Abstract
Endothelial dysfunction is a key feature of preeclampsia, and may contribute to increased cardiovascular disease risk years after pregnancy. Flow-mediated dilation (FMD) is a non-invasive endothelial function test that predicts cardiovascular event risk. New protocols allow researchers to measure three components of the FMD response: FMD, low flow-mediated constriction and the shear stimulus. This review encourages researchers to think beyond “low FMD” by examining how these three components may provide additional insights into the mechanisms and location of vascular dysfunction. The review then examines what FMD studies reveal about vascular dysfunction in preeclampsia, while highlighting opportunities to gain greater mechanistic insight from new protocols. Studies using traditional protocols show that FMD is low in mid-pregnancy prior to preeclampsia, at diagnosis, and for three years post-partum. However, FMD returns to normal by ten years post-partum. Studies using new protocols are needed to gain more mechanistic insight.
Keywords: Flow-mediated dilation, brachial artery reactivity testing, endothelial dysfunction, preeclampsia, pregnancy, low flow-mediated constriction, shear stimulus, shear rate area under the curve
Introduction
Endothelium-dependent flow-mediated dilation (FMD) is used to assess vascular function and cardiovascular risk in women with preeclampsia, and in many other patient populations [1]. FMD is measured non-invasively via ultrasound. Technological and methodological advances have significantly improved protocols since FMD was first proposed in 1992 [2]. New protocols and analysis techniques have the potential to provide additional insights into the mechanisms and location of vascular dysfunction. However, lack of awareness of these protocols among clinical investigators has limited their incorporation into clinical research.
This review provides a basic overview of recent methodological advances, focusing on what clinical investigators need to know to understand FMD studies. The review then examines what FMD studies reveal about vascular dysfunction in preeclampsia, while highlighting opportunities to gain greater mechanistic insight by using new protocols. We will accomplish these objectives by: 1. Explaining how FMD works, and why it is important, 2. Highlighting the primary features that distinguish new protocols from traditional protocols, 3. Showing how combining FMD with measurements of the shear stimulus and low flow-mediated constriction (LFMC) may provide additional insights into the mechanisms and location of vascular dysfunction, and 4. Examining what FMD studies reveal about vascular dysfunction before, during and after a preeclamptic pregnancy.
How Does FMD Work?
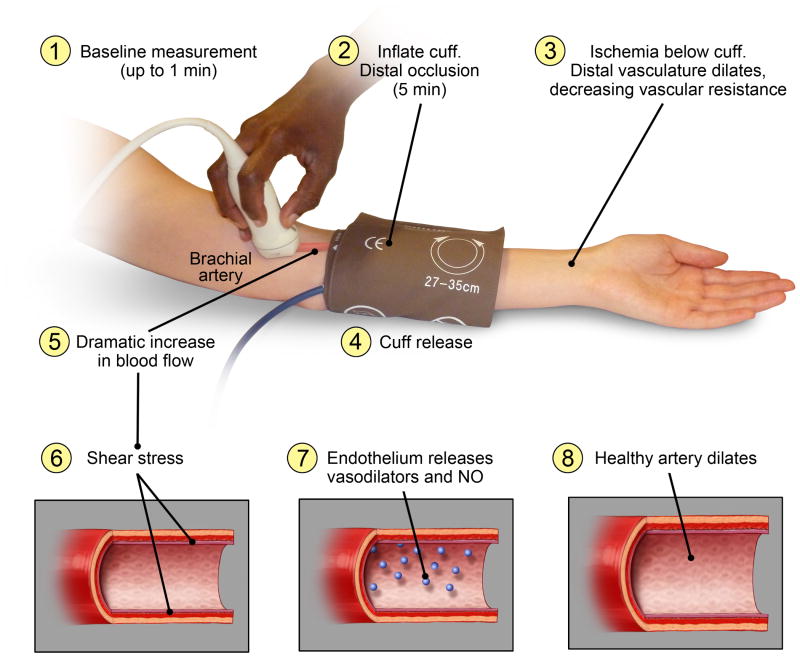
Flow-mediated dilation (FMD) is a non-invasive vascular function test that measures the change in artery diameter in response to reactive hyperemia. While FMD is traditionally performed in the brachial artery, some studies have also used the radial and femoral arteries [1]. During a typical FMD protocol, baseline artery diameter and blood flow velocity are measured using duplex ultrasound (Figure 1). An occlusion cuff is then inflated to stop blood flow to the lower arm for approximately 5 minutes. Ischemia in the tissue distal to the cuff causes the distal vessels to dilate, lowering vascular resistance. When the occlusion cuff is released, this reduction in downstream resistance dramatically increases blood flow to the arm. The endothelium responds to the resulting increase in shear stress by releasing vasodilators, including nitric oxide, which cause dilation in a healthy artery [3]. In a patient with vascular dysfunction, dilation is reduced or absent. FMD is the difference between the baseline diameter and the maximum diameter reached after cuff release (Figure 2). In addition to vascular health, FMD also depends on the artery examined, whether diameter is measured above or below the occlusion cuff, and how diameter is measured [1, 4].
Figure 1. How FMD Works.
The FMD protocol typically includes one minute of baseline measurement, 5 minutes of distal cuff occlusion, and up to five minutes of data collection following cuff release. In newer protocols, diameter and velocity are measured continuously throughout the test and used to calculate shear rate.
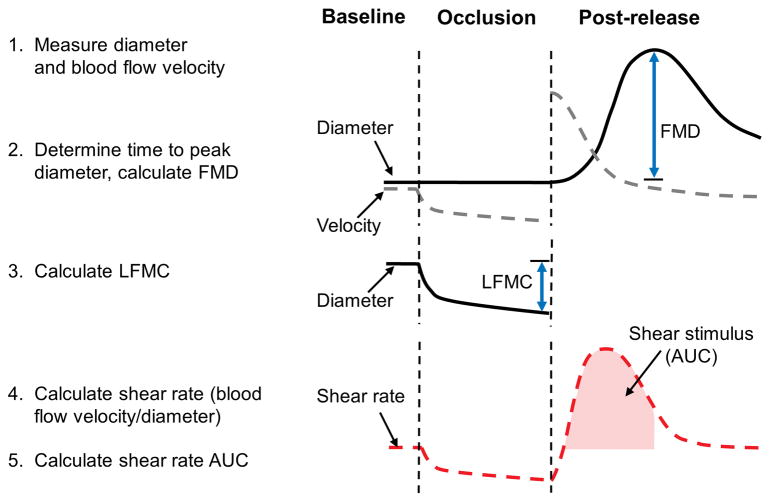
Figure 2. Measuring FMD, LFMC and Shear Rate AUC.
