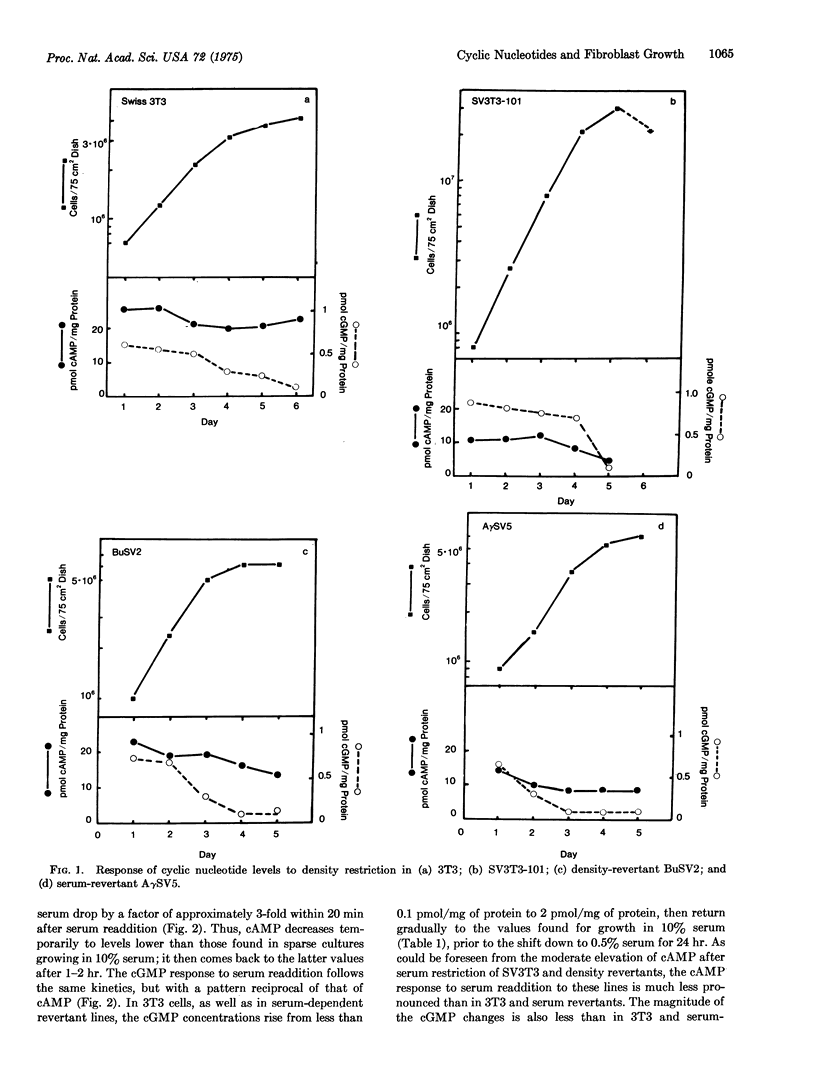
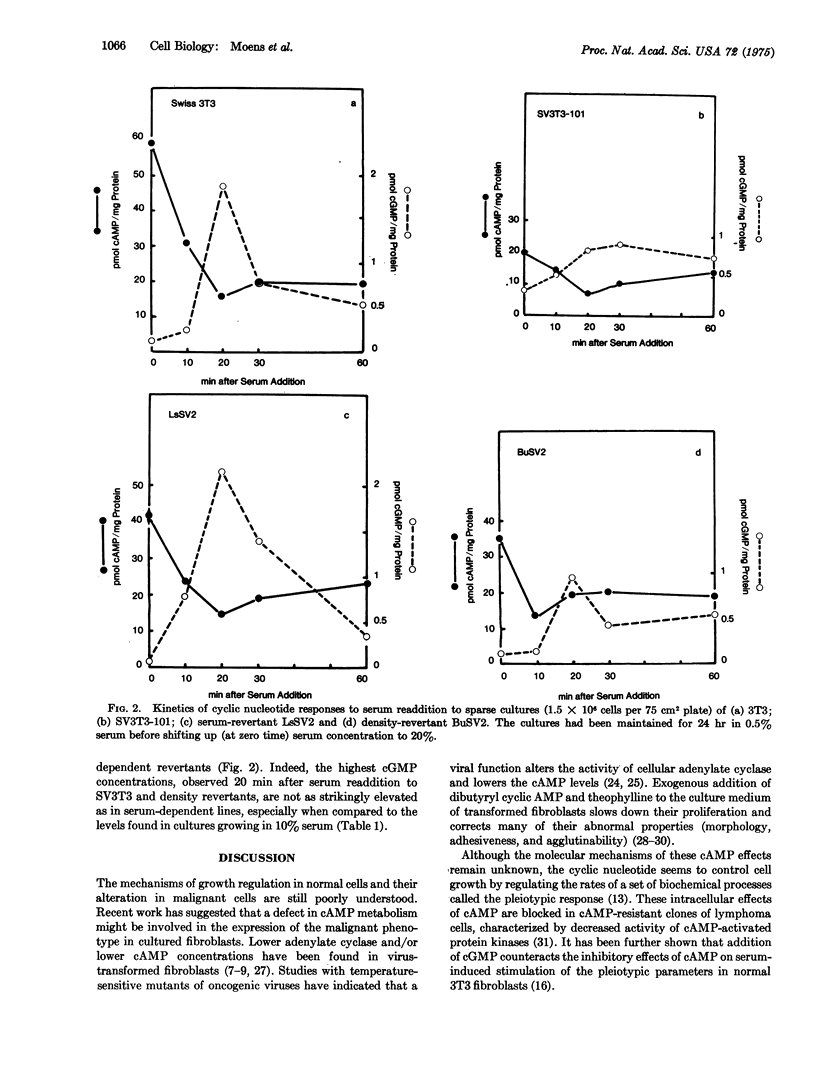
Abstract
Mouse fibroblasts transformed by simian virus 40 (SV3T3 cells) are characterized by cyclic AMP and cyclic GMP levels, respectively, about half and twice those found in growing untransformed 3T3 cells. Density-dependent inhibition of growth is correlated with reduced cyclic GMP concentrations in 3T3 and four different density-restricted revertant lines derived from SV3T3. The levels of cyclic AMP are not increased at confluence. Upon serum restriction, serum-dependent cell lines show a greater increase in intracellular cAMP than serum-insensitive lines. Cyclic GMP levels are greatly reduced, even in serum-insensitive density revertants, but not in SV3T3. Serum readdition to all serum-dependent lines is followed by a rapid decrease in cyclic AMP and increase in cyclic GMP concentrations. The magnitude of these responses is decreased in SV3T3 and density revertants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Johnson G. S., Pastan I. Transformation of chick-embryo fibroblasts by wild-type and temperature-sensitive Rous sarcoma virus alters adenylate cyclase activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1055–1059. doi: 10.1073/pnas.70.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Russell T. R., Carchman R. A., Pastan I. Interrelationship between adenylate cyclase activity, adenosine 3':5' cyclic monophosphate phosphodiesterase activity, adenosine 3':5' cyclic monophosphate levels, and growth of cells in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3802–3805. doi: 10.1073/pnas.70.12.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S., Sheppard J. R. Cyclic AMP, ATP and cell contact. Nature. 1974 Jul 5;250(461):62–64. doi: 10.1038/250062a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973 Nov 23;246(5430):197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez de Asúa L., Surian E. S., Flawia M. M., Torres H. N. Effect of insulin on the growth pattern and adenylate cyclase activity of BHK fibroblasts. Proc Natl Acad Sci U S A. 1973 May;70(5):1388–1392. doi: 10.1073/pnas.70.5.