Abstract
Intraduodenal fatty acids (FA) and bacterial overgrowth, which generate short-chain FAs (SCFAs), have been implicated in the generation of functional dyspepsia symptoms. We studied the mechanisms by which luminal SCFA perfusion affects duodenal HCO3− secretion (DBS), a measure of mucosal neurohumoral activation. Free fatty acid receptor (FFAR) 1 (FFA1), which binds long-chain FA (LCFA), and SCFA receptors FFA2 and FFA3 were immunolocalised to duodenal enteroendocrine cells. FFA3 colocalised with glucagon-like peptide (GLP)-1, whereas FFA2 colocalised with 5-HT. Luminal perfusion of the SCFA acetate or propionate increased DBS, enhanced by dipeptidyl peptidase-IV (DPPIV) inhibition, at the same time as increasing GLP-2 portal blood concentrations. Acetate-induced DBS was partially inhibited by monocarboxylate/HCO3− exchanger inhibition without affecting GLP-2 release, implicating acetate absorption in the partial mediation of DBS. A selective FFA2 agonist dose-dependently increased DBS, unaffected by DPPIV inhibition or by cholecystokinin or 5-HT3 receptor antagonists, but was inhibited by atropine and a 5-HT4 antagonist. By contrast, a selective FFA1 agonist increased DBS accompanied by GLP-2 release, enhanced by DPPIV inhibition and inhibited by a GLP-2 receptor antagonist. Activation of FFA1 by LCFA and presumably FFA3 by SCFA increased DBS via GLP-2 release, whereas FFA2 activation stimulated DBS via muscarinic and 5-HT4 receptor activation. SCFA/HCO3− exchange also appears to be present in the duodenum. The presence of duodenal fatty acid sensing receptors that signal hormone release and possibly signal neural activation may be implicated in the pathogenesis of functional dyspepsia.
Key points
Luminal lipid in the duodenum modulates gastroduodenal functions via the release of gut hormones and mediators such as cholecystokinin and 5-HT.
The effects of luminal short-chain fatty acids (SCFAs) in the foregut are unknown.
Free fatty acid receptors (FFARs) for long-chain fatty acids (LCFAs) and SCFAs are expressed in enteroendocrine cells. SCFA receptors, termed FFA2 and FFA3, are expressed in duodenal enterochromaffin cells and L cells, respectively.
Activation of LCFA receptor (FFA1) and presumed FFA3 stimulates duodenal HCO3− secretion via a glucagon-like peptide (GLP)-2 pathway, whereas FFA2 activation induces HCO3− secretion via muscarinic and 5-HT4 receptor activation.
The presence of SCFA sensing in the duodenum with GLP-2 and 5-HT signals further supports the hypothesis that luminal SCFA in the foregut may contribute towards the generation of functional symptoms.
Introduction
Postprandial nutrient sensing in the gastrointestinal mucosa is mediated by nutrient-sensing G protein-coupled receptors (GPCRs) expressed in the apical membranes of hormone-releasing enteroendocrine cells (Engelstoft et al. 2008). The presence of nutrients in the foregut lumen activates neuronal and endocrine mechanisms that bolster mucosal protective responses as a possible means to prevent post-prandial mucosal injury (Dockray, 2003; Akiba & Kaunitz, 2011a). Stimulation of nutrient-sensing receptors expressed on enteroendocrine L cells is emerging as a treatment for diabetes because L cells release an incretin, glucagon-like peptide (GLP)-1 (Drucker & Nauck, 2006). L cells also release GLP-2, another proglucagon-derived intestinotrophic hormone of clinical utility (Drucker et al. 1996).
We have previously demonstrated that the activation of an amino acid receptor, taste receptor 1 family heterodimer T1R1/T1R3, known as the umami receptor, by luminal perfusion of l-glutamate and 5′-inosine monophosphate increases duodenal HCO3− secretion via GLP-2 release and GLP-2 receptor activation, followed by nitric oxide and vasoactive intestinal peptide (VIP) release (Akiba et al. 2009; Wang et al. 2011). These studies suggest that the stimulation of nutrient-sensing GPCRs expressed on L cells by luminal small molecules not only affects glucose homeostasis, but also protects the duodenal mucosa from injury via the GLP-2 pathway. Furthermore, inhibition of dipeptidyl peptidase IV (DPPIV), the principal metabolic enzyme for numerous hormones, including GLP-1 and GLP-2 (Lambeir et al. 2003), enhances GLP-2 pathway-induced HCO3− secretion (Inoue et al. 2012), suggesting that the combination of DPPIV inhibition and luminal nutrient augments GLP-1 and -2 concentrations. Because experimental DPPIV inhibition prolongs the half-life of multiple hormones (Lambeir et al. 2003), enhancement of the rate of nutrient-responsive HCO3− secretion by DPPIV inhibition strongly supports the mediation of HCO3− secretion by peptide hormones. DPPIV inhibition prevents the formation and accelerates the healing of indomethacin-induced intestinal injury via GLP-2 release, and outcomes are enhanced further by luminal umami substances (Inoue et al. 2014), suggesting that DPPIV inhibition could be a useful therapeutic for the treatment of intestinal mucosal injury.
Intraduodenal lipid has been implicated in the generation of functional dyspepsia (FD) symptoms, such as epigastric pain, bloating and stomach fullness (Fried & Feinle, 2002; Akiba & Kaunitz, 2011b). These symptoms are attributed to cholecystokinin (CCK) release and vagal afferent activation via the CCK1 receptor (Fried & Feinle, 2002). The orphan GPCRs, GPR40 (also termed free fatty acid receptor 1; FFA1) and GPR120 (FFA4), were identified as long-chain fatty acid (LCFA) receptors (Briscoe et al. 2003) that represent attractive candidates for luminal lipid sensors. Activation of FFA1 by luminal LCFA releases CCK from endocrine cell lines or purified enteroendocrine I cells (Liou et al. 2011), whereas FFA1 is also expressed in GLP-1 secreting L cells (Edfalk et al. 2008). Nevertheless, the involvement of FFA1 has not yet been reported in the context of duodenal HCO3− secretion.
Other free fatty acid receptors (FFARs) include FFA2/GPR43 and FFA3/GPR41, which are activated by short-chain fatty acids (SCFAs) (Brown et al. 2003). FFA2 and FFA3 are expressed in L cells present in the ileum and colon, colocalised with peptide YY (Karaki et al. 2006; Tazoe et al. 2009). Luminal SCFAs directly activate FFA2 or FFA3, increasing GLP-1 release from L cells (Tolhurst et al. 2012). Although luminal SCFAs exist in the lower small intestine and large intestine in ∼100 mm concentrations as a consequence of bacterial fermentation of non-digestible fibres (Cummings et al. 1987), little is known about foregut SCFA physiology.
