Abstract
Acute weightlessness in space induces a fluid shift leading to central volume expansion. Simultaneously, blood pressure is either unchanged or decreased slightly. Whether these effects persist for months in space is unclear. Twenty-four hour ambulatory brachial arterial pressures were automatically recorded at 1–2 h intervals with portable equipment in eight male astronauts: once before launch, once between 85 and 192 days in space on the International Space Station and, finally, once at least 2 months after flight. During the same 24 h, cardiac output (rebreathing method) was measured two to five times (on the ground seated), and venous blood was sampled once (also seated on the ground) for determination of plasma catecholamine concentrations. The 24 h average systolic, diastolic and mean arterial pressures (mean ± se) in space were reduced by 8 ± 2 mmHg (P = 0.01; ANOVA), 9 ± 2 mmHg (P < 0.001) and 10 ± 3 mmHg (P = 0.006), respectively. The nightly blood pressure dip of 8 ± 3 mmHg (P = 0.015) was maintained. Cardiac stroke volume and output increased by 35 ± 10% and 41 ± 9% (P < 0.001); heart rate and catecholamine concentrations were unchanged; and systemic vascular resistance was reduced by 39 ± 4% (P < 0.001). The increase in cardiac stroke volume and output is more than previously observed during short duration flights and might be a precipitator for some of the vision problems encountered by the astronauts. The spaceflight vasodilatation mechanism needs to be explored further.
Key points
Weightlessness in space induces initially an increase in stroke volume and cardiac output, accompanied by unchanged or slightly reduced blood pressure.
It is unclear whether these changes persist throughout months of flight.
Here, we show that cardiac output and stroke volume increase by 35–41% between 3 and 6 months on the International Space Station, which is more than during shorter flights.
Twenty-four hour ambulatory brachial blood pressure is reduced by 8–10 mmHg by a decrease in systemic vascular resistance of 39%, which is not a result of the suppression of sympathetic nervous activity, and the nightly dip is maintained in space.
It remains a challenge to explore what causes the systemic vasodilatation leading to a reduction in blood pressure in space, and whether the unexpectedly high stroke volume and cardiac output can explain some vision acuity problems encountered by astronauts on the International Space Station.
Introduction
During spaceflight, weightlessness immediately induces a shift of blood and fluids from the lower segments of the body to the upper body, inducing puffy faces, as well as what has been termed chicken legs (Thornton & Hoffler, 1977). Approximately 2 litres of fluid are shifted upwards from the legs and, simultaneously, cardiac output is increased by 18–26% (Prisk et al. 1993; Shykoff et al. 1996; Norsk et al. 2006). The increase in cardiac output is induced by an increase in stroke volume because heart rate is unchanged or decreased (Fritsch-Yelle et al. 1996; Shykoff et al. 1996; Norsk et al. 2006). The increase in stroke volume is the result of an increase in cardiac preload induced by the weightlessness-induced venous translocation of blood from the lower body segments to the upper body. This is supported by an observed increase in transmural central venous pressure during the initial phase of weightlessness (Buckey et al. 1996; Videbaek & Norsk, 1997).
Simultaneous to the increase in central blood volume in weightlessness, blood pressure is either unchanged or slightly decreased (Fritsch-Yelle et al. 1996; Shykoff et al. 1996; Norsk et al. 2006). Fritsch-Yelle et al. (1996) conducted a study during short duration space shuttle flights, where ambulatory brachial blood pressure was measured over 24 h with portable equipment. A decrease in diastolic arterial pressure of 5 mmHg was reported within the initial 2 weeks of spaceflight, although there were no changes in systolic or mean arterial pressures. Therefore, because cardiac output is increased, mean arterial pressure is maintained unchanged by dilatation of the arterial resistance vessels inducing a decrease in systemic vascular resistance (Shykoff et al. 1996; Norsk et al. 2006).
These cardiovascular changes have been detected within the initial 2 weeks of weightlessness in space on board the space shuttle. Only little is known about whether they persist, or whether they are attenuated, abolished or even augmented, during longer duration flights. During up to 6 months of flight on the Russian space station Mir, Herault et al. (2000) observed a decrease in stroke volume of some 10–16%, as measured by echocardiography, compared to the ground-based supine body position. Hughson et al. (2012), using the finger blood pressure contour technique on the International Space Station (ISS), observed no changes in stroke volume throughout months of flight versus both the seated and supine posture on the ground. To what extent stroke volume and cardiac output adapt during longer duration spaceflights (i.e. of beyond a few weeks) remains unclear.
Therefore, the present study aimed to characterize the shift of blood and fluids from the lower to the upper body, as well as the effects on arterial pressures, during months of flight on the ISS. Based on our previous results obtained during short duration flights (Norsk et al. 2006), as well as the known vasoconstrictor effect of gravitational stress in upright individuals (Norsk, 2014), we tested the hypothesis that, during months of spaceflight, cardiac stroke volume and output are maintained increased to the same degree or (as a result of adaptation) less than during the initial 1–2 weeks of flight, and blood pressure is maintained unchanged or mildly reduced via a decrease in systemic vascular resistance.
Methods
Study design and subjects
In eight male astronauts (age 45–53 years, height 169–188 cm, weight 70–99 kg, body mass index 22.5–28.6 kg m–2), ambulatory brachial blood pressure, heart rate and cardiac output were measured over a 24 h period between 322 and 71 days before launch to the ISS and, once again, over a 24 h period between the 85th and 192nd day of flight. Finally, 2 months or more after landing (58–209 days), the 24 h measurements were repeated. During these 24 h periods, venous blood was sampled once for catecholamine concentration determination and urine was collected to measure 24 h fluid and electrolyte excretions. Two astronauts were treated for hypertension with angiotensin-converting enzyme inhibitors at the same dosage throughout the pre-, in- and post-flight observation periods. In addition, at least two of the astronauts were taking cholesterol-reducing medication (atorvastatin calcium, 10 mg day−1; Lipitor, Pfizer, New York, NY, USA). The protocol was performed in accordance with the declaration of Helsinki and approved annually or bi-annually by the Institutional Review Boards at NASA, the European Space Agency (ESA) and the Japanese Space Agency under the acronym CARD (HRMRB-12-KAM-40) and all subjects provided their written informed consent.