This figure illustrates the three components of the FMD test – FMD, LFMC and shear rate AUC. Diameter and velocity are measured continuously throughout the test. FMD and LFMC may be calculated as an absolute or a percent change in artery diameter. FMD is calculated as the change in diameter from baseline, to the maximum diameter reached after cuff release. LFMC is the change in diameter from baseline to the end of cuff inflation. Shear rate is calculated as blood flow velocity/diameter. Shear rate AUC is the area under the curve from the time of peak diameter, to the time of cuff release. Only increases in shear rate above baseline values are included.
FMD is usually calculated as a percent change in artery diameter, however, this approach was recently questioned [5, 6]. Percent FMD attempts to adjust for the effects of various factors, including body size, the size of the muscle bed, training status, etc., on baseline artery diameter [5]. This approach may underestimate FMD in large arteries and overestimate FMD in small arteries, contributing to spurious negative correlations between baseline diameter and percent FMD [5]. Alternative allometric scaling techniques were recently proposed [5], and are topics of extensive debate [7-9]. This review focuses on percent FMD, as the few studies that examined allometrically-scaled FMD did not include preeclamptic women.
Why is FMD important?
In addition to being a vascular function test, FMD is an established method of evaluating future cardiovascular disease risk in research studies. Low brachial artery FMD predicts cardiovascular event risk in healthy people and in patients with cardiovascular disease [10-13]. A recent meta-analysis concluded that for every 1% increase in brachial artery FMD, the relative risk of cardiovascular events was 0.87 (95% confidence interval 0.83 to 0.91) [14]. Average values for brachial artery FMD of 8 to 15% are commonly reported in healthy individuals. On average, FMD may be absent or reduced by half in individuals with co-morbidities. Whereas some studies suggest that FMD independently predicts cardiovascular events, others find that the strength of the relationship is attenuated after adjusting for traditional cardiovascular risk factors [15].
While FMD is a valuable research technique for investigating vascular function, significant limitations preclude its use in clinical settings [15]. The reader is referred to previous papers for more detailed discussions of the limitations of FMD [15, 1, 16]. Briefly, FMD requires expensive equipment and extensive operator training [15, 1]. Protocols differ among laboratories, making it difficult to establish standard cut off values. Testing conditions must be carefully standardized [15, 1]. This includes the time of day, room temperature, pre-test meal consumption, medication and caffeine use, and menstrual cycle phase in women. Day-to-day variability can be high, and FMD is not always reduced in individuals with risk factors or disease.
Evolving Methodologies
FMD was first proposed in 1992 [2]. Traditional protocols measured artery diameter for several heart cycles prior to cuff inflation, and again at a specified time point post-cuff release (usually 60 seconds). Arterial diameter was measured manually using electronic calipers at end diastole. Early attempts to quantify the stimulus for FMD focused on peak flow, which occurs within 15 seconds of cuff release. Ultrasound machines at the time could not measure diameter and velocity simultaneously. Investigators would measure velocity for 15 seconds post-release; then capture images to measure artery diameter at 60 seconds.
Advancements in ultrasound technology have significantly improved FMD protocols. Investigators can obtain simultaneous diameter and velocity data, record images continuously on an external computer, and analyze these large video files using semi-automated software. These improvements provide continuous diameter and velocity data throughout one minute of baseline, five minutes of cuff inflation, and up to five minutes after cuff release. They have also led to two additional measurements that may enhance our understanding of vascular health: the shear stimulus for FMD and LFMC (Figure 2). The shear stimulus, quantified as area under the curve, is the increase in shear stress from the time of cuff release to the time of peak dilation. LFMC refers to the change in diameter from pre-inflation baseline to the last minute of cuff release.
While newer protocols are often used in small physiology studies, their incorporation into clinical research has been slow. These approaches require expensive data capture systems and analysis software. Analysis is more complex and time-consuming. The use of newer protocols has accelerated since they were recommended by recent guidelines [2]. However, traditional protocols are well established in the clinical literature, and are effective in predicting cardiovascular risk [10]. This may dampen enthusiasm for adopting newer techniques.
The disadvantages of these new techniques are offset by their potential to offer additional insight into the mechanisms and location of vascular dysfunction. This article encourages investigators to think beyond “low FMD” by explaining how clinical investigators can potentially maximize mechanistic insight into vascular dysfunction by interpreting FMD in combination with the shear stimulus and LFMC. These measurements are part of the FMD protocol and do not require additional time; therefore they can be incorporated into clinical studies without increasing participant burden.
How to Identify New Protocols, and Why They Are Important
Five key components distinguish new protocols from traditional protocols. This section will help readers to identify these components, and understand why they are important.
1. Was diameter measured continuously to determine the maximum post-release diameter?
Traditional protocols measure post-release diameter at a specific time point (i.e. 60 seconds post-release). New protocols measure diameter continuously to determine the maximum post-release diameter. Maximum dilation occurs at 45-60 post-release seconds in most young healthy individuals [1]. However, there is substantial inter-individual variability [17]. Peak diameter often occurs later in older individuals or in individuals with disease [17]. If the time to peak diameter differs among groups, then differences in FMD observed with traditional protocols may be an artifact of the time selected for post-release diameter measurement. This problem can occur even when comparing young, healthy groups. Peak dilation occurred at 46 ± 16 (SD) seconds in non-pregnant women, vs. 57 ± 15 seconds in pregnant women [18]. FMD did not differ between pregnant and non-pregnant women when maximum dilation was determined from continuous measurements [18]. However, FMD was 2% greater in pregnant women when measured at 60 seconds post-release [18], as diameter was close to peak in pregnant women, but had begun decreasing in non-pregnant women.