Here, we show that luminal perfusion of the selective FFA1 and FFA2 agonists, as well as non-specific ligands for FFA2 and FFA3, differentially stimulates duodenal HCO3− secretion. Activation of FFA1, and presumably activation of FFA3, stimulates HCO3− secretion via GLP-2 release, whereas HCO3− secretion in response to FFA2 activation occurs via muscarinic and 5-HT4 receptors. Furthermore, the production of SCFAs by gut flora and the observation that luminal lipids activate the muscarinic, serotonergic and CCK pathways implicated in the generation of FD symptoms provides a novel and plausible mechanism by which small intestinal bacterial overgrowth can aggravate functional gastrointestinal symptoms.
Methods
Chemicals and animals
NVP DPP 728 dihydrochloride (NVP728), GW9508, tele-nzepine, J104129, GR113808, SR27897 and VIP6–28 were obtained from Tocris Bioscience (Ellisville, MO, USA). Phenylacetamide 1 [PA1; (S)-2-(4-chlorophenyl)-3-methyl-N-(thiazol-2-yl)butanamide] was synthesized, purified and verified in the Laboratory of Organic Chemistry, School of Pharmaceutical Sciences, University of Shizuoka, Japan, in accordance with the published chemical structure (Lee et al. 2008). Rat GLP-2(3–33) was synthesized by Bachem Americas, Inc. (Torrance, CA, USA). Atropine sulphate was obtained from Butler (Dublin, OH, USA). Sodium acetate, sodium propionate, ondansetron, Hepes and other chemicals were obtained from Sigma (St Louis, MO, USA). Krebs solution contained (in mm) 136 NaCl, 2.6 KCl, 1.8 CaCl2 and 10 Hepes at pH 7.0. Osmolality was adjusted to isotonicity by reducing the NaCl concentration. The pH of Krebs solution after dissolving the compound was adjusted to pH 7.0. All solutions were prewarmed at 37°C in a water bath; the temperature was maintained with a heating stirrer or heating pad. All studies were performed with the approval of the Veterans Affairs Institutional Animal Care and Use Committee. Antibody production was performed in accordance with the guidelines for the care and use of laboratory animals of Hokkaido University School of Medicine. Male Sprague–Dawley rats weighing 200–250 g (Harlan, San Diego, CA, USA) were fasted overnight, but had free access to water. Animals were killed by terminal exsanguination under deep isoflurane anaesthesia, followed by thoracotomy.
Antibody production
We produced the affinity-purified primary antibody RK1101, which was raised against rat FFA2. A cDNA fragment, which is preceded with a BamHI site and encodes C-terminal 30 amino acids of FFA2 (301–330 amino acid residues; GenBank NM 001005877), was obtained by PCR. After carrying out thymidine/adenosine cloning using a pGEM-T Easy Vector System I kit (Promega, Madison, WI, USA), the cDNA fragment was subcloned into the BamHI/EcoRI site of the pGEX4T-2 plasmid (Pharmacia Biotech AB, Uppsala, Sweden) and Escherichia coli BL21 for expression of glutathione S-transferase (GST) fusion proteins. GST fusion proteins were purified using glutathione-Sepharose 4B, in accordance with the manufacturer's instructions (Pharmacia Biotech AB). Fusion proteins emulsified with Freund's complete or incomplete adjuvant (Difco, Detroit, MI, USA) were injected s.c. into a female New Zealand White rabbit at intervals of 2 weeks. Antiserum sampled 2 weeks after the sixth injection was affinity-purified using CNBr-activated Sepharose 4B (Pharmacia Biotech AB) coupled with GST-free polypeptides as obtained by in-column thrombin digestion of fusion proteins.
Western blotting
Western blot analysis for FFA2 using RK1101 was performed as described previously (Akiba et al. 2008). The scraped mucosa of rat proximal duodenum was homogenized in 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.05% Triton X and 1% protease inhibitor cocktail (Sigma). After centrifugation at 10,000 g for 10 min at 4°C, supernatant protein samples were reduced and denatured in Laemmli buffer, followed by electrophoresis in a 4–20% gradient gel (Bio-Rad Laboratories, Hercules, CA, USA) and electroblotted onto polyvinylidene difluoride membranes (Thermo Fisher Scientific, Rockford, IL, USA). After blocking with 0.5% skimmed milk at 4°C overnight, the membranes were incubated with rabbit anti-FFA2 antibody (RK1101; 1 μg ml−1) for 2 h at room temperature, followed by incubation with alkaline phosphatase-conjugated secondary antibody at a dilution of 1:3000 (Chemicon, Temecula, CA, USA). The immunoreaction was visualized with chromogenic substrate solution (Sigma). As a negative control, pre-absorbed RK1101 solution was used after incubation with the GST-free antigen peptide described above at 100 μg ml−1 for 30 min.
Localisation of FFARs in rat duodenum
FFA1, FFA2 and FFA3 immunolocalisation was carried out on cryostat sections of Zamboni-fixed tissues incubated with goat anti-FFA1 antibody (dilution 1:100, sc-28417; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), rabbit anti-FFA2 antibody (RK1101; 1 μg ml−1) or rabbit anti-FFA3 antibody (dilution 1:100, sc-98332; Santa Cruz Biotechnology Inc.), followed by incubation with Alexa488 or Alexa594 secondary antibody (Molecular Probes, Eugene, OR, USA). Some were double-labelled with goat anti-GLP-1 antibody (dilution 1:200, sc-7782; Santa Cruz Biotechnology Inc.) or mouse anti-5-HT antibody (dilution 1:100, MCA3190Z; AbD Serotec, Kidlington, UK), followed by incubation with the corresponding Alexa488 secondary antibody (Molecular Probes). Fluorescence was observed with an Axio Observer Z1 microscope (Zeiss, Munich-Harbergmoons, Germany) or a confocal laser microscope (FV300; Olympus, Tokyo, Japan; LSM-710; Zeiss). Negative controls were processed identically, with the omission of the primary antibody or with incubation with primary antibody pre-absorbed with the immunizing peptide (100 μg ml−1). Furthermore, rat FFA2 and FFA3 cDNA were amplified by PCR, and inserted into the pTracer-CMV2 mammalian expression plasmid (Invitrogen, Carlsbad, CA, USA). The plasmid was transfected into human embryonic kidney (HEK293T) cells using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions. The transfected HEK293T cells were immunostained with RK1101 antibody.
