mol Biol. 2003;216:299–309. doi: 10.1385/1-59259-344-5:299. [DOI] [PubMed] [Google Scholar]
- 11.Zarlenga DS, Chute MB, Martin A, Kapel CMO. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int J Parasitol. 1999;29(11):1859–67. doi: 10.1016/S0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 12.Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83(4):575–83. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- 13.Reiczigel J. Confidence intervals for the binomial parameter: some new considerations. Stat Med. 2003;22(4):611–21. doi: 10.1002/sim.1320. [DOI] [PubMed] [Google Scholar]
- 14.Rozsa L, Reiczigel J, Majoros G. Quantifying parasites in samples of hosts. J Parasitol. 2000;86(2):228–32. doi: 10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Sokal RR, Rohlf FJ. Biometry. 3. New York: W. H. Freeman and Company; 1997. [Google Scholar]
- 16.Scholtens R, Norman L. Trichinella spiralis in Florida wildlife. J Parasitol. 1971;57(5):1103. doi: 10.2307/3277871. [DOI] [PubMed] [Google Scholar]
- 17.Pozio E, La Rosa G. Trichinella murrelli n. sp: etiological agent of sylvatic trichinellosis in temperate areas of North America. J Parasitol. 2000;86(1):134–9. doi: 10.1645/0022-3395(2000)086[0134:TMNSEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Hill DE, Dubey JP, Baroch JA, Swafford SR, Fournet VF, Hawkins-Cooper D, et al. Surveillance of feral swine for Trichinella spp. and Toxoplasmagondii in the USA and host-related factors associated with infection. Vet Parasitol. 2014;205(3–4):653–65. doi: 10.1016/j.vetpar.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay DS, Zarlenga DS, Gamble HR, Al-Yaman F, Smith PC, Blagburn BL. Isolation and characterization of Trichinella pseudospiralis Garkavi, 1972 from a black vulture (Coragyps atratus) J Parasitol. 1995;81(6):920–3. doi: 10.2307/3284041. [DOI] [PubMed] [Google Scholar]
- 20.Gajadhar AA, Forbes LB. A 10-year wildlife survey of 15 species of Canadian carnivores identifies new hosts or geographic locations for Trichinella genotypes T2, T4, T5, and T6. Vet Parasitol. 2010;168(1–2):78–83. doi: 10.1016/j.vetpar.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Wheeldon EB, Dick TA, Schulz TA. 1st report of Trichinella spiralis var. pseudospiralis in North America. J Parasitol. 1983;69(4):781–2. doi: 10.2307/3281161. [DOI] [PubMed] [Google Scholar]
- 22.Wheeldon EB, Kock MD, Tucker RL, Anderson M. Trichinellosis in a Cooper’s hawk. J Am Vet Med Assoc. 1982;181(11):1385–6. [PubMed] [Google Scholar]
- 23.Zimmermann WJ, Hubbard ED, Biester HE. Studies on trichiniasis in Iowa wildlife (1955–56 AND 1956–57 SEASONS) J Parasitol. 1959;45(1):87–90. doi: 10.2307/3274791. [DOI] [PubMed] [Google Scholar]
- 24.Rausch R, Babero BB, Rausch RV, Schiller EL. Studies on the helminth fauna of Alaska. XXVI. The occurrence of larvae of Trichinella spiralis in Alaskan mammals. J Parasitol. 1956;42(3):259–71. doi: 10.2307/3274850. [DOI] [PubMed] [Google Scholar]
- 25.La Rosa G, Marucci G, Zarlenga DS, Pozio E. Trichinella pseudospiralis populations of the Palearctic region and their relationship with populations of the Nearctic and Australian regions. Int J Parasitol. 2001;31(3):297–305. doi: 10.1016/S0020-7519(01)00110-2. [DOI] [PubMed] [Google Scholar]
- 26.Pozio E. Searching for Trichinella: not all pigs are created equal. Trends Parasitol. 2014;30(1):4–11. doi: 10.1016/j.pt.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Hill DE, Pierce V, Murrell KD, Ratliffe N, Rupp B, Fournet VM, et al. Cessation of Trichinella spiralis transmission among scavenging mammals after the removal of infected pigs from a poorly managed farm: Implications for trichinae transmission in the US. Zoonoses Public Health. 2010;57(7–8):E116–23. doi: 10.1111/j.1863-2378.2009.01296.x. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy ED, Hall RL, Montgomery SP, Pyburn DG, Jones JL, Centers for Disease Control and Prevention Trichinellosis surveillance - United States, 2002–2007. MMWR Surveill Summ. 2009;58(9):1–7. [PubMed] [Google Scholar]
- 29.Vanderleek ML, Dame JB, Littell RC, Shin SS. Seroepidemiology of trichinosis in Florida swine. Prev Vet Med. 1993;16(4):279–93. doi: 10.1016/0167-5877(93)90044-T. [DOI] [Google Scholar]
- 30.Gomez-Morales MA, Ludovisi A, Amati M, Blaga R, Zivojinovic M, Ribicich M, et al. A distinctive Western blot pattern to recognize Trichinella infections in humans and pigs. Int J Parasitol. 2012;42(11):1017–23. doi: 10.1016/j.ijpara.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Nockler K, Reckinger S, Pozio E. Trichinella spiralis and Trichinella pseudospiralis mixed infection in a wild boar (Sus scrofa) of Germany. Vet Parasitol. 2006;137(3–4):364–8. doi: 10.1016/j.vetpar.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Ooi HK, Kamiya M, Ohbayashi M. Cross resistance against challenge infection in mice infected with Trichinella spiralis or Trichinella pseudospiralis. Jpn J Vet Res. 1987;35(2):87–97. [PubMed] [Google Scholar]
- 33.Obendorf DL, Handlinger JH, Mason RW, Clarke KP, Forman AJ, Hooper PT, et al. Trichinella pseudospiralis infection in Tasmanian wildlife. Aust Vet J. 1990;67(3):108–10. doi: 10.1111/j.1751-0813.1990.tb16084.x. [DOI] [PubMed] [Google Scholar]