2. Was the shear stimulus measured and quantified as area under the curve?
The stimulus for FMD is the increase in shear stress following cuff release [19]. Early studies focused on peak flow or peak shear, the highest value attained after cuff release. While peak shear occurs within 15 seconds of cuff release, peak diameter occurs much later. Shear remains elevated well beyond 15 seconds post-release, and these post-peak elevations in shear contribute to FMD [19]. Hence, shear stimulus area under the curve (AUC), from the time of cuff release to the time of peak diameter better reflects the stimulus for FMD (Figure 2) [19]. However, one study reported that peak shear rate and shear rate AUC were highly correlated when FMD was measured below the cuff [20].
While shear stress is believed to be the stimulus for FMD, investigators often measure shear rate [21]. Shear stress reflects shear rate after accounting for viscosity. Shear rate is an acceptable approximation of shear stress when one group of participants is studied at one time point, as viscosity is unlikely to change during FMD testing [21]. When viscosity differs among groups or time points, investigators should consider measuring viscosity to calculate shear stress.
3. Was semi-automated analysis software used?
Traditional protocols use electronic calipers to manually measure diameter at baseline, and at a single time point post-release [2]. Newer protocols record continuous diameter and velocity data; then use semi-automated analysis software to measure diameter and velocity frame-by-frame [22].
4. Was FMD measured above or below the occlusion cuff?
The magnitude and mechanisms of dilation depend on whether FMD is measured above or below the cuff. The 2011 guidelines recommend measurement above the cuff [1]. Traditional interpretations suggested that proximal brachial artery diameter did not change during distal occlusion; therefore measurements above the cuff reflected shear-mediated dilation that occurred after cuff release [10]. Data suggesting that nitric oxide (NO) contributed to this shear-mediated dilation [23] implied that FMD measured above the cuff could be a bioassay of NO-mediated dilation [21]. In contrast, FMD measured below the cuff included two components which could not be separated; dilation due to ischemia during occlusion, and shear-mediated dilation following cuff release [10]. This additional ischemia-mediated dilation explained the greater FMD values observed when FMD was measured below the cuff, compared to above. However, a recent meta-analysis concluded that FMD measured below the cuff was more predictive or equally predictive of cardiovascular disease, compared to FMD measured above the cuff [10].
5. Was LFMC assessed?
LFMC, the change in artery diameter between baseline and the end of occlusion (Figure 2), was recently proposed as a vascular health indicator that could be measured concurrently with FMD [24]. LFMC has been examined in physiology studies and small clinical studies. However, the clinical significance of LFMC has not yet been assessed in large cohort studies.
Small studies suggest that LFMC depends on the artery examined and patient health. While FMD is traditionally assessed in the brachial artery, early LFMC studies focused on the radial artery [24-26]. Brachial artery studies provide insight into conduit artery function, whereas radial artery studies examine resistance artery function. In healthy individuals, the average radial artery LFMC is 4-7% [24-27], whereas brachial artery LFMC is minimal or absent (-2 to 2%) [28, 29, 19, 30, 27]. However, opposite results may be observed in patients with disease. Radial artery LFMC is attenuated in patients with hypertension [24] and coronary artery disease [31]. In contrast, LFMC has been reported in the brachial artery among smokers [30], and patients with hypercholesterolemia [32] and non-ST-segment elevation myocardial infarction [33]. Three months of pravastatin attenuated brachial artery LFMC in hypercholesterolemic patients [32]. The presence of brachial artery LFMC in patients with risk factors or cardiovascular disease questions the assumption that brachial artery diameter does not change during distal cuff occlusion (described in section 4, above).
Thinking Beyond Low FMD: Gaining Mechanistic Insight from New Protocols
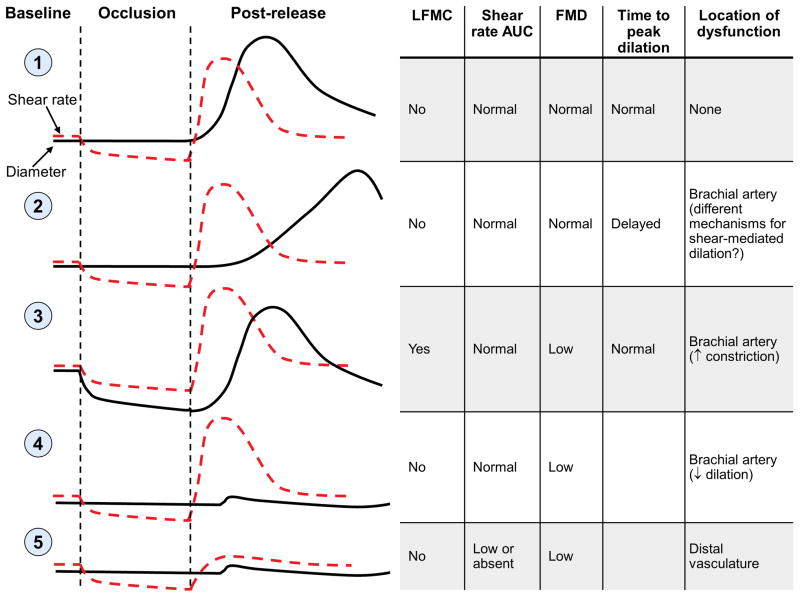
Low FMD has traditionally been interpreted as reduced dilation of the brachial artery in response to shear stress. However, several observations suggest that there may be multiple mechanistic pathways to low FMD. These pathways could potentially identify patients with different mechanisms and locations of vascular dysfunction. Figure 3 demonstrates how the combination of brachial artery LFMC, shear rate AUC, and FMD may provide more insights into vascular dysfunction than FMD alone. These hypothetical pathways are based on brachial artery FMD measured above the cuff.
Figure 3. Using FMD, LFMC and shear rate AUC to gain more insight into the nature and location of vascular dysfunction.
Profile 1 shows expected responses in the brachial artery of a healthy subject, when FMD is measured above the occlusion cuff. Profiles 2-5 show multiple pathways that could potentially result in low FMD. The pathways and evidence supporting each are described in detail in the text.