On the ISS, ambient temperature varied between 23 and 25ºC during the 24 h monitoring periods versus between 21 and 24ºC on the ground during the pre- and post-flight sessions. Humidity varied between 35% and 40% versus between 18% and 61% on the ground. The atmospheric pressure is the same for the ISS as it is at sea level on Earth, with same O2 partial pressure in N2, except that the CO2 concentration is an average of 10-fold higher (0.3–0.5%). The astronauts usually perform individualized aerobic and resistance exercise on a treadmill and an ergometer and also using equipment termed the Advanced Resistive Exercise Device, with body loads of up to 600 lbs (272 kg) and for up to 2 h daily, as a countermeasure against bone and muscle degradation (Wood et al. 2011; Smith et al. 2012). The content of the food intake is determined individually by each astronaut from a list of food items, although this is not regularly monitored.
Blood pressure
Ambulatory blood pressure was measured according to the same portable technique used by clinicians for diagnosis of hypertension and prediction of disease (Hansen et al. 2005). Systolic, diastolic and mean arterial pressures were automatically measured in an upper arm artery throughout 24 h periods during normal daily activities using portable equipment developed for use in space (oscillometry, portable blood pressure device; ESA and PAR Medizintechnik GmbH, Berlin, Germany). It was recorded at hourly intervals during the day (8–23 h) and bi-hourly during the night (23–8 h), and heart rate was simultaneously recorded by the oscillometric technique. The subjects were instructed to continue with whatever activity they were involved in when the cuff inflation was automatically initiated by the equipment, except that they were encouraged, if possible, to relax the arm to which the cuff was attached by passively hanging it alongside the body. In each individual, care was taken to ensure that measurements were performed in the same upper arm throughout the pre-, in- and post-flight 24 h periods.
Cardiac output
Cardiac output was measured using a foreign gas rebreathing technique developed for spaceflight (Pulmonary Function System; Danish Aerospace Company, Odense, Denmark & ESA) and as used in space previously (Norsk et al. 2006). In brief, subjects breathed through a mouthpiece back and forth into a bag with 1.5 litres of a gas mixture consisting of 1% Freon-22 (blood soluble tracer gas), 1% SF6 (non-blood soluble tracer gas) and 25% O2 in N2. The rate of disappearance of Freon-22 from the lung-bag system into the pulmonary blood during rebreathing was calculated from end-expiratory concentrations measured by an infrared photo-acoustic and magneto-acoustic multi-gas analyser connected to the mouth piece through a tube. Based on the blood solubility coefficient (Bunsen) of Freon-22, the rate of disappearance was used for calculation of pulmonary capillary blood flow, considered equal to cardiac output during steady-state conditions. End-expiratory concentrations of SF6 were used to correct for an inadequate mixing of residual air in the lungs with the gas mixture from the rebreathing bag, as well as for changes in rebreathing volume. Each rebreathing lasted some 20 s with a respiratory rate of 20 breaths min–1. Care was taken to ensure that exactly the same breathing frequency and tidal volume were used for all pre-, in- and post-flight measurements. The rebreathing method with same technique (i.e. as used in the present study) has been validated against the thermodilution and direct Fick method (Gabrielsen et al. 2003) and we have carried out tests to ensure that the breathing frequency and rebreathing volume do not increase the cardiac output measurements (Damgaard & Norsk, 2005). In a recent parabolic flight study, rebreathing during 20 s of weightlessness did not alter heart rate compared to rebreathing during 1 G in the seated posture (Petersen et al. 2011). Collectively, the results obtained in these studies show that the rebreathing technique employed both on ground and in space does not per se influence the measured variable (cardiac output).
Cardiac output was measured during the awake times of 24 h periods that were the same as those when ambulatory blood pressure was monitored: at 3–5 h intervals five times in five of the astronauts and two to four times in three of the astronauts and each time twice: shortly after ambulatory activities and again 5–7 min later after rest, and on the ground on both occasions, always in the seated position. In this way, we obtained close to ambulatory values, as well as values after rest. The seated posture for the cardiac output measurements was chosen because our purpose was to compare the effects of long duration spaceflight with the effects of being on the ground during normal daily activities. The upright seated relaxed posture is the best reflection of the average posture during the awake time because people are rarely supine during the day, when they are awake. We have also shown that the upright seated posture is stable with no changes in plasma volume and cardiovascular variables over an at least 12 h period (Johansen et al. 1992; Stadeager et al. 1992).
Systemic vascular resistance and stroke volume
Brachial blood pressure and heart rate were recorded in close conjunction with and just before or after the two rebreathing manoeuvres by manual or automatic activation of the portable equipment. These seated blood pressure measurements were used for the calculation of (i) systemic vascular resistance by dividing mean arterial pressure by the average of the two cardiac output measurements and (ii) stroke volume by dividing cardiac output by heart rate.
Sympathetic nerve activity
Sympathetic nerve activity was estimated from venous blood plasma concentration of noradrenaline. Blood was collected from an arm vein in the astronauts in the morning on the 2nd day of the 24 h blood pressure monitoring period and on the ground with the astronauts in a seated position during rest. The samples were immediately spun in a centrifuge to separate the plasma from the formed elements and then frozen at –80°C both on the ISS and on the ground. After the return of the sample to Earth, during which time the samples were kept frozen, the plasma concentration of noradrenaline was measured by an enzyme immunoassay (LDN, Nordhorn, Germany; Norepinephrine, Research Elisa BA E-5200) that was performed in quadruplicate for each sample and the mean was calculated. The analytical sensitivity was 1.3 pg mL−1 and the functional sensitivity was 2 pg mL−1. The cross-reactivity was minimal and the intra-assay coefficient of variation with this technique was 11.7%. The plasma concentrations of adrenaline were measured by same methodology in same samples (Elisa BA E-5100) and the mean values were calculated from two to four measurements.
Renal fluid and electrolyte excretions
For monitoring of the renal excretion rates of sodium and potassium, aiming to determine whether more or less salt was ingested during flight, as well as for estimation of the hydration level, 24 h urine volumes were determined by collection in plastic bags and, in space, by manually compressing the empty part of the bag and reading the volume of the filled part on a scale (Norsk et al. 2000). Each test subject carried out their own volume measurements in space and, on the ground, their scale readings had been correlated with the actual volumes estimated in measuring glasses. Urine samples from the 24 h collection bags were kept frozen at –80ºC until the time of analysis and urine sodium and potassium concentrations were then measured by an ion selective electrode system (EML 100; Radiometer Medical A/S, Copenhagen, Denmark). Urine osmolality was estimated in same samples by freezing point depression (Micro-Osmometer 3MO; Advanced Instruments, Norwood, MA, USA).
Statistical analysis
A one-way ANOVA for repeated measures followed by a pairwise planned contrast analysis after Bonferroni correction was conducted to determine whether changes of the mean values of a variable in the eight astronauts were statistically significant (P < 0.05) between the pre, in- and post-flight sessions. Normal distribution of the data was assured before the analyses.