Expression of FFA2 in the tissues was also analysed by real time RT-PCR as described previously (Akiba et al. 2009). The PCR primers of rat FFA2 were sense (5′-CACCGAGAACCAAATCACCT-3′) and anti-sense (5′-GAGGGACTCTGCCTCAAGTG-3′), giving rise to a 288 bp PCR product, and β-actin was used as an internal control. The expression level was presented as the fold induction per 103 copies of β-actin by the ΔCt method.
Measurement of duodenal HCO3− secretion
Duodenal loops were prepared and perfused under isoflurane anaesthesia as described previously (Mizumori et al. 2006; Akiba et al. 2007, 2009). After stabilisation with continuous perfusion of saline (pH 7.0) for ∼30 min, the time was set as t = 0. The duodenal loop was perfused with saline (pH 7.0) from t = 0 min until t = 10 min (basal period). The perfusate was then changed to Krebs solution (pH 7.0) with or without test compounds from t = 10 min until t = 35 min (challenge period). At t = 10 min, the system was gently flushed to rapidly change the perfusate. Duodenal HCO3− secretion was expressed as total CO2 output, calculated from the measured pH and [CO2] in the effluent solution as reported previously (Akiba et al. 2007, 2009).
Experimental protocol
We have previously reported that bolus i.v. injection of the DPPIV inhibitor NVP728 (3 μmol kg−1), 10-min before perfusion of luminal agonists (at t = 0 min), enhances amino acid or bile acid-induced HCO3− secretion. Injection of the GLP-2 receptor antagonist, GLP-2(3–33) (3 nmol kg−1, i.v.) at t = 10 min, just prior to the perfusion of luminal agonists, inhibits HCO3− secretion (Inoue et al. 2012). Therefore, to investigate the involvement of GLP-2 in stimulated HCO3− secretion, NVP728 (3 μmol kg−1) was bolus injected i.v. at t = 0 min, with or without GLP-2(3–33) i.v. at t = 10 min, followed by the luminal perfusion of FFAR agonists.
To examine the effect of LCFA on duodenal HCO3− secretion, the duodenal loop was perfused with the selective FFA1 agonist, GW9508 (10 μm) (Briscoe et al. 2006) with or without prior NVP728 or GLP2(3–33) injection.
To examine the effect of SCFAs on HCO3− secretion, the loop was perfused with acetate or propionate (10 μm to 1 mm), diluted with Krebs solution adjusted to pH 7.0 to eliminate any independent effects of luminal pH, or the selective FFA2 agonist PA1 (0.1–10 μm) (Lee et al. 2008) with or without prior NVP728 injection. To inhibit the monocarboxylate transporter 1 (MCT1), α-cyano-4-hydroxycinnamic acid (4-CHCA; 0.1 or 1 mm) (Wang et al. 1996) was co-perfused with acetate. To further assess the mechanism of PA1, neurotransmitter receptor antagonists were injected i.v. at t = 0: the muscarinic receptor antagonist atropine (0.5 mg kg−1); the M1 receptor antagonist telenzipine (1 mg kg−1); the M3 receptor antagonist J104129 (1 mg kg−1) (Mitsuya et al. 1999); the 5-HT3 receptor antagonist ondansetron (1 mg kg−1); the 5-HT4 receptor antagonist GR113808 (1 mg kg−1) (Gale et al. 1994); the CCK1 receptor antagonist SR27897 (1 mg kg−1) (Gully et al. 1993); or the VIP receptor antagonist VIP6–28 (100 nmol kg−1) (Fishbein et al. 1994; Wang et al. 2011). We also tested the effect of GR113808 or GLP-2(3–33) on acetate-induced HCO3− secretion.
Measurement of GLP-2 in portal venous (PV) blood
The plasma concentration of GLP-2 was measured in the PV blood samples as described previously (Wang et al. 2011; Inoue et al. 2012). The samples were collected after a 25 min challenge using a syringe containing 1 μl each of EDTA (0.5 mm) and NVP728 (10 μm). The samples were immediately centrifuged at 5000 g for 5 min; the separated plasma was stored at −80°C. Plasma was diluted with Tris-HCl buffer (50 mm, pH 7.4) containing a protease inhibitor cocktail (1 mg ml−1; Sigma) and NVP728 (10 μm). The total GLP-2 plasma concentration was measured using a rat GLP-2 enzyme immunoassay kit (ALPCO Diagnostics, Salem, NH, USA) in accordance with the manufacturer's instructions.
Statistical analysis
All data are expressed as the mean ± sem. Data were derived from six rats in each group. Comparisons between groups were made by one-way ANOVA, followed by Fischer's least significant difference test. P < 0.05 was considered statistically significant.
Results
Localisation of FFARs in rat duodenum
FFA2 immunoreactivity using RK1101 was observed in adipocytes in mesenteric white adipose tissue (Fig. 1A) as a positive control (Kimura et al. 2013), whereas pre-absorption with blocking peptide abolished the staining (Fig. 1B). By contrast, the oesophageal mucosa was negatively stained with RK1101 (Fig. 1C), consistent with low FFA2 expression in the oesophageal mucosa compared to the duodenal mucosa or white adipose tissue (Fig. 1D). A 35 kDa band was present in duodenal mucosa detected with RK1101, close to the predicted size of rat FFA2 (37 kDa), whereas pre-absorption abolished the band (Fig 1E). Furthermore, the RK1101 antibody positively immunostained FFA2-transfected HEK293T cells, whereas immunostaining of FFA2-transfected HEK293T cells with pre-absorbed antibody, or of FFA3-transfected HEK293T cells or of mock-transfected cells, was negative (Fig. 1F).
Figure 1. Detection of FFA2 using RK1101 antibody in rat tissues.