The first profile shows the expected response in healthy young individuals. There is no LFMC and a robust increase in shear rate following cuff release, leading to normal FMD. The participant has no dysfunction.
The second response shows a participant who has no LFMC, a normal shear stimulus, and normal FMD. However, peak dilation is delayed. Delayed time to peak dilation with normal FMD has been reported in older exercise-trained subjects [17], pregnant women [18], individuals with metabolic syndrome [34], and in 42% of patients with Type 2 diabetes [35]. It is unclear whether this profile has clinical significance. Liuni and colleagues reported high intra- and inter-individual variability in time to peak diameter [36]. In contrast, a large study reported greater Framingham risk scores in late dilators, compared to early dilators [37]. However, this study measured diameter at 50 seconds, two minutes and three minutes post-release [37, 35]. Time to peak diameter is normally distributed or right-skewed; hence selecting specific time points for post-release diameter measurements may create artificial groupings that would not be apparent if diameter were measured continuously. Large studies with continuous diameter data are needed to further explore the reliability, mechanisms and clinical significance of delayed time to peak dilation.
In profile three, low FMD is due to increased constriction during cuff inflation (greater LFMC), rather than to reduced dilation after cuff release. This profile is based on responses reported in smokers by Stadler and colleagues [30]. Brachial artery LFMC occurred during occlusion in smokers, but not in non-smokers. Following cuff release, the absolute magnitude of the dilatory response appeared similar in both groups. However, lower artery diameter in smokers at the time of cuff release (due to LFMC) led to a smaller increase in diameter when compared to the pre-inflation baseline; hence FMD was lower in smokers. These data suggest that low FMD in smokers may have been due to LFMC, rather than to failed dilation following cuff release.
The fourth response illustrates the traditional interpretation of low FMD. Shear rate AUC appears normal; yet the artery does not dilate. This patient has reduced shear-mediated dilation in the brachial artery.
The fifth profile shows a hypothetical participant who has no increase in shear rate following cuff release. Shear-mediated dilation (FMD) in the brachial artery was never tested because there was no shear stimulus. Two studies have identified a subset of “non-responders”; patients in whom distal cuff inflation causes limited reactive hyperemia and no dilation [35, 20]. These participants may have dysfunction in the distal vasculature, with failure to dilate in response to ischemia.
The profiles outlined in Figure 3, based on the existing literature, demonstrate how the combination of FMD, LFMC and shear rate AUC may provide more insights into the mechanisms and location of vascular dysfunction than FMD alone. Researchers have traditionally interpreted low FMD as reduced dilation in the brachial artery in response to shear stress (profile 4). However, existing data raise the intriguing possibility that “low FMD” may capture distinct subtypes of vascular dysfunction. If only FMD is measured, then investigators cannot distinguish among these potential subtypes. The opportunity to gain mechanistic insights may be a key advantage of newer FMD protocols and analysis methods, despite their additional complexities.
This proposal for FMD use in research studies has several caveats, in addition to the known limitations of FMD [15, 1, 16]. These hypothetical responses are based on published data from groups of subjects. Further research is needed to determine whether these types of profiles occur and whether they can reliably be identified in individual patients. High day-to-day variability is a significant limitation of FMD studies, and these individual response profiles will have limited value if they do not occur consistently within the same individuals. Researchers would also need to identify thresholds for determining whether each component is abnormal. Establishing thresholds for FMD is difficult because different laboratories use different protocols. Furthermore, each profile shows an abnormal response in a single component. However, it is possible that abnormalities in one component are associated with or contribute to abnormalities in other components. Finally, research would be needed to determine whether these response profiles have different implications for long-term disease risk. It is possible that FMD is an effective predictor because it detects abnormalities in several different vascular responses, any one of which identifies individuals at increased risk for cardiovascular disease. However, it is also possible that patients with no shear stimulus may have a worse prognosis than those with delayed dilation. In this case, newer protocols may allow researchers to obtain additional information about cardiovascular risk, in addition to the location and mechanisms of vascular dysfunction. This mechanistic insight could also help researchers and clinicians to identify new therapeutic targets for disease prevention and treatment. However, these questions can only be answered if investigators incorporate new protocols and analysis techniques into large clinical studies.
Preeclampsia: The Role of Endothelial Dysfunction
Endothelial dysfunction is a key feature of preeclampsia, and may contribute to increased cardiovascular disease risk years after pregnancy. The section examines what FMD studies reveal about vascular dysfunction in preeclampsia, while highlighting opportunities to gain greater mechanistic insight by using new methodologies.
Preeclampsia is a leading cause of maternal [38] and fetal [39] morbidity and mortality, which affects 2-7% of pregnancies [40, 41]. This disease is diagnosed in women presenting with new onset hypertension and proteinuria during the second half of pregnancy [42]. Preeclampsia can also be diagnosed in the absence of proteinuria in women with thrombocytopenia, impaired liver function, progressive renal insufficiency, pulmonary edema, or new onset cerebral or visual disturbances [42]. The only known cure for preeclampsia is delivery. Effective prevention strategies are lacking.
The pathophysiology of preeclampsia remains elusive; however endothelial dysfunction is believed to be a key feature. Maternal risk factors and abnormal placental development contribute to systemic maternal endothelial dysfunction, which leads to maternal symptoms [43]. Failed adaptation of the uterine spiral arteries that supply blood to the placenta may cause hypoxia [43], repeated ischemia-reperfusion injury [44], or high velocity blood flow in the intervillous space [45]. The damaged placenta is believed to release factors that contribute to vascular dysfunction into the maternal circulation [43]. Many cardiovascular disease risk factors also increase preeclampsia risk, possibly by contributing to inflammation, oxidative stress and vascular dysfunction [43]. The American Heart Association recently recognized preeclampsia as a risk factor for cardiovascular disease [46] and stroke [47]. Endothelial dysfunction is critically important to the pathophysiology of preeclampsia, and may also contribute to future cardiovascular disease in these women.
FMD and Preeclampsia
Preeclampsia presents a unique opportunity for researchers interested in using FMD to examine the mechanisms of vascular dysfunction, and their relationship to future cardiovascular risk. Preeclampsia and cardiovascular disease share several risk factors, such as obesity, which are associated with vascular dysfunction and low FMD [48]. Endothelial dysfunction is a crucial component of preeclampsia pathophysiology, and is also hypothesized to be one mechanism linking preeclampsia with future cardiovascular disease and stroke.
