Abstract
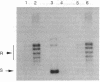
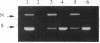
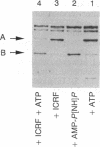
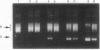
The mechanism of inhibition of eukaryotic DNA topoisomerase II [DNA topoisomerase (ATP-hydrolyzing), EC 5.99.1.3] by a member of the bisdioxopiperazine family of anticancer drugs, ICRF-193, was investigated by using purified yeast DNA topoisomerase II. In the absence of ATP, ICRF-193 has little effect on the binding of the enzyme to various forms of DNA. In the presence of ATP, the drug converts the enzyme to a form incapable of binding circular DNA. Incubation of a preformed circular DNA-enzyme complex with ICRF-193 and ATP converts the complex to a form stable in molar concentrations of salt. These results can be interpreted in terms of the ATP-modulated protein-clamp model of type II DNA topoisomerases [Roca, J. & Wang, J. C. (1992) Cell 71, 833-840]; ICRF-193 can bind to the closed-clamp form of the enzyme and prevents its conversion to the open-clamp form. This interpretation is further supported by the finding that whereas both ATP and the drug are needed to form the salt-stable circular DNA-enzyme complex, ATP is not needed for maintaining this complex; furthermore, a signature of the closed-clamp form of the enzyme, Staphylococcus aureus strain V8 endoproteinase cleavage site at Glu-680, is observed if the enzyme is incubated with both ATP and ICRF-193. Inhibition of interconversion between the open- and closed-clamp forms of type II DNA topoisomerases offers a new mechanism in the selection and design of therapeutics targeting this class of enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boritzki T. J., Wolfard T. S., Besserer J. A., Jackson R. C., Fry D. W. Inhibition of type II topoisomerase by fostriecin. Biochem Pharmacol. 1988 Nov 1;37(21):4063–4068. doi: 10.1016/0006-2952(88)90096-2. [DOI] [PubMed] [Google Scholar]
- Clarke D. J., Johnson R. T., Downes C. S. Topoisomerase II inhibition prevents anaphase chromatid segregation in mammalian cells independently of the generation of DNA strand breaks. J Cell Sci. 1993 Jun;105(Pt 2):563–569. doi: 10.1242/jcs.105.2.563. [DOI] [PubMed] [Google Scholar]
- Crenshaw D. G., Hsieh T. Function of the hydrophilic carboxyl terminus of type II DNA topoisomerase from Drosophila melanogaster. I. In vitro studies. J Biol Chem. 1993 Oct 5;268(28):21328–21334. [PubMed] [Google Scholar]
- D'Arpa P., Beardmore C., Liu L. F. Involvement of nucleic acid synthesis in cell killing mechanisms of topoisomerase poisons. Cancer Res. 1990 Nov 1;50(21):6919–6924. [PubMed] [Google Scholar]
- Drake F. H., Hofmann G. A., Mong S. M., Bartus J. O., Hertzberg R. P., Johnson R. K., Mattern M. R., Mirabelli C. K. In vitro and intracellular inhibition of topoisomerase II by the antitumor agent merbarone. Cancer Res. 1989 May 15;49(10):2578–2583. [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Holm C., Covey J. M., Kerrigan D., Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989 Nov 15;49(22):6365–6368. [PubMed] [Google Scholar]
- Ishida R., Miki T., Narita T., Yui R., Sato M., Utsumi K. R., Tanabe K., Andoh T. Inhibition of intracellular topoisomerase II by antitumor bis(2,6-dioxopiperazine) derivatives: mode of cell growth inhibition distinct from that of cleavable complex-forming type inhibitors. Cancer Res. 1991 Sep 15;51(18):4909–4916. [PubMed] [Google Scholar]
- Jensen P. B., Sørensen B. S., Demant E. J., Sehested M., Jensen P. S., Vindeløv L., Hansen H. H. Antagonistic effect of aclarubicin on the cytotoxicity of etoposide and 4'-(9-acridinylamino)methanesulfon-m-anisidide in human small cell lung cancer cell lines and on topoisomerase II-mediated DNA cleavage. Cancer Res. 1990 Jun 1;50(11):3311–3316. [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. On the coupling between ATP usage and DNA transport by yeast DNA topoisomerase II. J Biol Chem. 1993 Apr 15;268(11):8096–8104. [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. Proteolysis patterns of epitopically labeled yeast DNA topoisomerase II suggest an allosteric transition in the enzyme induced by ATP binding. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10485–10489. doi: 10.1073/pnas.88.23.10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley J. E., Wang J. C. Study of allosteric communication between protomers by immunotagging. Nature. 1993 Feb 25;361(6414):749–750. doi: 10.1038/361749a0. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- Maxwell A., Gellert M. Mechanistic aspects of DNA topoisomerases. Adv Protein Chem. 1986;38:69–107. doi: 10.1016/s0065-3233(08)60526-4. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Kohn K. Topological complexes between DNA and topoisomerase II and effects of polyamines. Biochemistry. 1989 Feb 7;28(3):995–1002. doi: 10.1021/bi00429a012. [DOI] [PubMed] [Google Scholar]
- Roca J., Wang J. C. The capture of a DNA double helix by an ATP-dependent protein clamp: a key step in DNA transport by type II DNA topoisomerases. Cell. 1992 Nov 27;71(5):833–840. doi: 10.1016/0092-8674(92)90558-t. [DOI] [PubMed] [Google Scholar]
- Sharpe H. B., Field E. O., Hellmann K. Mode of action of the cytostatic agent "ICRF 159". Nature. 1970 May 9;226(5245):524–526. doi: 10.1038/226524a0. [DOI] [PubMed] [Google Scholar]
- Shin C. G., Snapka R. M. Patterns of strongly protein-associated simian virus 40 DNA replication intermediates resulting from exposures to specific topoisomerase poisons. Biochemistry. 1990 Dec 11;29(49):10934–10939. doi: 10.1021/bi00501a011. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Ikegami Y., Ishida R., Andoh T. Inhibition of topoisomerase II by antitumor agents bis(2,6-dioxopiperazine) derivatives. Cancer Res. 1991 Sep 15;51(18):4903–4908. [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Melamed M. R. Effects of the L isomer (+)-1,2-bis(3,5-dioxopiperazine-1-yl)propane on cell survival and cell cycle progression of cultured mammalian cells. Cancer Res. 1981 Nov;41(11 Pt 1):4566–4576. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases: why so many? J Biol Chem. 1991 Apr 15;266(11):6659–6662. [PubMed] [Google Scholar]
- Worland S. T., Wang J. C. Inducible overexpression, purification, and active site mapping of DNA topoisomerase II from the yeast Saccharomyces cerevisiae. J Biol Chem. 1989 Mar 15;264(8):4412–4416. [PubMed] [Google Scholar]
- Zechiedrich E. L., Osheroff N. Eukaryotic topoisomerases recognize nucleic acid topology by preferentially interacting with DNA crossovers. EMBO J. 1990 Dec;9(13):4555–4562. doi: 10.1002/j.1460-2075.1990.tb07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]