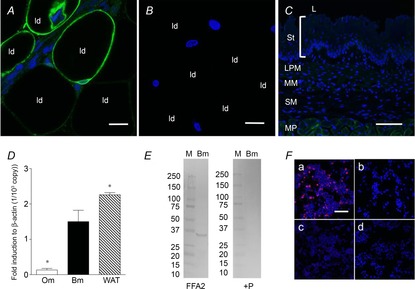
Whole mount mesenteric white adipose tissue or a cryostat section of oesophagus was incubated with anti-FFA2 antibody RK1101 (green) with or without blocking peptide, counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). Mesenteric adipocytes were positively stained (A), whereas pre-absorption abolished the staining (B). ld, lipid droplet. Internal bars: 20 μm. C, oesophageal mucosa, consisting of stratum layers (St), lamina propria mucosae (LPM), muscularis mucosae (MM) and submucosa (SM), was negatively stained, with faint staining observed in the muscularis propria (MP). L, lumen. Internal bars: 100 μm. D, expression of FFA2 in oesophageal mucosa (Om), duodenal bulb mucosa (Bm) and white adipose tissue (WAT) assessed by real time PCR with the ΔCt method. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. Bm. E, Western blot for FFA2 using RK1101 in duodenal mucosa (Bm) (left panel; FFA2) and pre-absorption with blocking peptide (right panel; + P). M, molecular marker with size (kDa) on the left. F, FFA2-transfected cells (a) were positively stained with RK1101 (red), whereas FFA2-transfected cells were negatively stained with pre-absorbed antibody (b). FFA3-transfected cells (c) and mock-transfected cells (d) were negatively stained with RK1101. Counterstained with DAPI (blue). Internal bars: 100 μm.
Immunoreactivity of FFA1, FFA2 and FFA3 was observed in cells of endocrine-like morphology in the duodenal mucosa (Fig. 2A–C). FFA2 colocalised with 5-HT (Fig. 2D) and FFA3 colocalised with GLP-1 (Fig. 2E), consistent with the FFA2 expression on enterochromaffin (EC) cells and FFA3 expression on L cells. These data demonstrate that duodenal endocrine cells express FFA1, 2 or 3.
Figure 2. Localisation of FFARs in rat duodenum.
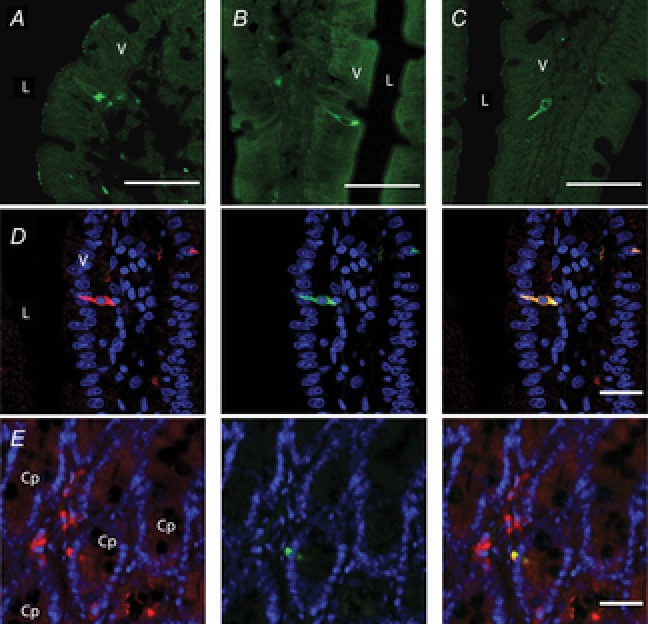
Frozen cryostat sections were incubated with the primary antibody for FFA1 (A), FFA2 (B) or FFA3 (C). L, lumen; V, villus. Internal bars: 100 μm. D, double staining with FFA2 (red; RK1101 antibody, left), 5-HT (green; middle) and merged image (right). Counterstained with DAPI (blue). L, lumen; V, villus. Internal bars: 20 μm. E, double staining with FFA3 (red; left), GLP-1 (green; middle) and merged image (right). Counterstained with DAPI (blue). Cp, crypt. Internal bars: 20 μm.
Effect of an FFA1 agonist on duodenal HCO3− secretion
Luminal perfusion of the FFA1 agonist GW9508 (10 μm) increased the rate of HCO3− secretion, as enhanced by prior injection of the DPPIV inhibitor NVP728 (Fig. 3A), although NVP728 injection had no effect on the basal rate of HCO3− secretion. Furthermore, augmented HCO3− secretion in response to NVP728 and GW9508 was inhibited by the GLP-2 receptor antagonist GLP-2(3–33) (Fig. 3B). These results support the proposed mechanism that activation of FFA1 in the duodenal mucosa increases HCO3− secretion via the GLP-2 pathway.
Figure 3. Effect of FFA1 agonist on duodenal HCO3− secretion in rats.
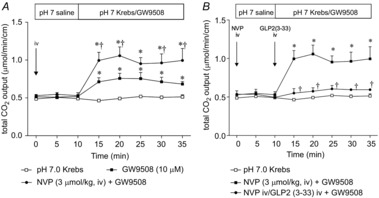
Duodenal HCO3− secretion was measured as total CO2 output using the flow-through pH and CO2 electrodes. The duodenal loop was perfused with GW9508 (10 μm). The DPPIV inhibitor NVP728 (NVP) was injected i.v. (3 μmol kg−1) at t = 0 min (A). The GLP-2 receptor antagonist GLP2(3–33) was additionally injected i.v. (3 nmol kg−1, i.v.) at t = 10 min (B). Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. †P < 0.05 vs. vehicle i.v. + GW9508 group.
Effect of SCFAs on duodenal HCO3− secretion
Next, we examined the effect of luminal perfusion of SCFAs on duodenal HCO3− secretion. Luminal perfusion of acetate dose-dependently increased HCO3− secretion (Fig. 4A), as increased by prior injection of NVP728 (Fig. 4B). Similarly, the perfusion of propionate (0.1 mm) increased HCO3− secretion, as also enhanced by NVP728 i.v. (Fig. 4C). Furthermore, acetate-induced HCO3− secretion was partially inhibited by the MCT1 inhibitor 4-CHCA (1 mm) (Fig. 4D). These results suggest that luminal SCFAs increase HCO3− secretion in part via GLP-2 release, and in part via SCFA transport via MCT1.
Figure 4. Effect of luminal perfusion of SCFA on duodenal HCO3− secretion.
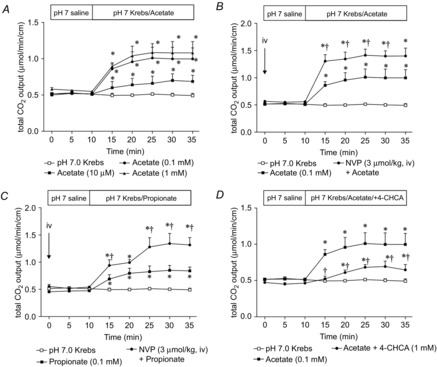
A, luminal perfusion of acetate (10 μm to 1 mm) dose-dependently increased HCO3− secretion. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. B, C, luminal perfusion of acetate (B) or propionate (C) (0.1 mm) increased HCO3− secretion, with the effect enhanced by the DPPIV inhibitor NVP pretreatment (3 μmol kg−1, i.v.). Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. †P < 0.05 vs. SCFA group. D, co-perfusion of 4-CHCA (1 mm) reduced acetate-induced HCO3− secretion. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. †P < 0.05 vs. Acetate group.