Studies examining FMD in preeclampsia have focused on three time periods; prior to disease (10-29 weeks gestation), during preeclampsia, and post-partum (2 weeks to 11 years). These studies provide considerable information about percent FMD. However, most studies used traditional protocols or modified traditional protocols that measured post-release diameter at a few additional time points, and/or peak flow. A few studies recorded continuous post-release diameter data [49-53], however they did not report time to peak diameter. Studies have measured FMD above [50, 51, 54, 55, 49, 53, 52, 56-64] and below [65-73] the cuff. Shear rate AUC and LFMC have not been examined, as many studies were conducted before these measurements were proposed or recognized in clinical research.
FMD Prior to Preeclampsia (10 to 29 weeks gestation)
Preeclampsia occurs in 2-7% of pregnancies [40, 41]. Prospective first and second trimester studies would need to measure FMD in hundreds of women to obtain data in the small number of women who would later develop preeclampsia. Most investigators addressed this challenge by studying high-risk women, or combining small high-risk and low-risk cohorts. While these approaches increase the proportion of women who develop preeclampsia, data on low risk women who develop preeclampsia are lacking. In addition, each study recruited women with different risk factors. These risk factors included a previous preeclamptic pregnancy, abnormal uterine artery Doppler, referral to a high-risk obstetrics clinic, Type 1 diabetes, obesity and hypertension. Risk factors, such as obesity, are themselves associated with lower FMD [48], and may confound study results.
Most studies observed lower FMD in the first and second trimesters among high-risk women who subsequently developed preeclampsia [50, 49, 64, 51, 63, 54]. These included studies in women with risk factors for preeclampsia [64], studies that combined low and high-risk women [54, 49, 74], and studies that compared low-risk women to high-risk women who subsequently developed preeclampsia [50, 51]. One small study observed a non-significant trend towards lower FMD in women who later developed preeclampsia [75]. Lower FMD may be more apparent closer to diagnosis. FMD did not differ between women who later developed preeclampsia and those who did not at 16-20 weeks gestation, but was lower in women who later developed preeclampsia at 24-28 weeks [63]. Among women with Type 1 diabetes, FMD was not different between those who later developed preeclampsia and those who did not [62].
Compared to women who remained normotensive, baseline diameter was not different [50, 51] or larger [54] in women who developed preeclampsia. Peak flow did not differ between groups [64, 50].
Women with Preeclampsia
Most studies report lower FMD in women with preeclampsia, compared to normotensive pregnant women [70, 66, 71, 72, 65, 52, 76, 77]. However, a few studies found no differences [67, 61, 68]. Protocol differences do not appear to explain these divergent results. Peak flow [70, 67] or shear rate [61] did not differ between preeclamptic and normotensive pregnant women.
Data regarding the severity of preeclampsia is also conflicting. FMD was lower in women with preeclampsia complicated by HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, eclampsia or stillbirth, compared to women who had preeclampsia without these complications [78]. FMD was also lower in preeclamptic women with an abnormal uterine artery Doppler at diagnosis, compared to preeclamptic women with a normal uterine artery Doppler [66]. However, FMD did not differ among women with normotensive pregnancies, mild preeclampsia and severe preeclampsia [67], or between women with preeclampsia and women with preeclampsia superimposed on chronic hypertension [79].
Post-partum
FMD increases by four to six weeks post-partum in women who had preeclampsia [77, 67], suggesting a partial reversal of the endothelial dysfunction observed at diagnosis. However, most studies suggest that FMD remains lower in women who had preeclampsia for up to three years post-partum [68, 52, 56, 69, 57, 55, 58], when compared to women who had normotensive pregnancies. The presence and magnitude of the effect may depend on disease severity. Several studies suggest that FMD is lowest in women who have had early onset or pre-term preeclampsia [52, 49, 53]. FMD in women who had late onset [53] or term preeclampsia [49] was not different from FMD in women who had normotensive pregnancies. FMD was lower in women who had recurrent preeclampsia, compared to women who had one preeclamptic pregnancy [58]. Chambers and colleagues reported that FMD was lower in women with previous preeclampsia, even when the analyses were restricted to non-obese, non-smoking, normotensive women with normal fasting glucose and cholesterol levels [58]. Peak flow did not differ between women who had normotensive pregnancies and those who had preeclampsia [58].
The persistent vascular dysfunction observed during the first three years post-partum may resolve by ten years post-partum. Hamad and colleagues evaluated the same women one year and eleven years postpartum. FMD was lower in women with previous preeclampsia at one year post-partum [56], but increased to levels observed in women with normotensive pregnancies by 11 years post-partum [59]. Despite this reversal of endothelial dysfunction, women with a history of preeclampsia had higher blood pressures and impaired glucose tolerance [59]. A second study also found no difference in FMD between women who had preeclampsia, and women who had normotensive pregnancies, ten years post-partum [60].
The timing of the post-release diameter measurement differed between the studies performed in the same women at one year and 11 years post-partum (50-60 seconds post-release [56], vs. maximum of 30, 60 or 90 seconds post-release [59]). This could potentially impact the results if the peak dilation for many women occurred at 30 or 90 seconds post-release in the later study. The study authors confirmed that the peak post-release diameter occurred at 60 seconds post-release in most women who were tested 11 years post-partum; therefore conclusions were not different when peak diameter was determined at 60 seconds post-release (Thomas Kahan, personal communication).
Future Directions
Existing studies suggest that women who develop preeclampsia have endothelial dysfunction in mid-pregnancy prior to the onset of disease and at the time of diagnosis. However, a few studies have not observed differences. This may be due to differences in study inclusion criteria or the timing of testing, differences in FMD protocols, or Type II error due to the small sample size of many studies. Post-partum studies raise the intriguing possibility that preeclampsia may cause persistent endothelial dysfunction that resolves between three and ten years post-partum. However, only two small studies have examined FMD in preeclamptic women ten years post-partum [59, 60]. Larger studies and studies which examine other time periods are needed. Very few studies have used continuous diameter measurements to account for potential between-group differences in time to peak diameter. Larger studies are needed to determine whether new protocols confirm these observations, while measuring the shear stimulus and LFMC. Researchers should also determine whether vascular dysfunction that resolves several years post-partum has lasting effects on cardiovascular risk. Pre-conception vascular function data are very difficult to obtain, but would be extremely valuable. Finally, combining FMD with other vascular assessment techniques may provide more comprehensive insight into vascular dysfunction before, during and after a preeclamptic pregnancy.