Results
Ambulatory blood pressure and heart rate
Ambulatory systolic, diastolic and mean arterial pressures were decreased during all phases of the 24 h cycle during flight compared to before flight on the ground (Fig. 1). The mean systolic arterial pressure of the 24 h period decreased from 129 ± 2 mmHg (mean ± sem) to 121 ± 2 mmHg (P = 0.01), diastolic arterial pressure from 85 ± 2 mmHg to 76 ± 2 mmHg (P < 0.001) and mean arterial pressure from 102 ± 3 mmHg to 92 ± 1 mmHg (P = 0.006). All arterial pressures returned to pre-flight levels after flight (132 ± 2, 86 ± 2 and 103 ± 2 mmHg, respectively). Mean arterial pressure decreased pre-flight from the awake to sleep period (Fig. 1) by 7 ± 3 mmHg (P = 0.056, paired t test), in-flight by 8 ± 3 mmHg (P = 0.015) and post-flight by 10 ± 3 mmHg (P = 0.007).
Figure 1. Mean ± sem of systolic (SAP), diastolic (DAP) and mean (MAP) 24 h ambulatory brachial arterial pressures recorded automatically at hourly (8–23 h) or bi-hourly (23–8 h) intervals (mean ± sem of N = 8) on the ISS and before and after flight.
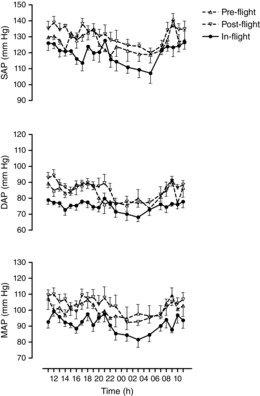
Measurements were conducted with portable oscillometric equipment between 85 and 192 days of flight on the ISS (in-flight, filled circles) and 322 to 71 days before (pre-flight, triangles) and 58 to 209 days after (post-flight, diamonds). The means of the 24 h SAP, DAP and MAP values on the space station were significantly lower than the same values during the pre- and post-flight 24-h monitoring periods.
The mean 24 h heart rate was unchanged by spaceflight (64 ± 2 beats min–1 versus 63 ± 2 beats min–1), and there was no preflight change between day and night (Fig. 2). The lowest value, however, with the least variation (Fig. 2) was observed during the sleep period (night) in space (58 ± 1 beats min–1), which was significantly different to the in-flight ambulatory day-time period (64 ± 3 beats min–1; P = 0.034) but not significantly so to the pre- and post-flight night-periods (Fig. 2). Post-flight, heart rate also decreased significantly during the night compared to the day (P = 0.027) (Fig. 2).
Figure 2. Individual and mean ± sem systolic (SAP) and diastolic (DAP) arterial pressures and heart rate (HR) measured on the ISS and before and after flight during ambulatory conditions when awake (day); in connection with seated cardiac output rebreathing measurements (CO day); and during the sleep period (night).
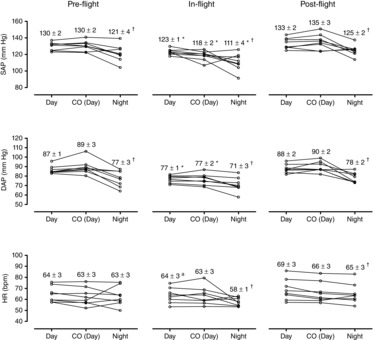
SAP, DAP and HR were measured by portable equipment in a brachial artery with an oscillation technique and during CO (day) using the same equipment activated by the subject and then in the seated posture. *Significant change (P < 0.05) from pre- and post-flight values at same time period [day, CO (day) or night]. †Significant change (P < 0.05) from day in same panel. aSignificant change (P < 0.05) from only post-flight value (day).
Of particular interest, systolic arterial pressure during spaceflight not only decreased when the subjects were ambulatory (8–23 h average), but also during the sleep period (23–8 h average) (Figs 1 and 2) from 121 ± 4 mmHg to 111 ± 4 mmHg (P = 0.012). Diastolic and mean arterial pressures also decreased during the sleep period by similar magnitudes from 77 ± 3 mmHg to 71 ± 3 mmHg and from 95 ± 5 mmHg to 85 ± 3 mmHg, although not statistically significantly (P = 0.054 and 0.075, respectively). During the day time, systolic arterial pressure decreased from 130 ± 2 mmHg to 123 ± 1 mmHg (P = 0.046), diastolic arterial pressure from 87 ± 1 mmHg to 77 ± 1 mmHg (P < 0.001) and mean arterial pressure from 103 ± 2 mmHg to 94 ± 1 mmHg (P = 0.006). The most pronounced decreases in space occurred from the ground-based upright seated and resting posture, when cardiac output was measured, from 130 ± 2 mmHg to 118 ± 2 mmHg (P = 0.017), from 89 ± 3 mmHg to 77 ± 2 mmHg (P < 0.001) and from 104 ± 3 mmHg to 92 ± 2 mmHg (P = 0.001) (Fig. 2), respectively. In all cases, the blood pressure values returned to pre-flight levels during post-flight monitoring (Figs 3).
Figure 3. Individual and mean ± sem mean arterial pressure (MAP), cardiac output (CO) and systemic vascular resistance (SVR) on the ISS and before and after flight.
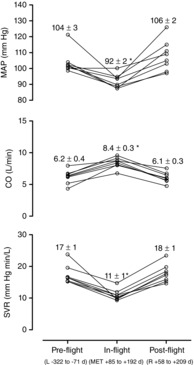
MAP was recorded by portable oscillometric equipment in the resting and on the ground seated posture at the time of CO measurements. SVR was calculated by MAP/CO. L–, days before launch; MET, days of mission elapsed time; R+, days after landing. *Significant change (P < 0.05) from all pre- and post-flight values.
Cardiac output, stroke volume and systemic vascular resistance
As indicated in Fig. 3, cardiac output increased in space from a pre-flight seated value (average of all the measurements over 24 h) of 6.2 ± 0.4 litres min–1 to 8.4 ± 0.3 litres min–1 (41 ± 9%; P < 0.001) and systemic vascular resistance decreased by 39 ± 4% (P < 0.001). Stroke volume increased by 33 ± 8 mL (35 ± 10%; P < 0.001). Cardiac output measured the first time immediately after ambulatory activities increased from 6.5 ± 0.4 litres min–1 to 8.5 ± 0.4 litres min–1 (P < 0.001) and the second time after 5–7 min of rest from 5.9 ± 0.4 litres min–1 to 8.4 ± 0.4 litres min–1 (P < 0.001). Thus, the increases in cardiac output in space immediately after ambulatory activities and after rest were very similar. All variables attained pre-flight levels after flight (Fig. 3).