Effect of an FFA2 agonist on duodenal HCO3− secretion
The FFA2 agonist PA1 dose-dependently stimulated HCO3− secretion (Fig. 5A), and was not affected by prior injection of NVP728 (Fig. 5B), suggesting that the prosecretory effect of FFA2 activation does not occur via peptide release. We therefore examined the involvement of cholinergic nerves in PA1-induced HCO3− secretion. Atropine injected i.v. inhibited PA1-induced HCO3− secretion (Fig. 6A). The M1 receptor antagonist telenzipine or the M3 antagonist J104129 reduced PA1-induced HCO3− secretion (Fig. 6B). Furthermore, the 5-HT4 receptor antagonist GR113808 abolished PA1-induced HCO3− secretion, whereas the 5-HT3 antagonist ondansetron had no effect (Fig. 6C). The CCK1 receptor antagonist SR27897 or the VIP receptor antagonist VIP6–28 had no effect on PA1-induced HCO3− secretion (Fig. 7A and B). These results suggest that, in contrast to SCFA-induced HCO3− secretion, FFA2 activation stimulates HCO3− secretion via muscarinic and 5-HT4 receptor activation, rather than via CCK, VIP or GLP-2 release.
Figure 5. Effect of luminal perfusion of FFA2 agonist on HCO3− secretion.
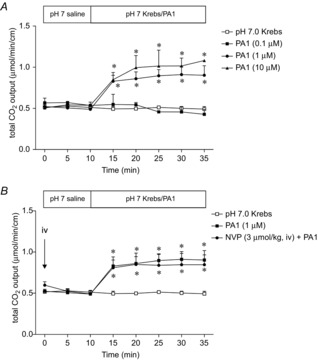
A, luminal perfusion of PA1 (0.1–10 μm) dose-dependently increased HCO3− secretion. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. B, PA1 (1 μm)-induced HCO3− secretion was not affected by the DPPIV inhibitor NVP (3 μmol kg−1, i.v.). Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group.
Figure 6. Effect of muscarinic or 5-HT receptor antagonist on PA1-induced HCO3− secretion.
Muscarinic or 5-HT receptor antagonist was injected i.v. at t = 0 min. PA1 (1 μm)-induced HCO3− secretion was inhibited by atropine (0.5 mg kg−1) (A), telenzipine or J104129 (1 mg kg−1) (B). C, 5-HT4 antagonist GR113808 (1 mg kg−1) abolished PA1-induced HCO3− secretion, whereas 5-HT3 receptor antagonist ondansetron (1 mg kg−1) had no effect. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group. †P < 0.05 vs. PA1 group.
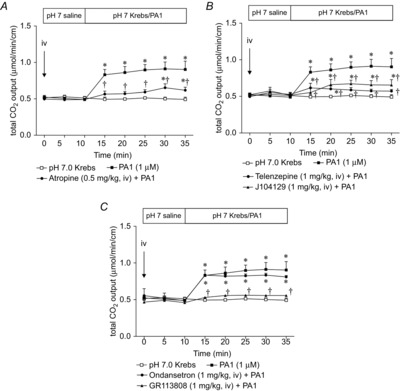
Figure 7. Effect of CCK or VIP receptor antagonist on PA1-induced HCO3− secretion.
CCK1 receptor antagonist SR27897 (1 mg kg−1) or VIP receptor antagonist VIP6–28 (100 nmol kg−1) was injected i.v. at t = 0 min. PA1 (1 μm)-induced HCO3− secretion was not affected by SR27897 (A) or VIP6–28 (B). Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group.
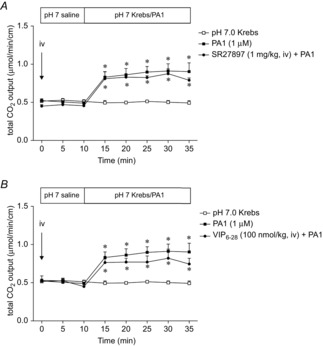
Furthermore, acetate-induced HCO3− secretion was reduced by combination of GR113808 and GLP-2(3–33), although each antagonist alone had little effect (Fig. 8A–C). These results support our hypothesis that luminal SCFAs activate both FFA2 and FFA3, followed by 5-HT and GLP-2 release, respectively.
Figure 8. Effect of 5-HT4 receptor or GLP-2 receptor antagonist on acetate-induced HCO3− secretion.
GR113808 (1 mg kg−1) was injected i.v. at t = 0 min, whereas GLP-2(3–33) was injected i.v. (3 nmol kg−1, i.v.) at t = 10 min, followed by luminal acetate (0.1 mm) perfusion. GR113808 (A) or GLP-2(3–33) (B) had little effect on acetate-induced HCO3− secretion. Pre-treatment of both antagonists reduced acetate-induced HCO3− secretion (C). Each data point represents the mean ± SEM (n = 4–6 rats). *P < 0.05 vs. pH 7.0 Krebs group. †P < 0.05 vs. Acetate group.
GLP-2 release into PV blood
We measured GLP-2 concentrations in PV blood plasma at baseline and after the challenge period (Fig. 9). Luminal perfusion of GW9508 increased PV GLP-2 concentrations, and was increased further by DPPIV inhibition (Fig. 9A), consistent with GLP-2-mediated HCO3− secretion (Fig. 3). Luminal acetate also increased PV GLP-2 concentrations, as enhanced further by NVP i.v. injection, whereas MCT1 inhibition with 4-CHCA or 5-HT4 receptor antagonism with GR113808 had no additional effect (Fig. 9B), supporting our hypothesis that acetate increases HCO3− secretion via GLP-2-dependent and independent pathways, and via SCFA absorption. By contrast, PA1 had no effect on GLP-2 release (Fig. 9C), which is consistent with HCO3− secretion via the GLP-2 independent, 5-HT/cholinergic pathway.
Figure 9. Effect of luminal perfusion of fatty acid receptor agonists on GLP-2 release.
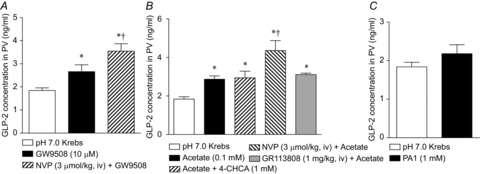
GLP-2 concentration was measured in PV plasma. The duodenal loop was perfused with GW9508 (10 μm, A), acetate (0.1 mm, B) or PA1 (1 μm, C) with or without inhibitors. PV blood was collected at t = 35 min. Each data point represents the mean ± SEM (n = 6 rats). *P < 0.05 vs. pH 7.0 Krebs group.