Conclusion
Combining FMD, LFMC and the shear stimulus may allow investigators to gain additional insight into the mechanisms and location of vascular dysfunction. Preeclampsia presents a unique opportunity for researchers interested in using FMD to examine the mechanisms of vascular dysfunction, and their relationships to long-term cardiovascular risk. Most studies suggest that women with preeclampsia have lower FMD than women with normotensive pregnancies, both prior to the onset of disease and at the time of diagnosis. While low FMD persists for at least three years after a preeclamptic pregnancy, small studies suggest that this endothelial dysfunction resolves by ten years post-partum. If confirmed, this observation would suggest that preeclampsia presents a unique opportunity to evaluate the effect of medium-term vascular dysfunction on cardiovascular risk. Studies using new FMD protocols in women prior to, during, and after a preeclamptic pregnancy may provide valuable mechanistic insights into preeclampsia and future disease risk, while enhancing our understanding of FMD.
Acknowledgments
TLW was supported by the Office of Women's Health Research (Building Interdisciplinary Careers in Women's Health award K12HD065987).
References
- 1**.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow mediated dilation in humans: a methodological and technical guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. This guideline describes provides detailed information on new methodologies recommended for FMD testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 3.Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension. 2014;63(2):376–82. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 4.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson G, Batterham AM. Allometric scaling of diameter change in the original flow-mediated dilation protocol. Atherosclerosis. 2013;226(2):425–7. doi: 10.1016/j.atherosclerosis.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 6*.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31(2):287–91. doi: 10.1097/HJH.0b013e32835b8164. This guideline describes detailed methodologies of new protocols for conducting FMD studies. [DOI] [PubMed] [Google Scholar]
- 7.Stoner L, Faulkner J, Sabatier MJ. Is allometric scaling really a panacea for flow-mediated dilation? Commentary on paper by Atkinson and Batterham. Atherosclerosis. 2013;228(1):280–1. doi: 10.1016/j.atherosclerosis.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Woodman RJ, Mangoni AA. Adjusting for brachial artery diameter in the analysis of flow-mediated dilatation: pitfalls of a landmark paper? Atherosclerosis. 2013;228(1):277–9. doi: 10.1016/j.atherosclerosis.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.DeVan AE, Pierce GL, Brooks FA, Seals DR. The dependence of FMD% on baseline diameter: a problem solved by allometric scaling - no problem in this case. Clin Sci (Lond) 2013;125(1):55–6. doi: 10.1042/CS20130047. [DOI] [PubMed] [Google Scholar]
- 10*.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9. doi: 10.1161/HYPERTENSIONAHA.110.167015. This meta-analysis shows that flow-mediated dilation measured above and below the cuff both predict future cardiovascular events. [DOI] [PubMed] [Google Scholar]
- 11.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134(1):52–8. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Shechter M, Marai I, Marai S, Sherer Y, Sela BA, Feinberg MS, et al. The association of endothelial dysfunction and cardiovascular events in healthy subjects and patients with cardiovascular disease. Isr Med Assoc J. 2007;9(4):271–6. [PubMed] [Google Scholar]
- 13.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 14.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 15.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Gokce N. Clinical assessment of endothelial function: ready for prime time? Circulation Cardiovascular imaging. 2011;4(4):348–50. doi: 10.1161/CIRCIMAGING.111.966218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension. 2008;51(2):203–10. doi: 10.1161/HYPERTENSIONAHA.107.101014. [DOI] [PubMed] [Google Scholar]
- 18.Weissgerber TL, Davies GA, Tschakovsky ME. Brachial artery flow-mediated dilation is not affected by pregnancy or regular exercise participation. Clin Sci (Lond) 2011;121(8):355–65. doi: 10.1042/CS20110008. [DOI] [PubMed] [Google Scholar]
- 19.Pyke KE, Tschakovsky ME. Peak vs total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102(4):1510–9. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs BB, Dobrosielski DA, Lima M, Bonekamp S, Stewart KJ, Clark JM. The association of arterial shear and flow-mediated dilation in diabetes. Vascular medicine. 2011;16(4):267–74. doi: 10.1177/1358863X11411361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, et al. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol. 2001;91(2):929–37. doi: 10.1152/jappl.2001.91.2.929. [DOI] [PubMed] [Google Scholar]
- 23.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 24.Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, et al. Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol. 2008;51(20):1953–8. doi: 10.1016/j.jacc.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 25.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88(2):145–51. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 26.Spieker LE, Luscher TF, Noll G. ETA receptors mediate vasoconstriction of large conduit arteries during reduced flow in humans. J Cardiovasc Pharmacol. 2003;42(3):315–8. doi: 10.1097/00005344-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Weissgerber TL, Davies GA, Tschakovsky ME. Low flow-mediated constriction occurs in the radial but not the brachial artery in healthy pregnant and nonpregnant women. J Appl Physiol. 2010;108(5):1097–105. doi: 10.1152/japplphysiol.00815.2009. [DOI] [PubMed] [Google Scholar]
- 28.Filitti V, Giral P, Simon A, Merli I, Del Pino M, Levenson J. Enhanced constriction of the peripheral large artery in response to acute induction of a low-flow state in human hypercholesterolemia. Arterioscler Thromb. 1991;11(1):161–6. doi: 10.1161/01.atv.11.1.161. [DOI] [PubMed] [Google Scholar]