Catecholamine and urine variables
There were no differences between pre-, in- and post-flight plasma noradrenaline or adrenaline concentrations, as well as 24 h urine volumes, sodium and potassium excretions and urine osmolalities (Table1).
Table 1.
Blood and urine variables during 3–6 months of spaceflight
| Pre-flight | In-flight | Post-flight | |
|---|---|---|---|
| Plasma noradrenaline (ng l−1) | 730 ± 130 | 720 ± 90 | 730 ± 100 |
| Plasma adrenaline (ng l−1) | 50 ± 10 | 60 ± 10 | 50 ± 10 |
| Urine volume (mL 24 h−1) | 1978 ± 394 | 1832 ± 232 | 2288 ± 359 |
| Urine sodium excretion (mmol 24 h−1) | 157 ± 26 | 172 ± 16 | 172 ± 18 |
| Urine potassium excretion (mmol 24 h−1) | 77 ± 10 | 76 ± 9 | 90 ± 7 |
| Urine osmolality (mosmol kg−1) | 623 ± 98 | 639 ± 98 | 564 ± 76 |
Plasma catecholamine concentrations (seated on the ground) and 24 h urine volume and electrolyte excretions and osmolality (N = 7–8 males) on the ISS and on the ground before and after flight. Data are the mean ± sem. Pre-flight: 322–71 days before flight. In-flight: 85–192 days into flight. Post-flight: 58–209 days after flight.
Discussion
Our hypothesis was partly confirmed because cardiac output and stroke volume increased during months of flight in space and 24 h ambulatory brachial blood pressure decreased. It was unexpected, however, to observe more pronounced increases in cardiac output than we had previously seen using similar technology on short duration shuttle flights (41 ± 9% versus 22 ± 8%; Norsk et al. 2006). Decreases in systolic, diastolic and mean arterial pressures by as much as 8–10 mmHg were also unexpected because, during previous shorter missions of 1–2 weeks, only ambulatory diastolic arterial pressure decreased significantly, and then only by 5 mmHg (Fritsch-Yelle et al. 1996). In the present study, the healthy nightly dipping in blood pressure was preserved unchanged in space (Figs 1 and 2). We had expected to find an attenuated dipping as observed on previous shuttle flights (Fritsch-Yelle et al. 1996) because of the lack of a posture-reducing blood pressure effect in weightlessness (Pump et al. 2001; Norsk, 2014). Thus, in these astronauts, the blood pressure-lowering effect of rest/sleep on the ISS was definitely maintained and was independent of gravitational loads.
Fluid shifts
It has always been assumed that, during spaceflight, acute weightlessness induces a fluid shift leading to an increase in cardiac preload, stroke volume and output, which, eventually over days, subsides to a lower level. There is controversy regarding how the initial increase subsides and adapts because almost no data are available from space (Norsk, 2014). The results of ground-based simulation models (e.g. head-down tilted bed rest and head-out water immersion) have indicated that, after an initial increase in central blood volume, natriuresis and diuresis seek to return the central blood volume back to the pre-intervention upright seated level, with data from head-down tilted bed rest indicating that intravascular volume adapts to below the level of that in the supine position (Johansen et al. 1992; Norsk, 2014). Prisk et al. (1993) measured cardiac output (acetylene gas rebreathing) during shuttle missions and found that, after an initial increase of 18%, it fell towards that of the pre-flight standing position within a few days. Subsequently, it was assumed that cardiac output would at least adapt to a lower level during weeks to months of spaceflight rather than during the initial days. Hughson et al. (2012), who used the continuous finger blood pressure contour technique, found no change in stroke volume compared to both the supine and seated postures on the ground during up to 6 months of flight. However, there was an increase in cardiac output towards the end of the mission. Hamilton et al. (2011), using echocardiography on the ISS, also observed no change in stroke volume and cardiac output in two astronauts. By contrast, Herault et al. (2000), also using ultrasound, reported a decrease in stroke volume during 5–6 months of flight on the Russian space station Mir compared to the ground-based supine position.
It was therefore unexpected that we found significant increases in stroke volume and cardiac output of as much as 35% and 41% after 3 months or more of flight, and similarly so in all astronauts independent of mission duration. Even though we did not have the opportunity to conduct early mission measurements in these subjects, this increase is substantially higher than we have previously seen in space after 1 week of flight (Norsk et al. 2006). It is comparable to very acute head-out water immersion (Stadeager et al. 1992). Furthermore, the increase is considerably higher compared to when changing from an upright seated posture to supine on the ground, where cardiac output only increases by 15–20% (Shiraishi et al. 2002).
The ostensible discrepancy between our results and those reported by Herault et al. (2000) and Hamilton et al. (2011) could stem from the different reference postures used on the ground. If the previous studies had used a lateral horizontal body position versus our upright seated posture as the reference posture on the ground, a discrepancy between the studies may not exist because an acute horizontal body posture increases cardiac output and stroke volume. Thus, all of these studies show that cardiac stroke volume and output are increased compared to the normal upright body posture on the ground, but apparently to varying degrees. The rather large increase that we observed in the present study could have been augmented compared to early in-flight and this might explain why the spaceflight-induced vision impairment syndrome occurs months into a mission on the ISS (Mader et al. 2011).
Ambulatory blood pressure
Two previous studies have monitored 24 h ambulatory, brachial blood pressures during short duration space shuttle flights of less than 2 weeks. Fritsch-Yelle et al. (1996) showed, as noted previously, that, within 2 weeks of flight in 12 astronauts, diastolic arterial pressure decreased by 5 mmHg with no changes in systolic and mean arterial pressures. Karemaker & Berecki-Gisolf (2009) showed a decrease also in diastolic arterial pressure in two subjects, but not in the systolic and mean. In both cases, the circadian blood pressure rhythm with dipping during the sleep period was present but in an attenuated fashion. Thus, compared to the outcome of the present study, it appears that diastolic arterial pressure decreases during the initial weeks of flight, although this is somehow augmented later in the mission with significant reductions also in the systolic and mean arterial pressures. Also, it appears that the nightly lowering of blood pressure is augmented throughout a long duration flight. These observations were unexpected.