Discussion
We have demonstrated that luminal perfusion of fatty acids differentially increased duodenal HCO3− secretion via several mechanisms (Fig. 10). LCFA increased HCO3− secretion via FFA1 activation that released GLP-2. SCFAs activated FFA2 and FFA3, which in turn activated different mechanisms: FFA3 agonists may release GLP-2, whereas FFA2 agonists activated a 5-HT/cholinergic pathway. Furthermore, SCFAs absorbed by epithelial cells may exchange for secreted HCO3− via an apical membrane exchange transporter. This is the first study to demonstrate that SCFA sensors are present in the duodenal mucosa and that activation of SCFA receptors increases the rate of protective HCO3− secretion via hormone release and possible neural mechanisms, similar to the mechanism in which duodenal LCFA releases CCK (Holzer et al. 1994; Liou et al. 2011).
Figure 10. Proposed mechanism of duodenal SCFA sensing.
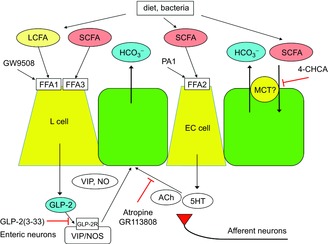
Luminal SCFAs, derived from diet or bacterial metabolism, activate multiple pathways. SCFAs may activate FFA3 on L cells, which release GLP-2, which in turn activates GLP-2 receptors expressed on myenteric neurons (Guan et al. 2006), followed by the release of VIP and NO (Wang et al. 2011), stimulating epithelial HCO3− secretion by established pathways. The actions of GLP-2 are enhanced by DPPIV inhibition (Inoue et al. 2012). SCFAs also activate FFA2 on EC cells, which release 5-HT and ACh, activating 5-HT4 and muscarinic receptors, respectively, with both expressed on enteric and afferent nerves, and on epithelial cells, also stimulating the rate of HCO3− secretion. SCFAs might be absorbed by SCFA/HCO3− exchanger, possibly by apical membrane MCT1, increasing the rate of HCO3− secretion. By contrast, luminal LCFA increases HCO3− secretion via activation of FFA1 expressed in L cells, which releases GLP-2, augmented by DPPIV inhibition, and inhibited by a GLP-2 receptor antagonist GLP-2(3–33). We propose that GLP-2, 5-HT and ACh, which are released by luminal SCFAs, not only activate the local mechanisms involved in duodenal mucosal defences, but also stimulate afferent nerves, which are implicated in the production of pathological symptoms. SCFA, short-chain fatty acid; LCFA, long-chain fatty acid; FFA1, free fatty acid receptor 1; GLP-2, glucagon-like peptide-2; VIP, vasoactive intestinal peptide; NO, nitric oxide; 5-HT, 5-hydroxytryptamine; Ach, acetylcholine; EC, enterochromaffin; MCT, monocarboxylate transporter; DPPIV, dipeptidyl peptidase IV.
Intestinal SCFA sensing and uptake has been studied primarily in the colon. The concentration of SCFA in hindgut lumen can reach ∼100 mm, especially in the cecum and proximal colon, where intestinal flora ferments undigested fibre and carbohydrates (Illman et al. 1986; Cummings et al. 1987). In the colon, butyrate is consumed in situ by the epithelial cells as a source of cellular energy, whereas acetate and propionate traverse the epithelium to enter the circulation (Cummings et al. 1987). FFA2 and FFA3 are expressed in L cells in the rat and human colon (Karaki et al. 2006, 2008; Tazoe et al. 2009; Kaji et al. 2011), which is consistent with the presence of abundant SCFA in the colonic lumen.
Few studies have addressed the SCFA content of the foregut lumen, under the assumption that the lack of bacterial flora should be associated with low luminal SCFA concentrations. SCFAs, however, are also present in the foregut lumen at 0.1–1 mm, mainly derived from the fermentation of nutrients by oral flora (Hoverstad et al. 1984). Furthermore, condiments, and fermented and preserved foods contain up to a molar range of SCFAs; for example, vinegar contains 4–7% v/v or 0.66–1.17 m acetic acid. Therefore, the foregut mucosa may be exposed to concentrations of SCFAs sufficient to activate cognate sensors such as FFA2 and FFA3. The present study showed that FFA3 was expressed in L cells, whereas FFA2 was expressed in EC cells in the rat duodenum. FFA2 did not colocalise with GLP-1, nor did FFA3 colocalise with 5-HT in the rat duodenum (data not shown), which is an expression pattern different from FFA2 localisation in the colon, and consistent with segmental differences in endocrine cell expression. Foregut SCFA sensing may sense ingested fermented foods, whereas colonic sensors likely sense the products of endogenous fermentation. Therefore, the presence of excessive foregut luminal SCFA concentrations as a result of bacterial overgrowth may stress homeostatic sensing mechanisms, producing unwanted symptomatology.
SCFAs are an important source of energy, especially for ruminants, although, unlike carbohydrates, SCFAs have a low glycaemic index. SCFA treatment improves glycaemic control in healthy subjects and in type 2 diabetic patients (Brighenti et al. 1995; Östman et al. 2005; Liatis et al. 2010). Furthermore, colonic SCFA absorption can provide up to 540 kcal day−1 in humans (Ruppin et al. 1980), helping to meet nutritional needs with ‘nature's afterburner’, especially for patients with short bowel syndrome.
FFA2 and FFA3 are expressed in the enteroendocrine cells, myenteric neurons, and leukocytes of transgenic fluorescent protein reporter mice (Nøhr et al. 2013). FFA3-positive endocrine cells also express GLP-1, which is consistent with our results, whereas FFA2 is weakly expressed in a fraction of enteroendocrine cells that have not yet been characterized. In the present study, activation of FFA2 expressed in 5-HT-positive EC cells with the selective FFA2 agonist PA1 increased the rate of HCO3− secretion via 5-HT4 and muscarinic receptor activation, strongly supporting our hypothesis that FFA2 is functionally expressed in EC cells and that FFA2 activation releases 5-HT, followed by acetylcholine release. The observations that an FFA2 agonist failed to increase GLP-2 release, whereas the mixed FFA2/3 agonist acetate increased GLP-2 release, and that FFA3 is expressed in GLP-1-positive L cells further support our hypothesis that FFA3 activation increases GLP-2 release, followed by GLP-2-mediated HCO3− secretion. The experimental use of selective FFA3 agonists and antagonists, when available, will help clarify this possibility.