- 29.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291(6):H3043–9. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- 30.Stadler RW, Ibrahim SF, Lees RS. Measurement of the time course of peripheral vasoactivity: results in cigarette smokers. Atherosclerosis. 1998;138(1):197–205. doi: 10.1016/s0021-9150(97)00267-0. [DOI] [PubMed] [Google Scholar]
- 31.Gori T, Muxel S, Damaske A, Radmacher MC, Fasola F, Schaefer S, et al. Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J. 2012;33(3):363–71. doi: 10.1093/eurheartj/ehr361. [DOI] [PubMed] [Google Scholar]
- 32.Megnien JL, Simon A, Andriani A, Segond P, Jeannin S, Levenson J. Cholesterol lowering therapy inhibits the low-flow mediated vasoconstriction of the brachial artery in hypercholesterolaemic subjects. Br J Clin Pharmacol. 1996;42(2):187–93. doi: 10.1046/j.1365-2125.1996.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Spiro JR, Digby JE, Ghimire G, Mason M, Mitchell AG, Ilsley C, et al. Brachial artery low-flow-mediated constriction is increased early after coronary intervention and reduces during recovery after acute coronary syndrome: characterization of a recently described index of vascular function. Eur Heart J. 2011;32(7):856–66. doi: 10.1093/eurheartj/ehq401. This paper examines brachial artery LFMC in patients with non-ST-segment elevation myocardial infarction, before and after percutaneous coronary intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes IA, Sales AR, Rocha NG, Silva BM, Vianna LC, da Nobrega AC. Preserved flow-mediated dilation but delayed time-to-peak diameter in individuals with metabolic syndrome. Clin Physiol Funct Imaging. 2014;34(4):270–6. doi: 10.1111/cpf.12092. [DOI] [PubMed] [Google Scholar]
- 35.Irace C, Tschakovsky ME, Carallo C, Cortese C, Gnasso A. Endothelial dysfunction or dysfunctions? Identification of three different FMD responses in males with type 2 diabetes. Atherosclerosis. 2008;200(2):439–45. doi: 10.1016/j.atherosclerosis.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Liuni A, Luca MC, Lisi M, Dragoni S, di Stolfo G, Mariani JA, et al. Observations of time-based measures of flow-mediated dilation of forearm conduit arteries: implications for the accurate assessment of endothelial function. Am J Physiol Heart Circ Physiol. 2010;299(3):H939–45. doi: 10.1152/ajpheart.00271.2010. [DOI] [PubMed] [Google Scholar]
- 37.Irace C, Padilla J, Carallo C, Scavelli F, Gnasso A. Delayed vasodilation is associated with cardiovascular risk. European journal of clinical investigation. 2014;44(6):549–56. doi: 10.1111/eci.12268. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. World Health Report: Make Every Mother, and Child Count. Geneva: WHO; 2005. [Google Scholar]
- 39.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321):1877–90. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 40.Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet. 1998;61(2):127–33. doi: 10.1016/s0020-7292(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 41.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95(1):24–8. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 42**.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. This guideline outlines new ACOG criteria for preeclampsia diagnosis. [DOI] [PubMed] [Google Scholar]
- 43.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–72. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 44.Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162(1):115–25. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–82. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women-2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–23. doi: 10.1016/j.jacc.2011.02.005. This guideline lists preeclampsia as a major risk factor for cardiovascular disease in women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(5):1545–88. doi: 10.1161/01.str.0000442009.06663.48. This guideline identifies preeclampsia as a risk factor for stroke in women. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suboc TM, Dharmashankar K, Wang J, Ying R, Couillard A, Tanner MJ, et al. Moderate Obesity and Endothelial Dysfunction in Humans: Influence of Gender and Systemic Inflammation. Physiol Rep. 2013;1(3) doi: 10.1002/phy2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122(5):478–87. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 50.Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361(9368):1511–7. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- 51.Savvidou MD, Noori M, Anderson JM, Hingorani AD, Nicolaides KH. Maternal endothelial function and serum concentrations of placental growth factor and soluble endoglin in women with abnormal placentation. Ultrasound Obstet Gynecol. 2008;32(7):871–6. doi: 10.1002/uog.6126. [DOI] [PubMed] [Google Scholar]
- 52.Hamad RR, Eriksson MJ, Berg E, Larsson A, Bremme K. Impaired endothelial function and elevated levels of pentraxin 3 in early-onset preeclampsia. Acta Obstet Gynecol Scand. 2011;91(1):50–6. doi: 10.1111/j.1600-0412.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- 53.Yinon Y, Kingdom JC, Odutayo A, Moineddin R, Drewlo S, Lai V, et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;122(18):1846–53. doi: 10.1161/CIRCULATIONAHA.110.948455. [DOI] [PubMed] [Google Scholar]
- 54.Takase B, Goto T, Hamabe A, Uehata A, Kuroda K, Satomura K, et al. Flow-mediated dilation in brachial artery in the second half of pregnancy and prediction of pre-eclampsia. J Hum Hypertens. 2003;17(10):697–704. doi: 10.1038/sj.jhh.1001599. [DOI] [PubMed] [Google Scholar]
- 55.Paez O, Alfie J, Gorosito M, Puleio P, de Maria M, Prieto N, et al. Parallel decrease in arterial distensibility and in endothelium-dependent dilatation in young women with a history of pre-eclampsia. Clin Exp Hypertens. 2009;31(7):544–52. doi: 10.3109/10641960902890176. [DOI] [PubMed] [Google Scholar]
- 56.Hamad RR, Eriksson MJ, Silveira A, Hamsten A, Bremme K. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25(11):2301–7. doi: 10.1097/HJH.0b013e3282ef5fc0. [DOI] [PubMed] [Google Scholar]