The results of other studies during short duration shuttle flights using the continuous peripheral photoplethysmographic finger-technique (Fina- and Portapres; Finapres Medical Systems BV, Amsterdam, The Netherlands) have indicated no changes or even temporary increases in blood pressure compared to the ground-based seated posture (Norsk et al. 2006; Di Rienzo et al. 2008) and no changes or increases compared to the supine position on the ground (Ertl et al. 2002; Cox et al. 2002; Eckberg et al. 2010). Watenpaugh et al. (2001) using a brachial auscultative technique also found an increase in mean arterial pressure of 5 mmHg compared to being acute supine on the ground. However, all other studies using an automatic oscillometric or auscultative technique in a brachial artery during short duration spaceflights have demonstrated decreases in blood pressure to varying degrees compared to during either ambulatory activities (Fritsch-Yelle et al. 1996) or when being passively upright seated on the ground (Shykoff et al. 1996). This indicates that there might be a difference in the changes to spaceflight of the arterial pressures in a large versus smaller and more peripheral artery. These considerations are supported by the results reported by Verheyden et al. (2009), who compared finger versus brachial blood pressures in space and found that in-flight mean arterial pressure assessments resulted in discrepancies of up to 30 mmHg.
This is the first time that ambulatory 24 h brachial blood pressure monitoring has been conducted during a long duration spaceflight on the ISS, although it is not the first time blood pressure has been measured during months of flight. Baevsky et al. (2007) observed that, compared to a semi-supine body position on the ground, brachial diastolic arterial pressure measured by an oscillometric technique was significantly decreased throughout 6-month missions in eight astronauts and by the same magnitude of ∼10 mmHg from beginning to the end. Systolic and mean arterial pressures, however, were not significantly reduced. Simultaneously, heart rate was unchanged. Verheyden et al. (2010), using automated brachial measurements at regular intervals (not ambulatory), did not observe any statistically significant changes in blood pressure during 5–6 months of flight on the ISS compared to the supine posture on the ground prior to flight, but did find a trend of a decrease compared to the standing posture. Hughson et al. (2012), who used a peripheral, finger photo-plethysmographic method (Finapres) on the ISS in six astronauts, did not observe any changes in blood pressure during the first and last month of the 2–6 months of flight compared to both the supine and seated posture on the ground. Thus, our data are more in line with those of Baevsky et al. (2007) and Verheyden et al. (2010) showing that blood pressure is reduced compared to that in the upright posture on the ground, and closer to that when supine.
Systemic vascular resistance
Because cardiac output was increased by 41% and mean arterial pressure was decreased by 10%, systemic vascular resistance decreased by 39% (Fig. 3) as a result of systemic arterial vasodilatation. A decrease in systemic vascular resistance has also been observed in young males during maximal bicycle exercise immediately after simulation of weightlessness by 35 days of 6° head-down bed rest, where the lowest vascular resistance was observed when exercising supine (Bringard et al. 2010). Our observed magnitude of the decrease in space compared to the upright seated posture is considerably larger than when attaining the acute supine position on the ground from an upright seated posture, for which numerous studies in our laboratory have shown a decrease in systemic vascular resistance of 15–20% (Pump et al. 1999; Shiraishi et al. 2002). The decrease in systemic vascular resistance in the present study in space is also considerably larger than that reported in four astronauts during a previous shorter duration shuttle flight, where we observed a decrease compared to a ground-based upright seated posture of 14% within 1 week of flight (Norsk et al. 2006). Thus, systemic vascular resistance is considerably lower during months of spaceflight compared to being acute supine on the ground, as well as lower than during the initial days of flight.
It is unclear how the peripheral vasodilatation is accomplished because, usually, based on ground-based experiments, such vasodilatation would be expected to be induced by suppression of efferent sympathetic nervous activity (Stadeager et al. 1992; Johansen et al. 1997; Norsk, 2014). Possible physiological mechanisms explaining the decrease in systemic vascular resistance entail the chronic blood and fluid shift to the upper body as indicated by the increase in cardiac stroke volume and output leading to less stretching of the small arteries and veins in the legs and to expansion of the cardiac chambers. Less stretching of the dependent vessels reduces the arteriolar smooth muscle contractility and circulatory resistance (Henriksen, 1977; Zhang, 2001) and distension of the cardiac chambers releases vasodilatory and natriuretic peptides into the bloodstream (de Bold, 2011). All of these effects could result in a decrease in systemic vascular resistance. The weakness of this hypothesis is that it is not supported by the results of previous investigations on shorter shuttle flights (Leach et al. 1996; Watenpaugh et al. 2001; Gabrielsen & Norsk, 2007)
Ambient, nutritional and/or exercise factors on the ISS were probably not responsible for induced arterial dilatation because the temperature and humidity were very similar in space and on the ground when the 24 h measurements were conducted, and urine volumes and sodium excretions were also very similar between space and the ground (Table1). Dehydration and/or differences in salt intake therefore do not explain our findings. Data from the ISS have recently indicated that astronauts only lose a few kilogrammes of body mass during months of flight (Zwart et al. 2014) and this cannot explain the decrease in blood pressure of 8–10 mmHg. The exercise countermeasures (Wood et al. 2011; Smith et al. 2012) on the ISS probably also did not account for our observations by themselves because (i) 24 h heart rate and blood concentration of noradrenaline should then have been lower (Leosco et al. 2013); (ii) aerobic exercise for several months in normotensive individuals of the same age as our astronauts does not decrease blood pressure (Mortensen et al. 2014); and (iii) aerobic capacity is decreased by 17% in astronauts on the ISS despite the use of exercise countermeasures (Moore et al. 2014). Thus, a lower aerobic fitness cannot contribute to decreased systemic vascular resistance and blood pressure.
Heart rate
Ambulatory heart rate did not change in space, which is in contrast to the results reported by Fritsch-Yelle et al. (1996), who observed a significant decrease within 12 days of flight on the space shuttle. Our data thus show that systemic arterial vasodilatation was the cause of the decrease in blood pressure. Gundel et al. (2002) showed, in one cosmonaut during 438 days of spaceflight on the Russian space station Mir in the mid-1990s, that heart rate decreased more in space during the night than on the ground because, during sleep, the RR-interval increased by some 176 ms. We also observed a significant decrease in heart rate during the sleep period on the ISS, with the lowest values of the whole study and with the least inter-individual variations (Fig. 2). Thus, as noted by Gundel et al. (2002), the parasympathetic drive to the heart is probably stronger in space during sleep than on the ground. This could be a result of the concomitant upper body fluid shift with an increased preload to the heart that we observed.