The significance of the HCO3− secretory response to luminal fatty acids remains unknown. Because the pKa of SCFA is ∼5, SCFAs are mostly dissociated at neutral pH, existing in the anionic form, which may be absorbed via transporters such as the MCT, rather than, as has been historically proposed, absorbed passively in the undissociated form through the colonic epithelium at luminal pH < 6 (Ruppin et al. 1980; Umesaki et al. 1980). Therefore, the stimulation of HCO3− secretion by luminal SCFA may help the duodenal mucosa absorb SCFA anions by raising the luminal pH, and thus increasing the relative concentration of the anionic form.
Because the presence of luminal SCFAs can signify the presence of luminal bacterial growth, elevated SCFA concentrations in the foregut lumen may represent bacterial overgrowth, which is implicated in the pathogenesis of FD (Costa et al. 2012). We showed that SCFA activation of luminal sensors followed by GLP-2 and 5-HT release with subsequent presumed neuronal activation forms the basis of a plausible pathophysiological mechanism incorporating three well-documented clinical observations: (1) irritable bowel syndrome and FD can be associated with small intestinal bacterial overgrowth (Costa et al. 2012; Ghoshal & Srivastava, 2014); (2) FD symptoms such as nausea, epigastralgia, bloating and fullness can be treated with 5-HT receptor modulators (Beattie & Smith, 2008); and (3) lipids introduced into the duodenal lumen often reproduce dyspeptic symptoms in FD patients (Barbera et al. 1995). Therefore, the results of the present study suggest that 5-HT release via FFA2 activation by luminal SCFA derived from bacterial overgrowth may contribute to the generation of FD symptoms. Similarly, the expression of FFA1 in CCK-positive I cells that release CCK in response to LCFA (Liou et al. 2011), further supports the hypothesis that luminal fat produces FD symptoms via CCK release and CCK1 activation (Feinle et al. 2001).
Controversy still surrounds the existence of FFAR expression and hormone release in response to FAs. FFA1 activation releases CCK, which may increase duodenal HCO3− secretion (Flemström et al. 1982; Konturek et al. 1985), whereas GW9508-induced HCO3− secretion was not affected by CCK1 receptor antagonist SR27897 (data not shown). FFA2 activation by PA1 increased GLP-1 release from primary colonic cultures (Tolhurst et al. 2012), whereas PA1, which had no effect on GLP-2 release in the duodenum, activated EC cells in the present study. These differences can be explained by the overlapping expression of FFARs and hormones in enteroendocrine cells, presumably dependent upon the endocrine cell lineage (Egerod et al. 2012; Habib et al. 2012). For example, although EC cells and I cells are assumed to be distinct, 5-HT/CCK double-positive endocrine cells are present in rat duodenum with up to 50% overlap (Cho et al. 2014), suggesting that the release of 5-HT or CCK by receptor activation may be depend on whether the cells predominantly express FFA1 or FFA2. These findings support the presumed variability of a hormonal and mediator response to luminal FAs, in addition to segmental or species differences, which may underlie the marked variation of clinical irritable bowel syndrome or FD.
The presence of SCFA sensing in the duodenum with the release of GLP-2 and stimulated HCO3− secretion suggests that the described pathways differentially activated by FFA1 and FFA3 agonists might be useful clinically. Exogenous potent FFA1 and FFA3 agonists may ameliorate small intestinal injury via GLP-2 release, similar to the previously described mechanism by which nutrient receptor activation combined with DPPIV inhibition accelerates the healing of non-steroidal anti-inflammatory drug-induced enteropathy (Inoue et al. 2014) or increases the rate of mucosal proliferation in the treatment of short bowel syndrome (Tappenden et al. 2003). Conversely, FFA2 receptor antagonists might be useful in the treatment of FD by kerbing the 5-HT and neutrally mediated responses to foregut SCFAs.
In conclusion, duodenal enteroendocrine cells express functional FFARs, which, when activated, release GLP-2 in addition to the highly bioactive mediators 5-HT and acetylcholine. This novel pathway, with its inherent ability to locally regulate hormone and mediator release, may serve important functions in regard to mucosal protection and repair but, when deregulated, may contribute towards the genesis of FD-related symptoms.
Acknowledgments
We thank Bea Palileo for her assistance with the preparation of the manuscript.
Glossary
Abbreviations
- CCK
cholecystokinin
- 4-CHCA
α-cyano-4-hydroxycinnamic acid
- DPPIV
dipeptidyl peptidase-IV
- EC
enterochromaffin
- FD
functional dyspepsia
- FFAR
free fatty acid receptor
- GLP
glucagon-like peptide
- GPCR
G protein-coupled
- GST
glutathione S-transferase
- LCFA
long-chain fatty acid
- MCT1
monocarboxylate transporter 1
- PV
portal venous
- SCFA
short-chain fatty acid
- VIP
vasoactive intestinal peptide
Additional information
Competing interests
All authors declare that they have no conflicts of interest.
Author contributions
Y.A. and J.D.K. were responsible for the study concept and design. Y.A., T.I., I.K., M.H., K.I. and M.W. were responsible for the acquisition of data. Y.A., T.I., I.K., M.H., P.H.G., E.E., A.K. and J.D.K. were responsible for data analysis. Y.A. and J.D.K. were responsible for manuscript preparation and revision. I.K. and M.W. were responsible for antibody production. K.N. was responsible for the acquisition of additional data. K.I. was responsible for chemical design and synthesis. P.H.G., E.E., A.K. and J.D.K. were responsible for the interpretation of data.
Funding
This work was supported by a Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J. Kaunitz), a Grant-in-aid for Japanese Society for the Promotion of Science Fellow (I. Kaji, 24-5317) and the animal core of NIH-NIDDK P30 DK0413 (J. E. Rozengurt).