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Restoration of several morphological characteristics of normal fibroblasts in sarcoma cells treated with adenosine-3':5'-cyclic monphosphate and its derivatives. Proc Natl Acad Sci U S A. 1971 Feb;68(2):425–429. doi: 10.1073/pnas.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram R., Tomkins G. M. Pleiotypic control by cyclic AMP: interaction with cyclic GMP and possible role of microtubules. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1659–1663. doi: 10.1073/pnas.70.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey J., Vogel A., Pollack R. Intracellular cyclic AMP concentration responds specifically to growth regulation by serum. Proc Natl Acad Sci U S A. 1974 Mar;71(3):694–698. doi: 10.1073/pnas.71.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudland P. S., Gospodarowicz D., Seifert W. Activation of guanyl cyclase and intracellular cyclic GMP by fibroblast growth factor. Nature. 1974 Aug 30;250(5469):741-2, 773-4. doi: 10.1038/250741a0. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Seeley M., Seifert W. Cyclic GMP and cyclic AMP levels in normal and transformed fibroblasts. Nature. 1974 Oct 4;251(5474):417–419. doi: 10.1038/251417a0. [DOI] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Davis J. W., Sutherland E. W. A new enzymatic assay for guanosine 3':5'-cyclic monophosphate and its application to the ductus deferens of the rat. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1721–1725. doi: 10.1073/pnas.70.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Seifert W., Paul D. Levels of cyclic AMP in sparse and dense cultures of growing and quiescent 3T3 cells. Nat New Biol. 1972 Dec 27;240(104):281–283. doi: 10.1038/newbio240281a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Restoration of contact-inhibited growth to transformed cells by dibutyryl adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1316–1320. doi: 10.1073/pnas.68.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]