Spontaneous baroreflexes
In the present study, we did not measure blood pressure or heart rate continuously to estimate heart rate variability and spontaneous baroreflex adaptation to long duration spaceflight. Studies on the Russian space station Mir in the 1990s have shown attenuated baroreflexes during long duration flights (Cooke et al. 2000; Herault et al. 2000), whereas studies on the ISS have not shown any changes compared to ground conditions (Baevsky et al. 2007; Hughson et al. 2012). This discrepancy was suggested to be a result of the efficiency of the exercise countermeasures on the ISS (Hughson et al. 2012). Thus, attenuated baroreflexes probably cannot account for the systemic vasodilatation and blood pressure reduction that we observed. Verheyden et al. (2010) investigated spontaneous baroreflex activity on the ISS and concluded that the operational point of neural cardiovascular regulation in space sets to a level close to that of an Earth-based supine position, suggesting that the arterial resistance vessels are chronically dilated for at least 6 months of spaceflight. This is supported by our data.
Limitations
A criticism of the present study could be that two of our test astronauts were treated for hypertension by angiotensin-converting enzyme inhibitors. We did not, however, observe any differences in their responses versus the other test astronauts, and excluding them would be wrong because we intended to investigate the astronaut population exactly as it is during normal everyday life, although we made sure that no changes in medication occurred over the course of the study (pre-, in-, and post-flight).
Our estimations of sympathetic nervous activity by noradrenaline concentrations in plasma of forearm venous blood could be criticized for not accurately reflecting changes in sympathetic nervous activity because changes in noradrenaline metabolism affect such estimations. However, high sympathetic nervous activity measured invasively by microneurography in peroneal nerves simultaneously with plasma noradrenaline concentrations has also been detected in space in three astronauts by Ertl et al. (2002) compared to the ground-based supine posture, and the changes in sympathetic microneural activity and plasma noradrenaline exhibited similar patterns of increase during lower body negative pressure. It has also been reported that changes in venous plasma noradrenaline concentrations and sympathetic activity estimated by peroneal microneurography correlate during lower body negative pressure in humans (Davy et al. 1998). Our unchanged 24 h heart rate data also indicate that sympathetic nervous activity is not suppressed in space.
Whether changes in ambient conditions could have influenced the results needs to be considered. Ambient CO2 levels on the ISS fluctuate at an average of ∼10 times the values on the ground (0.5% = 3–4 mmHg). Short-term (30 min) exposure to very high levels of inhaled CO2 can increase cardiac output by 1 l min−1, as well as blood pressure and heart rate (Kiely et al. 1996). Thus, it cannot be excluded that the higher CO2 on the ISS somehow contributed to the increase in cardiac output, although it cannot account for the decrease in blood pressure and systemic vascular resistance that we observed.
Perspectives and clinical implications
The major perspective of the present study is that the fluid shift inducing the increases in stroke volume and thus cardiac output was unexpectedly high compared to several previous results obtained from short duration shuttle missions of 1–2 weeks. The mechanisms for this are not clear but could explain why astronauts begin to experience decreases in vision acuity some months into flight and not immediately upon entering weightlessness (Mader et al. 2011). The higher cardiac output in combination with the higher upper body venous pressures in space compared to the awake upright body posture on the ground could induce chronic increases in intracranial pressures in space that are augmented over time and eventually manifest themselves as ocular changes. A future task would be to correlate the severity of vision changes in space with changes in stroke volume and cardiac output compared to the upright body posture on the ground.
Other perspectives regarding the outcomes of the present study are that, within the normal healthy range, a decrease of 10 mmHg and 5 mmHg in 24 h ambulatory systolic and diastolic arterial pressure, respectively, could reduce the relative risk of cardiovascular mortality (Chobanian et al. 2003) and as indicated by Hansen et al. (2005) by some 34% and 30%. Therefore, from a health perspective, the systemic vasodilatation and lower ambulatory blood pressure in space and the maintained nightly dipping might be beneficial for astronauts during long duration missions.
Finally, our results provide a more fundamental understanding of the chronic effects of gravity on Earth on an individual's blood pressure. That weightlessness in space during months of flight leads to a decrease in ambulatory blood pressure indicates that gravity per se raises blood pressure. Whether this has any significance for the development of hypertension with age is worthy of consideration.
Conclusions
In conclusion, in the present study, we show that 3–6 months of weightlessness in space leads to (i) an increase in cardiac stroke volume and output of 35% and 41%; (ii) a decrease in 24 h ambulatory brachial systolic, diastolic and mean arterial pressures of 8–10 mmHg; and (iii) the maintainance of a nightly blood pressure dipping of 8 mmHg during the sleep period. All of these changes are more pronounced than reported previously from earlier shorter space shuttle flights. Systolic arterial pressure was significantly decreased even during the sleep period in space compared to the same period on the ground. The reduction in blood pressure is induced by a decrease in systemic vascular resistance of 39% by dilatation of the arterial resistance vessels. Because sympathetic nervous activity as monitored by the venous plasma concentration of noradrenaline is unchanged in space, the mechanism responsible for the vasodilatation is unknown.
Acknowledgments
We are grateful to the astronauts for participating as operators and test subjects; to Poul Knudsen and colleagues at Danish Aerospace Company, Odense, Denmark; to Alain Maillet and colleagues at CADMOS, CNES (French National Space Center), Toulouse, France; to Jacob Utzon Frank and Inge Hornung Pedersen at the Department of Biomedical Sciences, University of Copenhagen, Denmark; to Ulla Kjaerulff-Hansen, Endocrine Laboratory, Herlev Hospital, Denmark; to Patrik Sundblad, Simone Thomas and Jennifer Ngo-Anh at ESTEC, ESA, Noordwijk, The Netherlands; and to Suzanne McCollum and Kathleen McMonigal and their staffs at Johnson Space Center, NASA, Houston, TX, USA.
Glossary
Abbreviations
- ESA
European Space Agency
- ISS
International Space Station
Additional information
Competing interests
None.
Author contributions
P.N. conceived and designed the experiment, supervised and participated in the collection, analysis and interpretation of the data, and wrote the manuscript. A.A. participated in data collection, analysis, statistics and interpretation, and also contributed to the writing of the manuscript. M.D. participated in data collection and interpretation, as well as the final preparation of the manuscript. N.J.C. participated in designing the experiment and in data collection and interpretation, performed the catecholamine analyses, and contributed to the writing of the manuscript. All authors approved the final version of the manuscript submitted for publication. The experiments were conducted at (i) the European Astronaut Centre in Cologne, Germany; (ii) Building nine at Johnson Space Centre, Houston, TX, USA; and (iii) on the ISS.
Funding
This work was supported by grant numbers 2105-04-0006 and 272-07-0614 from the Danish Research Agency and the Danish Agency for Science, Technology and Innovation, Copenhagen, Denmark.