References
- Akiba Y. Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion. 2011;83(Suppl 1):25–31. doi: 10.1159/000323401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y. Kaunitz JD. Duodenal chemosensing: Master control for epigastric sensation. J Gastroenterol Hepatol. 2011;26:6–7. doi: 10.1111/j.1440-1746.2010.06580.x. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Mizumori M, Guth PH, Engel E. Kaunitz JD. Duodenal brush border intestinal alkaline phosphatase activity affects bicarbonate secretion in rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1223–G1233. doi: 10.1152/ajpgi.00313.2007. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Mizumori M, Kuo M, Ham M, Guth PH, Engel E. Kaunitz JD. CO2 chemosensing in rat oesophagus. Gut. 2008;57:1654–1664. doi: 10.1136/gut.2007.144378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiba Y, Watanabe C, Mizumori M. Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G791. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera R, Feinle C. Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol. 1995;7:1051–1057. doi: 10.1097/00042737-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Beattie DT. Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedeberg's Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- Brighenti F, Castellani G, Benini L, Casiraghi MC, Leopardi E, Crovetti R. Testolin G. Effect of neutralized and native vinegar on blood glucose and acetate responses to a mixed meal in healthy subjects. Eur J Clin Nutr. 1995;49:242–247. [PubMed] [Google Scholar]
- Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM. Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S. Muir AI. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A. Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Callaghan B, Bron R, Bravo DM. Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 2014;356:77–82. doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- Costa MB, Azeredo IL, Jr, Marciano RD, Caldeira LM. Bafutto M. Evaluation of small intestine bacterial overgrowth in patients with functional dyspepsia through H2 breath test. Arq Gastroenterol. 2012;49:279–283. doi: 10.1590/s0004-28032012000400009. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP. Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. Luminal sensing in the gut: an overview. J Physiol Pharmacol. 2003;54:9–17. [PubMed] [Google Scholar]
- Drucker DJ, Erlich P, Asa SL. Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- Edfalk S, Steneberg P. Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS. Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelstoft MS, Egerod KL, Holst B. Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Feinle C, Meier O, Otto B, D'Amato M. Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. 2001;48:347–355. doi: 10.1136/gut.48.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein VA, Coy DH, Hocart SJ, Jiang NY, Mrozinski JE, Jr, Mantey SA. Jensen RT. A chimeric VIP-PACAP analogue but not VIP pseudopeptides function as VIP receptor antagonists. Peptides. 1994;15:95–100. doi: 10.1016/0196-9781(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Flemström G, Heylings JR. Garner A. Gastric and duodenal HCO3− transport in vitro: effects of hormones and local transmitters. Am J Physiol Gastrointest Liver Physiol. 1982;242:G100–G110. doi: 10.1152/ajpgi.1982.242.2.G100. [DOI] [PubMed] [Google Scholar]
- Fried M. Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. 2002;51(Suppl 1):i54–i57. doi: 10.1136/gut.51.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JD, Grossman CJ, Whitehead JW, Oxford AW, Bunce KT. Humphrey PP. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br J Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC. Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol. 2014;20:2482–2491. doi: 10.3748/wjg.v20.i10.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL. Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Gully D, Frehel D, Marcy C, Spinazze A, Lespy L, Neliat G, Maffrand JP. Le FG. Peripheral biological activity of SR 27897: a new potent non-peptide antagonist of CCKA receptors. Eur J Pharmacol. 1993;232:13–19. doi: 10.1016/0014-2999(93)90722-t. [DOI] [PubMed] [Google Scholar]
- Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F. Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer HH, Turkelson CM, Solomon TE. Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol Gastrointest Liver Physiol. 1994;267:G625–G629. doi: 10.1152/ajpgi.1994.267.4.G625. [DOI] [PubMed] [Google Scholar]
- Hoverstad T, Bjorneklett A, Midtvedt T, Fausa O. Bohmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol. 1984;19:1053–1058. [PubMed] [Google Scholar]
- Illman RJ, Topping DL. Trimble RP. Effects of food restriction and starvation-refeeding on volatile fatty acid concentrations in the rat. J Nutr. 1986;116:1694–1700. doi: 10.1093/jn/116.9.1694. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higashiyama M, Kaji I, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD. Akiba Y. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci. 2014;59:1286–1295. doi: 10.1007/s10620-013-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD. Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Karaki S, Tanaka R. Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol. 2011;42:27–38. doi: 10.1007/s10735-010-9304-4. [DOI] [PubMed] [Google Scholar]
- Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB. Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y. Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H. Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek SJ, Bilski J, Tasler J. Laskiewicz J. Gut hormones in stimulation of gastroduodenal alkaline secretion in conscious dogs. Am J Physiol Gastrointest Liver Physiol. 1985;248:G687–G691. doi: 10.1152/ajpgi.1985.248.6.G687. [DOI] [PubMed] [Google Scholar]
- Lambeir AM, Durinx C, Scharpe S. De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, Liu J, Kayser F, Tian H. Li Y. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol. 2008;74:1599–1609. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- Liatis S, Grammatikou S, Poulia KA, Perrea D, Makrilakis K, Diakoumopoulou E. Katsilambros N. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur J Clin Nutr. 2010;64:727–732. doi: 10.1038/ejcn.2010.89. [DOI] [PubMed] [Google Scholar]
- Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE. Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya M, Mase T, Tsuchiya Y, Kawakami K, Hattori H, Kobayashi K, Ogino Y, Fujikawa T, Satoh A, Kimura T, Noguchi K, Ohtake N. Tomimoto K. J-104129, a novel muscarinic M3 receptor antagonist with high selectivity for M3 over M2 receptors. Bioorg Med Chem. 1999;7:2555–2567. doi: 10.1016/s0968-0896(99)00177-7. [DOI] [PubMed] [Google Scholar]
- Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD. Akiba Y. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol. 2006;573:827–842. doi: 10.1113/jphysiol.2006.107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier Poulsen S, Han S, Jones RM, Offermanns S. Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in nteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- Östman E, Granfeldt Y, Persson L. Bjorck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–988. doi: 10.1038/sj.ejcn.1602197. [DOI] [PubMed] [Google Scholar]
- Ruppin H, Bar-Meir S, Soergel KH, Wood CM. Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78:1500–1507. [PubMed] [Google Scholar]
- Tappenden KA, Albin DM, Bartholome AL. Mangian HF. Glucagon-like peptide-2 and short-chain fatty acids: a new twist to an old story. J Nutr. 2003;133:3717–3720. doi: 10.1093/jn/133.11.3717. [DOI] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M. Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F. Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesaki Y, Yajima T, Tohyama K. Mutai M. Characterization of acetate uptake by the colonic epithelial cells of the rat. Pflügers Arch. 1980;388:205–209. doi: 10.1007/BF00658482. [DOI] [PubMed] [Google Scholar]
- Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD. Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Levi AJ. Halestrap AP. Substrate and inhibitor specificities of the monocarboxylate transporters of single rat heart cells. Am J Physiol Heart Circ Physiol. 1996;270:H476–H484. doi: 10.1152/ajpheart.1996.270.2.H476. [DOI] [PubMed] [Google Scholar]