- 57.Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49(1):90–5. doi: 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- 58.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285(12):1607–12. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 59**.Ostlund E, Al-Nashi M, Hamad RR, Larsson A, Eriksson M, Bremme K, et al. Normalized endothelial function but sustained cardiovascular risk profile 11 years following a pregnancy complicated by preeclampsia. Hypertens Res. 2013;36(12):1081–7. doi: 10.1038/hr.2013.81. This paper shows that the vascular dysfunction observed in women with preeclampsia one year post-partum resolved by 11 years post-partum. [DOI] [PubMed] [Google Scholar]
- 60.Sandvik MK, Leirgul E, Nygard O, Ueland PM, Berg A, Svarstad E, et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol. 2013;209(6):569 e1–e10. doi: 10.1016/j.ajog.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 61.Torrado J, Farro I, Farro F, Bia D, Zocalo Y, Sosa C, et al. Carotid-radial pulse wave velocity as an alternative tool for the evaluation of endothelial function during pregnancy: potential role in identifying hypertensive disorders of pregnancy. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5603–6. doi: 10.1109/EMBC.2012.6347264. [DOI] [PubMed] [Google Scholar]
- 62.Clausen P, Ekbom P, Damm P, Feldt-Rasmussen U, Nielsen B, Mathiesen ER, et al. Signs of maternal vascular dysfunction precede preeclampsia in women with type 1 diabetes. Journal of diabetes and its complications. 2007;21(5):288–93. doi: 10.1016/j.jdiacomp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Brandao AH, Felix LR, do Carmo Patricio E, Leite HV, Cabral AC. Difference of endothelial function during pregnancies as a method to predict preeclampsia. Arch Gynecol Obstet. 2014 doi: 10.1007/s00404-014-3243-3. [DOI] [PubMed] [Google Scholar]
- 64.Garcia RG, Celedon J, Sierra-Laguado J, Alarcon MA, Luengas C, Silva F, et al. Raised C-reactive protein and impaired flow-mediated vasodilation precede the development of preeclampsia. Am J Hypertens. 2007;20(1):98–103. doi: 10.1016/j.amjhyper.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Brodszki J, Lanne T, Laurini R, Strevens H, Wide-Swensson D, Marsal K. Vascular mechanical properties and endothelial function in pre-eclampsia with special reference to bilateral uterine artery notch. Acta Obstet Gynecol Scand. 2008;87(2):154–62. doi: 10.1080/00016340701733646. [DOI] [PubMed] [Google Scholar]
- 66.Adali E, Kurdoglu M, Adali F, Cim N, Yildizhan R, Kolusari A. The relationship between brachial artery flow-mediated dilatation, high sensitivity C-reactive protein, and uterine artery doppler velocimetry in women with pre-eclampsia. J Clin Ultrasound. 2011;39(4):191–7. doi: 10.1002/jcu.20781. [DOI] [PubMed] [Google Scholar]
- 67.Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K, et al. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens Res. 2014;37(2):145–50. doi: 10.1038/hr.2013.131. [DOI] [PubMed] [Google Scholar]
- 68.Tyldum EV, Backe B, Stoylen A, Slordahl SA. Maternal left ventricular and endothelial functions in preeclampsia. Acta Obstet Gynecol Scand. 2011;91(5):566–73. doi: 10.1111/j.1600-0412.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 69.Goynumer G, Yucel N, Adali E, Tan T, Baskent E, Karadag C. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound. 2012;41(3):145–50. doi: 10.1002/jcu.21962. [DOI] [PubMed] [Google Scholar]
- 70.Mori T, Shinohara K, Wakatsuki A, Watanabe K, Fujimaki A. Adipocytokines and endothelial function in preeclamptic women. Hypertens Res. 2010;33(3):250–4. doi: 10.1038/hr.2009.222. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto T, Suzuki Y, Kojima K, Suzumori K. Reduced flow-mediated vasodilation is not due to a decrease in production of nitric oxide in preeclampsia. Am J Obstet Gynecol. 2005;192(2):558–63. doi: 10.1016/j.ajog.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 72.Yoshida A, Nakao S, Kobayashi M, Kobayashi H. Flow-mediated vasodilation and plasma fibronectin levels in preeclampsia. Hypertension. 2000;36(3):400–4. doi: 10.1161/01.hyp.36.3.400. [DOI] [PubMed] [Google Scholar]
- 73.Kinzler WL, Smulian JC, Ananth CV, Vintzileos AM. Noninvasive ultrasound assessment of maternal vascular reactivity during pregnancy: a longitudinal study. Obstetrics and gynecology. 2004;104(2):362–6. doi: 10.1097/01.AOG.0000134787.24959.9b. [DOI] [PubMed] [Google Scholar]
- 74.Brandao AH, Cabral MA, Leite HV, Cabral AC. Endothelial function, uterine perfusion and central flow in pregnancies complicated by Preeclampsia. Arq Bras Cardiol. 2012;99(4):931–5. doi: 10.1590/s0066-782x2012005000087. [DOI] [PubMed] [Google Scholar]
- 75.Anastasakis E, Paraskevas KI, Papantoniou N, Daskalakis G, Mesogitis S, Mikhailidis DP, et al. Association between abnormal uterine artery Doppler flow velocimetry, risk of preeclampsia, and indices of arterial structure and function: a pilot study. Angiology. 2008;59(4):493–9. doi: 10.1177/0003319708316008. [DOI] [PubMed] [Google Scholar]
- 76.de Resende Guimaraes MF, Brandao AH, de Lima Rezende CA, Cabral AC, Brum AP, Leite HV, et al. Assessment of endothelial function in pregnant women with preeclampsia and gestational diabetes mellitus by flow-mediated dilation of brachial artery. Arch Gynecol Obstet. 2014 doi: 10.1007/s00404-014-3220-x. [DOI] [PubMed] [Google Scholar]
- 77.Kuscu NK, Kurhan Z, Yildirim Y, Tavli T, Koyuncu F. Detection of endothelial dysfunction in preeclamptic patients by using color Doppler sonography. Archives of gynecology and obstetrics. 2003;268(2):113–6. doi: 10.1007/s00404-002-0351-2. [DOI] [PubMed] [Google Scholar]
- 78.Vieira MC, da Cunha Filho EV, Paula LG, Hentschke MR, Poli-de-Figueiredo CE, Pinheiro da Costa BE. Flow-mediated dilatation of brachial artery as marker of preeclampsia morbidity. International journal of cardiology. 2013;168(4):4424–5. doi: 10.1016/j.ijcard.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 79.Filho EV, Mohr C, Filho BJ, Gadonski G, Paula LG, Antonello IC, et al. [Flow-mediated dilatation in the differential diagnosis of preeclampsia syndrome] Arquivos brasileiros de cardiologia. 2010;94(2):182–6. 95–200, 185–9. [PubMed] [Google Scholar]