References
- Baevsky RM, Baranov VM, Funtova II, Diedrich A, Pashenko AV, Chernikova AG, Drescher J, Jordan J. Tank J. Autonomic cardiovascular and respiratory control during prolonged spaceflights aboard the International Space Station. J Appl Physiol. 2007;103:156–161. doi: 10.1152/japplphysiol.00137.2007. [DOI] [PubMed] [Google Scholar]
- Bringard A, Pogliaghi S, Adami A, Roia GD, Lador F, Lucini D, Pizzinelli P, Capelli C. Ferretti G. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Resp Physiol Neurobiol. 2010;172:53–62. doi: 10.1016/j.resp.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Buckey JC, Jr, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW, Jr, Meyer DM. Blomqvist CG. Central venous pressure in space. J Appl Physiol. 1996;81:19–25. doi: 10.1152/jappl.1996.81.1.19. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT., Jr Rocella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Ames IVJE, Crossman AA, Cox JF, Kuusela TA, Tahvanainen KUO, Moon LB, Drescher J, Baisch FJ, Mano T, Levine BD, Blomqvist CG. Eckberg DL. Nine months in space: effects on human autonomic cardiovascular regulation. J Appl Physiol. 2000;89:1039–1045. doi: 10.1152/jappl.2000.89.3.1039. [DOI] [PubMed] [Google Scholar]
- Cox JF, Tahvanainen KUO, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC, Baisch FJ, Blomqvist CG, Robertson D. Eckberg DL. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva's manoeuvre. J Physiol. 2002;538:309–320. doi: 10.1113/jphysiol.2001.012574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard M. Norsk P. Effects of ventilation on cardiac output determined by inert gas rebreathing. Clin Physiol Funct Imaging. 2005;25:142–147. doi: 10.1111/j.1475-097X.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Davy KP, Seals DR. Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- de Bold AJ. Thirty years of research on atrial natriuretic factor: historical background and emerging concepts. Can J Physiol Pharmacol. 2011;89:527–531. doi: 10.1139/y11-019. [DOI] [PubMed] [Google Scholar]
- Di Rienzo M, Castiglioni P, Iellamo F, Volterrani M, Pagani M, Mancia G, Karemaker JM. Parati G. Dynamic adaptation of cardiac baroreflex sensitivity to prolonged exposure to microgravity: data from a 16-day spaceflight. J Appl Physiol. 2008;105:1569–1575. doi: 10.1152/japplphysiol.90625.2008. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Halliwill JR, Beigthol LA, Brown TE, Taylor JA. Goble R. Human vagal baroreflex mechanisms in space. J Physiol. 2010;588:1129–1138. doi: 10.1113/jphysiol.2009.186650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl AC, Diedrich A, Biaggioni I, Levine BD, Robertson RM, Cox JF, Zuckerman JH, Pawelczyk JA, Ray CA, Buckey JC, Jr, Lane LD, Shiavi R, Gaffney FA, Costa F, Holt C, Blomqvist CG, Eckberg DL, Baisch FJ. Robertson D. Human muscle sympathetic nerve activity and plasma norepinephrine kinetics in space. J Physiol. 2002;538:321–329. doi: 10.1113/jphysiol.2001.012576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch-Yelle JM, Charles JB, Jones MM. Wood ML. Microgravity decreases heart rate and arterial pressure in humans. J Appl Physiol. 1996;80:910–914. doi: 10.1152/jappl.1996.80.3.910. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A. Norsk P. Effect of spaceflight on the subcutaneous venoarteriolar reflex in the human lower leg. J Appl Physiol. 2007;103:959–962. doi: 10.1152/japplphysiol.00899.2006. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A, Videbaek R, Schou M, Damgaard M, Kastrup J. Norsk P. Non-invasive measurement of cardiac output in heart failure patients using a new foreign gas rebreathing technique. Clin Sci. 2003;102:247–252. [PubMed] [Google Scholar]
- Gundel A, Drescher J, Spatenko YA. Polyakov VV. Changes in basal heart rate in spaceflights up to 438 days. Aviat Space Environ Med. 2002;73:17–21. [PubMed] [Google Scholar]
- Hamilton DR, Sargsyan AE, Martin DS, Garcia KM, Melton SL, Feiveson A. Dulchavsky SA. On-orbit prospective echocardiography on International Space Station crew. Echocardiography. 2011;28:491–501. doi: 10.1111/j.1540-8175.2011.01385.x. [DOI] [PubMed] [Google Scholar]
- Hansen TW, Jeppesen J, Rasmussen S, Ibsen H. Torp-Pedersen C. Ambulatory blood pressure and mortality – a population-based study. Hypertension. 2005;45:499–504. doi: 10.1161/01.HYP.0000160402.39597.3b. [DOI] [PubMed] [Google Scholar]
- Henriksen O. Local sympathetic reflex mechanism in regulation of blood flow in human subcutaneous adipose tissue. Acta Physiol Scand (Suppl) 1977;450:1–48. [PubMed] [Google Scholar]
- Herault S, Fomina G, Alferova I, Kotovskaya A, Poliakov V. Arbeille P. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets) Eur J Appl Physiol. 2000;81:384–390. doi: 10.1007/s004210050058. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Shoemaker JH, Blaber AP, Arbeille P, Greaves DK, Pereira-Junior PP. Xu D. Cardiovascular regulation during long-duration spaceflights to the International Space Station. J Appl Physiol. 2012;112:719–727. doi: 10.1152/japplphysiol.01196.2011. [DOI] [PubMed] [Google Scholar]
- Johansen LB, Foldager N, Stadeager C, Kristensen MS, Bie P, Warberg J, Kamegai M. Norsk P. Plasma volume, fluid shifts, and renal responses in humans during 12 hours of head-out water immersion. J Appl Physiol. 1992;73:539–544. doi: 10.1152/jappl.1992.73.2.539. [DOI] [PubMed] [Google Scholar]
- Johansen LB, Gharib C, Allevard AM, Sigaudo D, Christensen NJ, Drummer C. Norsk P. Hematocrit, plasma volume and norepinephrine in humans during simulated weightlessness for 42 days. Clin Physiol. 1997;17:203–210. doi: 10.1046/j.1365-2281.1997.02626.x. [DOI] [PubMed] [Google Scholar]
- Karemaker JM. Berecki-Gisolf J. 24-h blood pressure in space: the dark side of being an astronaut. Resp Physiol Neurobiol. 2009;169S:S55–S58. doi: 10.1016/j.resp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Kiely DG, Cargill RI. Lipworth BJ. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109:1215–1221. doi: 10.1378/chest.109.5.1215. [DOI] [PubMed] [Google Scholar]
- Leach CS, Alfrey CP, Suki WN, Leonard JI, Rambaut PC, Inners LD, Smith SM, Lane HW. Krauhs JM. Regulation of body fluid compartments during short-term spaceflight. J Appl Physiol. 1996;81:105–116. doi: 10.1152/jappl.1996.81.1.105. [DOI] [PubMed] [Google Scholar]
- Leosco D, Parisi V, Femminella GD, Formisano R, Petraglia L, Allocca E. Bonaduce D. Effects of exercise training on cardiovascular adrenergic system. Front Physiol. 2013;4:348. doi: 10.3389/fphys.2013.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R. Polk JD. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in Astronauts after long-duration space flight . Ophthalmology. 2011;118:2058–2069. doi: 10.1016/j.ophtha.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Moore AD, Downs ME, Lee SM, Feiveson AH, Knudsen P. Ploutz-Snyder L. Peak exercise oxygen uptake during and following long-duration spaceflight. J Appl Physiol. 2014;117:231–238. doi: 10.1152/japplphysiol.01251.2013. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Gliemann L, Thaning P, Saltin B. Hellsten Y. Exercise training modulates functional sympatholysis and α-adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J Physiol. 2014;592:3063–3073. doi: 10.1113/jphysiol.2014.273722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsk P. Blood pressure regulation IV: adaptive responses to weightlessness. Eur J Appl Physiol. 2014;114:481–497. doi: 10.1007/s00421-013-2797-2. [DOI] [PubMed] [Google Scholar]
- Norsk P, Christensen NJ, Bie P, Gabrielsen A, Heer M. Drummer C. Unexpected renal responses in space. Lancet. 2000;356:1577–1578. doi: 10.1016/s0140-6736(00)03135-4. [DOI] [PubMed] [Google Scholar]
- Norsk P, Damgaard M, Petersen L, Gybel M, Pump B, Gabrielsen A. Christensen NJ. Vasorelaxation in space. Hypertension. 2006;47:69–73. doi: 10.1161/01.HYP.0000194332.98674.57. [DOI] [PubMed] [Google Scholar]
- Petersen LG, Damgaard M, Petersen JC. Norsk P. Mechanisms of increase in cardiac output during acute weightlessness in humans. J Appl Physiol. 2011;111:407–411. doi: 10.1152/japplphysiol.01188.2010. [DOI] [PubMed] [Google Scholar]
- Prisk GK, Guy HJ, Elliott AR, Deutschman RA. West JB. Pulmonary diffusing capacity, capillary blood volume and cardiac output during sustained microgravity. J Appl Physiol. 1993;75:15–26. doi: 10.1152/jappl.1993.75.1.15. [DOI] [PubMed] [Google Scholar]
- Pump B, Kamo T, Gabrielsen A, Bie P, Christensen NJ. Norsk P. Central volume expansion is pivotal for sustained decrease in heart rate during seated to supine posture change. Am J Physiol Heart Circ Physiol. 2001;281:H1274–H1279. doi: 10.1152/ajpheart.2001.281.3.H1274. [DOI] [PubMed] [Google Scholar]
- Pump B, Schou M, Gabrielsen A. Norsk P. Contribution of the leg vasculature to hypotensive effects of an antiorthostatic posture change in humans. J Physiol. 1999;519:623–628. doi: 10.1111/j.1469-7793.1999.0623m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi M, Schou M, Gybel M, Christensen NJ. Norsk P. Comparison of acute cardiovascular responses to water immersion and head-down tilt in humans. J Appl Physiol. 2002;92:264–268. doi: 10.1152/jappl.2002.92.1.264. [DOI] [PubMed] [Google Scholar]
- Shykoff BE, Fahri LE, Olszowka AJ, Pendergast DR, Rokitka MA, Eisenhardt CG. Morin RA. Cardiovascular response to submaximal exercise in sustained microgravity. J Appl Physiol. 1996;81:26–32. doi: 10.1152/jappl.1996.81.1.26. [DOI] [PubMed] [Google Scholar]
- Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L. Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–1906. doi: 10.1002/jbmr.1647. [DOI] [PubMed] [Google Scholar]
- Stadeager C, Johansen LB, Warberg J, Christensen NJ, Foldager N, Bie P. Norsk P. Circulation, kidney function and volume-regulating hormones during prolonged water immersion in humans. J Appl Physiol. 1992;73:530–538. doi: 10.1152/jappl.1992.73.2.530. [DOI] [PubMed] [Google Scholar]
- Thornton WE. Hoffler GW. Hemodynamic studies of the legs under weightlessness. In: Dietlein LF, editor; Johnston RS, editor. Biomedical Results from Skylab. NASA SP-377. Washington, DC, USA: NASA; 1977. pp. 324–329. [Google Scholar]
- Verheyden B, Liu J, Beckers F. Aubert AE. Adaptation of heart rate and blood pressure to short and long duration space missions. Resp Physiol Neurobiol. 2009;169S:S13–S16. doi: 10.1016/j.resp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Verheyden B, Liu J, Beckers F. Aubert AE. Operational point of neural cardiovascular regulation in humans up to 6 months in space. J Appl Physiol. 2010;108:646–654. doi: 10.1152/japplphysiol.00883.2009. [DOI] [PubMed] [Google Scholar]
- Videbaek R. Norsk P. Atrial distension in humans during microgravity induced by parabolic flights. J Appl Physiol. 1997;83:1862–1866. doi: 10.1152/jappl.1997.83.6.1862. [DOI] [PubMed] [Google Scholar]
- Watenpaugh DE, Buckey JC, Lane LD, Gaffney FA, Levine BD, Moore WE, Wright SJ. Blomqvist CG. Effects of spaceflight on human calf hemodynamics. J Appl Physiol. 2001;90:1552–1558. doi: 10.1152/jappl.2001.90.4.1552. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Loehr JA. Guilliams ME. Sensorimotor reconditioning during and after spaceflight. NeuroRehab. 2011;29:185–195. doi: 10.3233/NRE-2011-0694. [DOI] [PubMed] [Google Scholar]
- Zhang LF. Vascular adaptation to microgravity: what have we learned. J Appl Physiol. 2001;91:2415–2430. doi: 10.1152/jappl.2001.91.6.2415. [DOI] [PubMed] [Google Scholar]
- Zwart SR, Launius RD, Coen GK, Morgan JLL, Charles JB. Smith SM. Body mass changes during long-duration spaceflight. Aviat Space Environ Med. 2014;85:897–904. doi: 10.3357/ASEM.3979.2014. [DOI] [PubMed] [Google Scholar]


